Abstract
The causes of stress urinary incontinence are not completely known. Recent papers have stressed the importance of more anatomical information, which may help to elucidate the mechanism of stress urinary incontinence. The purpose of this study was to evaluate the prevalence of lesions of the urethral support mechanism and lesions (defects and scars, thinning) of levator ani muscle with endovaginal MRI in a case-control study. Forty women (median age 52 years, age range 40–65 years)—20 patients with stress urinary incontinence (cases) and 20 age-matched healthy volunteers (controls)—underwent endovaginal MRI: axial, coronal, and sagittal T2-weighted turbo spin echo. The examinations were evaluated for the presence of lesions of urethral supporting structures and levator ani and scar tissue of the levator ani. The thickness of the levator ani muscle was measured. Lesions of the urethral support system and levator ani were significantly more prevalent in cases than in controls (p<0.01). Median levator ani thickness in patients was significantly lower than in healthy controls [2.5 mm (range 0.9–4.1 mm) vs 3.9 mm (range 1.4–7 mm)] (p<0.01). This study indicates a relationship between stress urine incontinence and the presence of lesions of the urethral support and levator ani and levator ani thinning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence is a relatively frequent disorder (prevalence approximately 5%), which has considerable social, medical, and economical impact. Incidence increases with age [1, 2] and approximately half of the women older than 45 years have symptoms of urinary incontinence [3]. This has substantial impact on the quality of life [3, 4] and leads to social withdrawal in approximately 20% of the involved individuals [5]. Incontinence is commonly stress, urge, or a combination of the two [5].
Stress urinary incontinence is the complaint of involuntary leakage upon effort or exertion, or upon sneezing or coughing [6]. Urinary stress incontinence can be caused by hypermobility of the bladder neck, primarily due to weakened pelvic floor support, either to denervation, musculofascial defects, or both [7, 8, 9, 10, 11]. Bladder neck hypermobility results in ineffective transfer of intra-abdominal pressure onto the urethra, preventing closure. Deficiency of the sphincter can be caused by nerve injury, surgical injury (e.g., bladder neck suspension), or trauma.
Differentiation between the types of stress urinary incontinence is of importance, as these types of urinary incontinence have different surgical treatment after failed conservative treatment (e.g., pelvic-floor muscle training). Patients with hypermobility of the bladder neck are treated by one of the different types of colposuspension operations, whereas more recently the tension-free vaginal tape procedure is gaining popularity. Incontinence due to intrinsic sphincter deficiency is treated by injection of bulking agents, a classical pubovaginal sling procedure, or an artificial urinary sphincter.
Current workup of patients with stress urinary incontinence comprises clinical history, physical examination, voiding diary, and urodynamic studies (e.g., Valsalva leak point pressure, urethra pressure profile, and bladder volume at first leak). Urodynamics are performed to exclude detrusor instability and voiding difficulties. Despite this extensively predominantly functional workup, results of surgery in patients with stress urinary incontinence are less optimal than might be expected [12].
Expanding the diagnostic workup with a technique revealing a detailed visualization of the urethra, bladder neck, and supporting structures might result in more insight leading to a better differentiation between the types of stress incontinence, and may result in a better stratification for treatment. Recent studies have demonstrated the accurate demonstration of the urethra, urethral support system, and levator ani muscle with high-resolution MR imaging [13, 14, 15].
The purpose of this case-control study was to compare the findings concerning lesions in the urethra-support structures and the levator ani and the thickness of the levator ani at endovaginal MRI in patients with stress urinary incontinence and normal age-matched individuals. The authors chose this study design because confirmation of the findings at surgery is not possible. Many patients are treated without surgery or have minimally invasive surgery. In open surgery the urethra and supporting structures cannot be evaluated without extending the procedure beyond ranges appropriate for incontinence surgery, which would result in damaging the urethra, its supportive structures, and nerves, whereas part of the supportive structures is not discernible at surgery.
Materials and methods
In the period between March 1998 and January 2001, 40 women (median age 52 years, age range 40–65 years) were included. These 40 women concerned 20 patients with stress urinary incontinence and 20 age-matched normal volunteers. Patients were included when the diagnosis stress urinary incontinence was established at routine workup, comprising clinical history, physical examination, and urodynamic examinations (Valsalva leak pressure, urethra pressure profile, and bladder volume at first leak). Controls were recruited from a population volunteering for pelvic-floor research. Normal continence in the control group was established by a questionnaire. Normal volunteers were age matched within a range of 3 years of the age of the case. In the patient group all patients had one or more deliveries (median 1.4, range 1–5). In the control group all but 7 patients had one or more deliveries (median 1.2, range 0–6). None of the 40 participants had surgery and all patients were treated with conservative treatment (primarily physiotherapy).
The institutional review boards of the participating hospitals approved the study and written informed consent was obtained in all patients and controls.
Endovaginal MRI procedure
Patients and controls underwent endovaginal MRI at 1 T (Gyroscan NT 10, Philips Medical Systems, Best, The Netherlands) and 1.5 T (Philips Gyroscan NT 15; Horizon Echospeed, General Electric, Milwaukee, Wis.). Endovaginal MRI was performed with a dedicated rigid cylindrical coil (diameter 15–17 mm diameter, length 10 cm). Preparation included fasting for 4 h and information on the procedure. The endoluminal coil was covered with a condom and a small amount of lubricant (sonography gel) was applied. The coil was inserted in supine position. For a stable position, the coil was secured by pads or sandbags. After coil introduction, 20 mg butylscopalaminebromide (Buscopan, Boehringer Ingelheim, Germany) was injected intramuscularly to reduce bowel motion artifacts. Individuals were asked to relax the pelvic-floor muscles to reduce motion artifacts.
The studies were performed at 1.0 T for all controls and 2 cases. All other cases were studied at 1.5 T at the three participating institutions. Imaging comprised axial, coronal, and sagittal T2-weighted turbo-spin-echo sequences (TSE; TR/TE 2800–3000 ms/90–120 ms; echo train length 11–15; field of view 90×120 mm to 120–140 mm; 226×256 imaging matrix; 3-mm slice thickness; 0.3-mm interslice gap; number of excitations 4–8). The sequences were angulated to be parallel or orthogonal to the urethra from bladder neck to external meatus.
Image evaluation
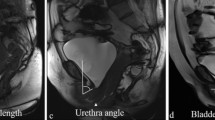
All images were evaluated in consensus by two radiologists with experience in endoluminal MR imaging of the pelvic floor. Examinations were studied blinded for the clinical condition (case or control), patient data, institution and MR machine. Image quality was scored as good, adequate, or inadequate. The examinations were evaluated for the presence of lesions of the urethral supporting structures, defects (paravaginal defect), or scar tissue of the levator ani muscle, and for the presence of atrophy of the levator ani muscle. The urethral support structures were evaluated as one entity and considered normal when there was no distortion or defect. Distortion was defined as an internal architectural change with waviness of the ligament and a defect as discontinuity of the ligament with visualization of the torn parts. Subtle changes as well as asymmetry without obvious lesions were not considered abnormal. No attempt was made to separately evaluate the two primary structures of the urethral support system as defined by Tan et al. [14]: periurethral ligament and paraurethral ligaments. This refinement in classification was not attempted, as there is a wide variation in presentation forms of these structures, even in normal individuals without urogynecological medical history or symptoms [14]. This variation includes the absence of part of the structures in normal volunteers; although in each normal individual at least part of the normal urethral support system is visible [14]; therefore, the urethral support system was evaluated as an entity (Fig. 1). Visualization of normal urethral support at one side with asymmetry at the other side was considered normal when no obvious lesion at the other side was visible (Fig. 2). The levator ani muscle (iliococcygeus and pubococcygeus parts) was evaluated for the presence of defects, which is disruption of the normal close relationship of the pubovisceral (puborectal) and levator ani muscle, and for scar tissue, which is distorted normal anatomy with hypointense scar tissue. The thickness of the levator ani muscle was measured at maximal thickness on the coronal sequences. All evaluations and measurements were performed at a workstation (Easy Vision, Philips Medical Systems, Best, The Netherlands) by one of the observers, using electronic calipers for measurements.
Two consecutive images of an axial T2–weighted turbo spin echo demonstrates the normal anatomy of the urethral support system in a normal control. Curved arrow indicates urethral support system. U urethra, LA levator ani, A anterior, L left. In most normal individuals the anatomy of the urethral support system is variable between both sides, as in this control. This variation may be subtle or may be more pronounced. A normal urethral support at one side with asymmetry at the other side, but no obvious lesion, is considered a normal finding
Axial T2-weighted turbo-spin-echo image in a patient with stress urinary incontinence reveals a relatively subtle lesion of the urethral support system at the left side (arrows) and slight waviness at the right side. The morphology at the right side can be considered a variation of normal. U urethra
Statistical evaluation
Differences between patients and controls in the presence of lesions of the urethral support system and levator ani muscle were assessed by means of the Fischer exact test. Differences in thickness of the levator ani muscle were assessed with the Mann-Whitney U test. For all statistical analyses, a p value of less than 0.05 was considered to indicate a statistically significant difference.
Results
All procedures were performed uneventfully. No case or control experienced any significant discomfort. Image quality was good in 32 and adequate in 8, with 5 of the adequate exams in the patient group.
The combined evaluation of the imaging sequences revealed lesions of the urethral support structures in 9 of 20 cases (Figs. 3, 4). In controls lesions of the urethral support structures were found in 2 of 20 (one of these two had previous deliveries). Defects of the levator ani muscle (paravaginal defects) only were present in cases (13 cases) and not in controls. Scar tissue of the levator ani muscle was present in 5 cases and in 1 control. Lesions were significantly more prevalent in cases than in controls (p<0.01). Median levator ani thickness in cases was significantly lower than in controls [2.5 mm (range 0.9–4.1) vs 3.9 mm (range 1.4–7)] (p<0.01).
Two consecutive images of an axial T2-weighted turbo spin echo in a patient with stress urinary incontinence. Gross distortion of the urethral support mechanism at both sides. At the right the urethral support is partly fragmented and partly not discernable, whereas there is extensive scar tissue at the left (arrows; compare with Fig. 1). U urethra
a Coronal and b axial T2-weighted turbo-spin-echo image in a patient with stress urinary incontinence demonstrates normal anatomy of the levator ani muscle at the right side (LA; black and white straight arrows in a) and a paravaginal defect (arrows) and thinning of the levator ani muscle at the left side. L left, H head, A anterior. Measurement of the levator ani thickness is indicated in a
Discussion
The urethra support structures connect the urethra to the pubovisceral (puborectal) muscle and the arcus tendineus fascia pelvis (tendineus arch at the levator ani surface). These connections are important in immobilizing the urethra against downward force by increased abdominal pressure. The levator ani borders the urogenital hiatus, with levator ani contraction leading to enforcement of the closure at increased abdominal pressure.
This study demonstrated that in stress-urine-incontinent women there is a higher prevalence of lesions of the urethral support system and levator ani muscle as well as a thinner levator ani muscle as compared with age-matched controls. The significantly higher number of lesions of the urethral support structures and levator ani in this study supports the hypothesis that these supportive structures are important in preserving continence. The vagina anchored to the pelvic sidewall by these supportive structures is considered to act as a hammock supporting the urethra [16].
Recently, several papers have reported on the visualization of the urethra support system with MR imaging, both with endovaginal MRI and external coil MRI [14, 15, 17]. In the radiology literature two principal urethral support structures have been described, the periurethral and paraurethral ligament [14]. In the urogynecology literature a different descriptive nomenclature approach is used. The structure defined as periurethral ligament in the radiology literature probably is identical to the structure defined as pubovesical muscle in the urogynecology literature [15]. The paraurethral ligaments may be identical to the endopelvic fascia in the urogynaecology literature [18].
In this study we evaluated the urethral support as an entity. Separate evaluation of principal urethral support structures is cumbersome because, although urethral support structures can be identified in normal volunteers, there is variation in presence and form of these structures [14]. Evaluation of the urethral support structures as an entity and scrutinizing this for tautness and lesions overcomes this problem. This also prevents confusion concerning nomenclature. Further studies are needed to more precisely define the normal and abnormal anatomy of the urethral support system. In some normal controls in this study lesions of the urethral support were found. Until now no MRI data are available to determine whether these lesions may be a predicting factor for urinary incontinence with increasing age.
Several studies using MRI have studied the levator ani, including the visualization of defects of the vaginal support (paravaginal defect) [19, 20]. The paravaginal defect is considered an important cause of stress urinary incontinence and is defined as detachment of the urethra and vaginal support structures (endopelvic fascia) from the arcus tendineus fascia pelvis during delivery. The tendineus arch anchors the urethra and vagina to the pelvic sidewall. This study confirms the potential of MRI in visualizing defects of the urethral support structures in stress-incontinent women, which may have consequences for the choice of treatment modality [21]. Findings in this study also may be valuable for the prevention of pelvic-floor defects as more insight is gained into the relevance of these defects [22]. A recent study on endovaginal MRI studied the paravaginal fascia in 11 continent and 10 stress-incontinent women and reported changes of the paravaginal fascia in incontinent individuals [23]. These findings support our findings.
In the present study there was a significant thinning of the levator ani muscle in patients with stress urinary incontinence as compared with age-matched controls. Atrophy of pelvic-floor muscles is an important finding. Although this study was not designed to address the potential influence of levator ani atrophy on management, a potential important role of levator ani atrophy on stress urinary incontinence and management outcome is hypothesized. In fecal incontinence atrophy of the external anal sphincter at endoluminal MRI has been demonstrated to be important for patient management [24]. Further studies may address this important issue.
The number of cases and controls included were limited. Nevertheless, a significant difference between cases and controls was present. Matching was performed for age and not for a combination for both age and deliveries. We chose to match for age, as matching for both age and number of deliveries was very cumbersome. There was no major difference in the overall number of deliveries between cases and controls, although 7 of 20 controls had not had a delivery.
In this study the role of phased-array coils was not addressed. Although the local spatial resolution of an endovaginal coil is superior to an external phased array, recent studies have demonstrated that the image quality of phased-array-coil MRI may suffice for evaluation of the urethral support system. Whether the use of an endovaginal coil is advantageous as compared with the more widely available phased-array coils in the evaluation of patients with stress urinary incontinence remains to be addressed.
The findings of this study suggest a relationship between stress urine incontinence and the presence of lesions of the urethral support and levator ani and levator ani thinning as visualized at endovaginal MRI. Based on these findings other studies, including outcome-related studies, may be initiated to further elucidate the role of MRI in the management of patients with stress urinary incontinence.
References
Castleden CM, Duffin HM (1985) Factors influencing outcome in elderly patients with urinary incontinence and detrusor instability. Aging 14:303
Thomas TM, Plymat KR, Blannin J, Meade (1980) TW prevalence of urinary incontinence. Br Med J 281:1243–1245
Vaart van der CH, Leeuw de JRJ, Roovers JPWR, Heintz APM (2000) De invloed van urine–incontinentie op de kwaliteit van leven bij thuiswonende vrouwen Nederlandse vrouwen van 45–70 jaar. Ned Tijdsch Geneesk 144:894–897
Rekers H, Drogendijk AC, Valkenburg, HA, Riphagen F (1992) The menopause, urinary incontinence and other symptoms of the genito-urinary tract. Maturitas 15:101–111
Payne CK (1998) Epidemiology, pathophysiology and evaluation of urinary incontinence and overactive bladder. Urology 51 (Suppl 2A):3–10
Abrams P, Cardozo L, Fall M et al. (2002) The standardisation of terminology of lower urinary tract function. Neuro-urology and urodynamics 21:167–178
Smith ARB, Hosker GL, Warrell DW (1989) The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine. A neurophysiological study. Br J Obstet Gynecol 96:24–28
Smith ARB, Hosker GL, Warrell DW (1989) The role of pudendal nerve damage in the aetiology of genitourinary prolapse and stress incontinence of urine. A neurophysiological study. Br J Obstet Gynaecol 96:29–32
Gilpin SA, Gosling JA, Smith ARB, Warrell DW (1989) The pathogenesis of genitourinary prolapse and stress incontinence of urine. A histological and histochemical study. Br J Obstet Gynaecol 96:15–23
Snooks SJ, Barnes, Swash M (1984) Damage to the innervation of the voluntary anal and periurethral sphincter musculature in incontinence: an electrophysiological study. J Neurol Neurosurg Psychiatry 47:1269–1273
Snooks SJ, Badenoch DF, Tiptaft RC, Swash M (1985) Perineal damage in genuine stress urinary incontinence. An electrophysiological study. Br J Urol 57:422–426
Stoker J, Halligan S, Bartram CI (2001) Pelvic floor imaging. Radiology 218:621–641
Tan IL, Stoker J, Laméris JS (1997) Magnetic resonance imaging of the female pelvic floor and urethra: body coil versus endovaginal coil. MAGMA 5:59–63
Tan IL, Stoker J, Zwamborn AW, Entius KA, Calame JJ, Laméris JS (1998) Female pelvic floor: endovaginal MR imaging of normal anatomy. Radiology 206:777–783
Tunn R, DeLancey JOL, Quint EE (1998) Visibility of pelvic organ support system structures in magnetic resonance images without an endovaginal coil. Am J Obstet Gynecol 184:1156–1163
DeLancey JO (1994) Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 170:1713–1720
Chou Q, DeLancey JO (2001) A structured system to evaluate urethral support anatomy in magnetic resonance images. Am J Obstet Gynecol 185:44–50
Stoker J (2003) The anatomy of the pelvic floor and sphincters. In: Bartram CI, DeLancey JOL (eds) Imaging pelvic floor disorders. Springer, Berlin Heidelberg New York, pp 1–26
Huddleston HT, Dunnihoo DR, Huddleston PM, Meyers PC (1995) Magnetic resonance imaging of defects in DeLancey's vaginal support levels I, II and III. Am J Obstet Gynecol 172:1778–1784
Strohbehn K, Ellis J, Strohbehn JA, DeLancey JOL (1996) Magnetic resonance imaging of the levator ani with anatomic correlation. Obstet Gynecol 87:277–285
Bruce RG (1999) El–Galley RES, Galloway NTM. Paravaginal defect repair in the treatment of female stress urinary incontinence and cystocele. Urology 54:647–651
Handa VL, Harris TA, Ostergard DR (1996) Protecting the pelvic floor: obstetric management to prevent incontinence and pelvic organ prolapse. Obstet Gynecol 88:470–478
DeSouza NM, Daniels OJ, Williams AD, Gilderdale DJ, Abel PD (2002) Female urinary genuine stress incontinence: anatomic considerations at MR imaging of the paravaginal fascia and urethra. Initial observations. Radiology 225:433–439
Briel JW, Stoker J, Rociu E, Laméris JS, Hop WCJ, Schouten WR (1999) External anal sphincter atrophy on endoanal magnetic resonance imaging adversely affects continence after sphincteroplasty. Br J Surg 86:1322–1327
Acknowledgement
J. Stoker acknowledges the ECR for the ECR Research and Education Fund grant 1999 for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoker, J., Rociu, E., Bosch, J.L.H.R. et al. High-resolution endovaginal MR imaging in stress urinary incontinence. Eur Radiol 13, 2031–2037 (2003). https://doi.org/10.1007/s00330-003-1855-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-1855-2