Abstract
Amongst the nototheniid subfamily Pleuragramminae, Aethotaxis mitopteryx is an infrequently collected high Antarctic species with an array of morphological and physiological adaptations supporting an evolutionarily derived benthopelagic lifestyle. The present study deals with some poorly known life history traits of this species, counting on 79 specimens collected in the Weddell Sea during 2014 and 2015 austral summer. Annulation pattern in sagittal otoliths were used to assess population age structure and growth rate, while macroscopic and histological analyses of gonads were performed to estimate reproductive status and features of gametogenesis. The sex ratio of the sampled population was close to parity, with females significantly larger than males. Based on the von Bertalanffy growth model, females attained a larger maximum size (40 vs. 27 cm) at a lower rate (0.05 vs. 0.12 years−1) than males. Individual longevity was remarkable in both sexes, females and males attaining 62 and 32 years of age, respectively. Females showed group synchronous oocyte development and presumed high reproductive effort, as indicated by the large size of residual hydrated oocytes in regressing individuals (4.6–4.8 mm). Body sizes at sexual maturity were 33 and 19 cm in females and males, corresponding to 32 and 11 years of age, respectively. All specimens were caught far from the reproductive season. From an evolutionary perspective, it appears that the process of pelagization similarly influenced the life strategies of the species within the clade Pleuragramminae, which shared high reproductive effort linked to early sexual maturity, slow somatic growth and long life span.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The modern fish fauna inhabiting the Antarctic continental shelf is largely dominated by a single endemic perciform suborder, the Notothenioidei (Kock 1992). In the early Miocene, a series of tectonic and oceanographic events progressively isolated the Antarctic Continent, altering the local faunal composition. Loss of habitat, low temperature regimes and changes in the trophic structure of the ecosystem probably led to the extinction of the Eocene components of the fish fauna (Eastman 2005). Since then, in filling habitats newly devoid of competitors, the notothenioids underwent an adaptive radiation exhibiting a remarkable morphological and ecological diversification. Although notothenioids shared a benthic, bladderless ancestor (Eastman 1993), some species evolved an array of morphological and physiological characters to utilize unfilled niches in the water column, an evolutionary process sometimes referred to as pelagization (Klingenberg and Ekau 1996). Despite lacking swim bladders, some lineages of Nototheniidae, most notably the Pleuragramminae, adapted to a pelagic lifestyle by evolving towards neutral buoyancy through a combination of reduced skeletal mineralization and intramuscular lipid deposition (Eastman 1993).
Based on morphological characters and molecular data, the “pelagic” clade was found to be monophyletic and basal amongst the Nototheniidae, including four genera (Aethotaxis, Dissostichus, Gvozdarus and Pleuragramma) within the subfamily Pleuragramminae (Andersen 1984) or Pleuragrammatinae (Balushkin 2000; Sanchez et al. 2007). Except for Dissostichus, which includes two large species with partially overlapping circum-Antarctic distributions, the other genera are monospecific and attain small to medium size (DeWitt et al. 1990). This clade has developed rather recently, between 10 and 20 million years ago (Near et al. 2012), evolving physiological adaptations (e.g. neutral buoyancy) to accomplish recent changes in lifestyle (e.g. Wöhrmann 1998). Although they share the same pelagic environment, data on blood physiology and oxygen-carrying capacity indicated that some genera (Aethotaxis and Pleuragramma) are sluggish with a limited scope for activity, while others (Dissostichus) are regarded as more active (e.g. Kunzmann 1991).
Due to their dominance and key role in the pelagic food web of the Antarctic continental shelf (Pleuragramma antarctica) and/or their importance for commercial fisheries purposes (Dissostichus eleginoides and D. mawsoni), a huge amount of data have been reported on biological and ecological features of these species (recently reviewed by Collins et al. 2010; La Mesa and Eastman 2012; Hanchet et al. 2015). On the other hand, Aethotaxis mitopteryx is one of the lesser-known species of the family Nototheniidae (Kunzmann and Zimmermann 1992), while Gvozdarus svetovidovi is known only from the holotype taken in the Ross Sea and another specimen collected in the Cooperation Sea (DeWitt et al. 1990).
Compared to other nototheniids, A. mitopteryx was described somewhat recently from McMurdo Sound, Ross Sea (DeWitt 1962). The common name, threadfin pithead, is due to the unique elongated spines and rays associated with the first dorsal and pelvic fins, as well as to the pores on head appearing as large holes or pits (DeWitt et al. 1990). It is a rare species with a supposed circum-Antarctic distribution, having been sparsely recorded from the Ross Sea, Cooperation Sea, Lazarev Sea, Weddell Sea and along the islands of the Scotia Sea (DeWitt et al. 1990, Zimmermann 1997). As an example, four Polarstern expeditions carried out between 1985 and 1992 in the Weddell and Lazarev Seas yielded a total of 99 A. mitopteryx, representing 90% of total catches of this species reported until then (Kunzmann and Zimmermann 1992).
The streamlined appearance of body placed A. mitopteryx at the lowest rank of morphological characteristics standing for pelagic life style (Ekau 1988). On the basis of skeletal reduction and lipid deposition in typical subcutaneous adipose cells, Eastman and DeVries (1982) argued that this species was probably close to neutral buoyancy, as recently confirmed by direct measurements on fresh specimens collected from the Bransfield Strait (Near et al. 2007). Accordingly, A. mitopteryx feed almost exclusively on small zooplanktonic prey such as krill, copepods and amphipods (Kunzmann and Zimmermann 1992), as suggested by the numerous long and slender gill rakers (Eastman 1993). Like P. antarctica and G. svetovidovi, A. mitopteryx exhibit extensive peritoneal and gut melanism reducing potential predation due to zooplanktonic prey bioluminescence (Eastman and DeVries 1997).
In an evolutionary perspective, the pelagic mode of life similarly influenced the life history strategies of the species within the clade Pleuragramminae. Compared to other nototheniids, they attain sexual maturity earlier (i.e. at 30–50% of maximum age) and exhibit higher absolute (Dissostichus spp.) or relative fecundity (P. antarctica), producing pelagic eggs floating free in the water column or layer under the platelet ice (Evseenko et al. 1995; Vacchi et al. 2004). In turn, a large parental investments in reproduction largely contribute at determining their slow somatic growth after sexual maturity linked to a long life-span (La Mesa and Vacchi 2001).
Whether A. mitopteryx evolved similar life history strategies such as the relative timing of sexual maturity and long life span representative of other clades of Pleuragramminae inhabiting the same pelagic environments is not known. To address this issue, we aim to study the reproductive traits from juvenile and adult specimens of A. mitopteryx collected in the Weddell Sea using macroscopic and histological analyses of gonads. In addition, we examine the age structure and growth rate of local population through the sagittal otoliths growth increment pattern.
Materials and methods
Fish sampling
Samples of A. mitopteryx were collected during two Polarstern expeditions carried out in the southern Weddell Sea (PS82, ANT-XXIX/9 during December 2013–March 2014; PS96, ANT-XXXI/2 during December 2015–February 2016). All the specimens were sampled in two stations (PS82/341-1, 72°47,93′S, 19°29,71′W, 14 February 2014; PS96/090-11, 72°19,24′S, 16°53,74′W, 29 January 2016) located off the Riiser–Larsen Ice Shelf at 740 and 882 m depth (Fig. 1, Table 1). The gear was a bottom trawl equipped with 40-mm mesh in the cod end and towed at 3 knots for 30 min (Knust and Schröder 2014; Schröder 2016).
After species identification and sorting, each specimen was measured to the nearest half cm below as total length (TL) and standard length (SL), weighed as total weight (TW, g) and sexed macroscopically through direct examination of gonads. Stage of gonad maturity was assessed according to the five-point scale proposed for nototheniids (Kock and Kellermann 1991). Gonads were excised from the abdominal cavity, weighed (GW, 0.1 g precision) and stored in Dietrich solution (60% distilled water, 30% ethanol, 9% formaldehyde, 1% acetic acid) for further analyses. Sagittal otolith pairs were removed from each specimen and stored dry in numbered vials.
Laboratory activities
For histological analysis of gonads, subsamples of gonad tissue were dehydrated through ascending concentrations of ethanol and embedded in paraplast. Serial transverse sections of 5–7 μm thickness were put on slides, dried at 50 °C and stained with Harrys’ haematoxylin and eosin (Pearse 1985). The histological sections were observed at 50 to ×1000 magnification using a light microscope (LEICA DM400B) linked to a software video analysis system (LAS, Leica Application Suite). Based on the presence of different cellular types and morphological structures within the gonad tissue, each specimen was staged according to five phases of gonad development (Brown-Peterson et al. 2011). For both sexes, the phases were defined as follows: (1) immature (never spawned); (2) developing (gonads beginning to develop, but not ready to spawn); (3) spawning capable (fish are developmentally and physiologically able to spawn); (4) regressing (post spawning); (5) regenerating (sexually mature but reproductively inactive). Representative subsamples of gonads from regressing females (i.e. the only specimens with vitellogenic oocytes) were removed and weighed to determine their oocytes size–frequency distributions. Oocytes were manually separated into a Petri dish, counted and diameter measured using a stereomicroscope.
From each specimen, the weights of left and right sagittal otoliths were recorded (OW, 0.1 mg) and compared to each other using a t test for paired comparisons. As they differed significantly (t = 3.06, df = 71, p < 0.01), right otoliths were arbitrarily selected and their maximum length (OL, 0.01 mm precision) measured using a stereomicroscope linked to the LAS software. As commonly observed in notothenioids (e.g. White 1991), sagittal otoliths of A. mitopteryx were opaque with a dense calcareous matrix, requiring to be sectioned or ground to reveal the inner structure. Right otoliths from all specimens were first burned in an oven at 350 °C for a few minutes to enhance the contrast between growth rings. They were individually embedded in resin (Crystalbond 509 Amber, Aremo Products, Inc.), ground using abrasive paper and polished with a lapping film covered by alumina powder (0.05 µm particle size). Once the annulation pattern was clearly visible from the core to the otolith margin, the transverse sections were fixed on glass slides and observed under reflected light using a stereomicroscope at 25 to ×40 magnification. The annulation pattern consisted of an opaque nucleus surrounded by alternating opaque (light rings) and translucent zones (dark rings). The combination of each pair of opaque and translucent zones was considered to form an annulus, as generally reported for other species of notothenioids (e.g. North 1988). Individual age was assigned by counting all translucent zones, assuming the annual periodicity of annulus formation. Two blind counts were made by the a single reader for each otolith, taking the mean value as individual age estimate.
Data analysis
The length–frequency distributions of sexes were compared by the Kolmogorov–Smirnov’s two-sample test. The departure from the expected 1:1 sex ratio was assessed using a χ2 goodness-of-fit test. The length–weight relationships of fish were calculated for each sex, fitting experimental data to the equation TW = aSL^b, where TW is total weight (g), SL is the standard length (cm) and a and b are the regression parameters. An F-test was applied to test for differences of allometric coefficients (b) between sexes. In addition, a t-test was applied to determine the deviation from isometric growth (b = 3) using the equation t = (b – 3) sb−1, where sb is the standard error of b. The relationship between fish length (SL) and otolith maximum length (OL) was determined by simple linear regression analysis (Hecht 1987). Assumptions of normality and homogeneity of variance were verified by the Shapiro–Wilk test and analysis of residuals for all relationships.
As a measure of reproductive investment, the gonadosomatic index (GSI) was individually calculated as GW/TW × 100. The fish size at sexual maturity was estimated for both sexes by taking into account the smallest maturing/mature male and female (i.e. in phases 2–5), as the relatively few specimens caught did not allow for fitting a logistic model to the proportion of mature fish over the whole size range. The size–frequency distributions of oocytes were recorded in regressing females, to infer their pattern of development (Wallace and Selman 1981).
The average percentage error (APE) and the coefficient of variation (CV) (Beamish and Fournier 1981; Chang 1982) were calculated as a measure of age estimates precision. Age–length data pairs estimated for each sex were fitted by the von Bertalanffy growth model, and the growth parameters (L∞, k, t0) compared by the likelihood ratio test (Kimura 1980). For best fitting, three larvae of A. mitopteryx (17.4, 21.5 and 34.1 mm SL) collected in the Weddell Sea from November to February were added to age estimates of juveniles and adults, assuming they hatched 2–5 months before capture (Kellermann 1990). The growth performance index (Φ′ = 2 log L∞ + log k, Munro and Pauly 1983) was calculated for comparison with other nototheniids.
Results
Fish samples
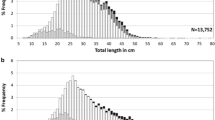
Overall, catches yielded 44 females and 35 males. Females were significantly larger than males (Kolmogorov–Smirnov test, D = 0.83, p < 0.01), ranging between 18 and 45 cm SL and 13 and 31 cm SL, respectively (Fig. 2). Total body weight (TW) ranged from 59 to 1159 g and from 26 to 314 g in females and males. The departure of sex ratio from the proportion 1:1 was not statistically significant (χ2 = 1.02, df = 1, p = 0.311). The length–weight relationships for females and males were TW = 0.0057SL^3.20 and TW = 0.0111SL^2.99, respectively (Fig. 3). The body growth of females was positively allometric (i.e. b > 3) (t = 4.30, df = 42, p < 0.0001), whereas that of males tended towards isometry (b = 3) (t = 0.14, df = 33, p = 0.888). Allometric coefficients (b) were statistically different between sexes (F = 4.71, df = 1,75, p = 0.033).
Reproductive traits
Given the histological examination of gonads, we arrived at the following characteristics to define the phases of gonad maturity.
Females
-
(1)
Immature: small ovaries, with follicles consisting exclusively of oogonia and previtellogenic or primary growth oocytes (Pg) at two stages of development, namely chromatin nucleolar and larger perinucleolar (Fig. 4a).
Fig. 4 Photomicrographs of gonad histology, illustrating the reproductive phases recorded in both sexes of Aethotaxis mitopteryx. a Immature female with sparse oogonia (Oo) and previtellogenic oocytes (Pg), b developing female with follicles consisting of previtellogenic oocytes (Pg) and cortical alveolar oocytes (Ca), c regressing female showing previtellogenic (Pg) and cortical alveolar oocytes (Ca), vitellogenic oocyte at an early stage of development (Vtg) and postovulatory follicles (POF), d regenerating female characterized by a few previtellogenic oocytes (Pg) and oocytes at various stages of atresia (A), e immature male with cysts of spermatogonia (Sg), f, g developing male in active spermatogenesis, with lobules filled by peripheral spermatogonia (Sg), spermatocytes I and II (ScI and ScII) and spermatids (St), h regenerating male with lobules consisting exclusively of spermatogonia (Sg), lumen of lobules and sperm duct (sd) empty
-
(2)
Developing: ovaries increasing in size, with follicles consisting of primary growth and endogenous vitellogenic or cortical alveolar oocytes (Ca), characterized by a large nucleus with several peripheral nucleoli and chromophobic vesicles (cortical alveoli) forming several rows at the periphery of cytoplasm (Fig. 4b).
-
(3)
not recorded
-
(4)
Regressing: small and flaccid ovaries, containing primary growth and cortical alveolar oocytes, as well as early vitellogenic oocytes (Vtg) with yolk granules filling the cytoplasm before coalescence; presence of postovulatory follicles (POF) in varying stages of degeneration (Fig. 4c).
-
(5)
Regenerating: small ovaries, with primary growth and cortical alveolar oocytes; several atretic oocytes (A) at different stages of resorption (Fig. 4d).
Males (unrestricted testes)
-
(1)
Immature: small testes with densely packed lobules without evident lumina, filled by cysts of spermatogonia (Sg) (Fig. 4e).
-
(2)
Developing: testes increasing in size, with the germinal epithelium consisting of cysts of spermatocytes I and II (Sc) and spermatids (St), and spermatogonia located at the periphery of the gonad (Fig. 4f, g).
-
(3)
not recorded
-
(4)
not recorded
-
(5)
Regenerating: small testes, with continuous germinal epithelium made up of spermatogonia throughout lobules with partially collapsed lumina; residual spermatozoa not present into the sperm ducts (Fig. 4h).
The GSI patterns in relation to fish size and different phases of gonad development were determined for each sex (Fig. 5). In agreement with the gonad cycle, the GSI increased from immature (mean ± SD, 0.17 ± 0.09, n = 13) to developing (0.41 ± 0.12, n = 3) and regressing females (0.73 ± 0.28, n = 14), decreasing in regenerating ones (0.67 ± 0.21, n = 5) (Fig. 5a). In males, the GSI increased from immature (0.02 ± 0.01, n = 5) to developing specimens (1.08 ± 0.31, n = 3), declining in regenerating ones (0.06 ± 0.03, n = 19) (Fig. 5b). Fish size at sexual maturity was 33 and 19 cm SL in females and males, corresponding to 73 and 61% of their maximum standard length, respectively. Considering the size–frequency distribution of oocytes in regressing females, this species showed a group synchronous development, consisting of two discrete groups of oocytes separated by a threshold size of ~ 1 mm (Fig. 6). Based on the histological analysis (see below), the smaller group included previtellogenic and cortical alveoli oocytes, whereas the larger one was composed of vitellogenic oocytes at an early stage of development. The presence of postovulatory follicles (POF) and atretic oocytes in regressing females prevented any estimation of individual fecundity. A few residual eggs as large as 4.6–4.8 mm at the final process of hydration were found in a single specimen.
Age and growth
The relationship between fish standard length (SL) and maximum otolith size (OL) was linear, being described by the equation SL = − 1.95 + 5.24 OL (r2 = 0.91, n = 73) (Fig. 7). Through the otolith sectioning, a total of 75 specimens of A. mitopteryx were successfully aged (Fig. 8). Indices of precision of age estimates were both relatively low (CV = 3.7%; APE = 5.2%), revealing a good consistency between readings. The von Bertalanffy growth curves fitted to age–length data pairs were separately plotted for each sex (Fig. 9). Age estimates of juveniles and adults ranged from 7 to 62 years for females and from 7 to 36 years for males. Von Bertalanffy growth parameters and index of growth performance are reported in Table 2. Applying the likelihood ratio test, growth curves differed significantly between sexes, females attained larger size and grew slower than males (Table 3). Based on Von Bertalanffy growth curves, age at sexual maturity was about 32 and 11 years in females and males, corresponding to 50–30% of their maximum estimated age, respectively.
Discussion
Considering the relatively few records of A. mitopteryx reported in the past throughout the Antarctic continental shelves (Kunzmann and Zimmermann 1992), the present collection was worthwhile for the large number of samples, enabling us to shed more light on the biology of this infrequently collected species. The fish sample collected in the Weddell Sea consisted of juveniles and adults, with a balanced sex ratio. It was characterized by a remarkable sexual dimorphism, being females significantly larger than males, as found in other components of the pelagic clade such as Pleuragramma and Dissostichus spp. In addition, maximum size and weight of A. mitopteryx was larger than previously reported, being 52 cm TL and 1159 g respectively. Despite the considerable fishing effort (a total of 56 hauls) spent over a wide area located in the eastern and south-eastern Weddell Sea, A. mitopteryx was caught only in two stations north of Vestkapp, indicating a patchy distribution in relatively deep waters. In agreement with previous records from the Weddell Sea (reviewed in Kunzmann and Zimmermann 1992), the species was mainly distributed on the shelf at depths of more than 700 m. As most specimens were caught by bottom trawl, A. mitopteryx may be considered a benthopelagic species, inhabiting at least temporarily in close proximity to the sea bottom. Nevertheless, the attainment of neutral buoyancy through skeletal reduction and lipid deposition allowed this species to move easily throughout the water column to feed on zooplanktonic prey (e.g. Takahashi and Iwami 1997).
Based on macroscopic and histological analyses of gonads, the specimens collected in late summer in the Weddell Sea were out of the reproductive season. However, the presence of several regressing females with postovulatory follicles (POF) at various stages of resorption suggests that spawning likely occurred 1–2 months before sampling. It was unclear whether the early vitellogenic oocytes forming the larger batch found in regressing females will be spawned later in the season or resorbed undergoing atretic processes. Lacking mature females in our samples, we were unable to estimate in this species the reproductive investment in terms of fecundity. In any case, the large size of hydrated eggs observed in a single regressing female could support the hypothesis of a marked reproductive effort, as elsewhere reported in the few other pelagic nototheniids such as Dissostichus and Pleuragramma (e.g. Collins et al. 2010; La Mesa and Eastman 2012). In A. mitopteryx, large eggs give rise to relatively large yolk–sac larvae of approximately 17.5 mm SL, collected in the Weddell Sea in November (Kellermann 1990). Unlike adults, larvae of A. mitopteryx are particularly widespread, ranking second in abundance in the inner shelf of the south-eastern Weddell Sea (White and Piatkowski 1993).
The most striking result of the present study concerned the life span and the strong sexual dimorphism in size and age estimated for this species. Although age estimates were only presumed lacking validation, A. mitopteryx exhibited the longest life span reported so far amongst notothenioid fishes (Kock and Everson 1998). However, the annulation patterns in the sagittal otoliths and ageing criteria used for this species closely resembled those reported in Dissostichus mawsoni, in which age estimates have been recently validated using radiometrics (Brooks et al. 2011). Sexual dimorphism has been observed in a few number of notothenioids, generally associated to different body colouration or fins morphology in mating fishes (Kock and Kellermann 1991). A remarkable dimorphism in size was described in the Scotia Sea icefish, females being larger and slightly older than males (La Mesa et al. 2004).
In the Weddell Sea, the age structure of local population of A. mitopteryx consisted of very old fishes, most of them being older than 20 (males) and 40 (females) years of age. Attaining a moderate maximum size coupled with a low instantaneous growth rate (k), the growth performance of A. mitopteryx was at the lower end reported for notothenioids inhabiting the high-Antarctic Zone (La Mesa and Vacchi 2001). Although late in absolute terms due to slow body growth rate of juveniles, A. mitopteryx reached sexual maturity early if compared with the long life span estimated in this study. Most high-Antarctic fish spawn for the first time at 50-80% of their maximum age (La Mesa and Vacchi 2001), whereas A. mitopteryx reach sexual maturity at 30–50% of their life span. In turn, an early sexual maturity associated to high parental gonadal investment spread over a long life span would likely produce a great reproductive potential for this species, as reported elsewhere for P. antarctica (La Mesa et al. 2015). Instead of investing energy reserves in somatic growth, A. mitopteryx utilized most of metabolic energy to assuring an adequate number of offsprings (Hubold 1991), as well as the production of antifreeze glycopeptides and lipid storage for buoyancy.
In conclusion, the evolutionary trend towards a complete pelagic life style which involved the few species within the clade Pleuragramminae shaped their life strategies through unique morphological, physiological and ecological adaptations. In adapting to the physical and biological constraints of the pelagic environment, they evolved common characteristics such as streamlined shape, skeletal reduction and lipid storage for buoyancy, zooplanktonic to piscivorous feeding, high reproductive effort linked to early sexual maturity, slow somatic growth and long life span.
References
Andersen NC (1984) Genera and subfamilies of the family Nototheniidae from the Antarctic and Sub-Antarctic. Steenstrupia 10:1–34
Balushkin AV (2000) Morphology, classification, and evolution of notothenioid fishes of the Southern Ocean (Notothenioidei, Perciformes). J Ichthyol 40:74–109
Beamish RJ, Fournier DA (1981) A method of comparing the precision of a set of age determinations. Can J Fish Aquat Sci 38:982–983
Brooks CM, Andrews AH, Ashford JR, Ramanna N, Jones CD, Lundstrom CC, Cailliet GM (2011) Age estimation and lead–radium dating of Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea. Polar Biol 34:329–338
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish 3:52–70
Chang WYB (1982) A statistical method for evaluating the reproducibility of age determination. Can J Fish Aquat Sci 39:1208–1210
Collins MA, Brickle P, Brown J, Belchier M (2010) The Patagonian toothfish: biology, ecology and fishery. Adv Mar Biol 58:227–300
DeWitt HH (1962) A new Antarctic nototheniid fish with notes on two recently described nototheniiforms. Copeia 1962:826–833
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Eastman JT (1993) Antarctic fish biology. Academic Press, San Diego
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT, DeVries AL (1982) Buoyancy studies of notothenioid fishes in McMurdo Sound, Antarctica. Copeia 1982:385–393
Eastman JT, DeVries AL (1997) Morphology of the digestive system of Antarctic nototheniid fishes. Polar Biol 17:1–13
Ekau W (1988) Ecomorphology of nototheniid fish from the Weddell Sea, Antarctica. Ber Polarforsch 51:1–140
Evseenko SA, Kock KH, Nevinsky MM (1995) Early life history of the Patagonian toothfish, Dissostichus eleginoides Smitt, 1898 in the Atlantic Sector of the Southern Ocean. Antarct Sci 7:221–226
Hanchet S, Dunn A, Parker S, Horn P, Stevens D, Mormede S (2015) The Antarctic toothfish (Dissostichus mawsoni): biology, ecology, and life history in the Ross Sea region. Hydrobiologia 761:397–414
Hecht T (1987) A guide to the otoliths of Southern Ocean fishes. South Afr J Antarct Res 17:1–87
Hubold G (1991) Ecology of notothenioid fish in the Weddell Sea. In: Di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer Science and Business Media, Berlin, pp 3–22
Kellermann A (1990) Catalogue of early life stages of Antarctic notothenioid fishes. Ber Polarforsch 67:45–136
Kimura DK (1980) Likelihood methods for the von Bertalanffy growth curve. Fish Bull 77:765–776
Klingenberg CP, Ekau W (1996) A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol J Linn Soc 59:143–177
Knust R, Schröder M (2014) The expedition PS82 of the research vessel Polarstern to the southern Weddell Sea in 2013/2014. Ber Polar- Meeresforsch 680:1–155
Kock K-H (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge
Kock K-H, Everson I (1998) Age, growth and maximum size of Antarctic notothenioid fish—revisited. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. Springer, Berlin, pp 29–40
Kock K-H, Kellermann A (1991) Reproduction in Antarctic notothenioid fish. Antarct Sci 3:125–150
Kunzmann A (1991) Blood physiology and ecological consequences in Weddell Sea fishes. Ber Polarforsch 91:1–79
Kunzmann A, Zimmermann C (1992) Aethotaxis mitopteryx, a high-Antarctic fish with benthopelagic mode of life. Mar Ecol Progr Ser 88:33–40
La Mesa M, Eastman JT (2012) Antarctic silverfish: life strategies of a key species in the high-Antarctic ecosystem. Fish Fish 13:241–266
La Mesa M, Vacchi M (2001) Review—and growth of high Antarctic notothenioid fish. Antarct Sci 13:227–235
La Mesa M, Ashford J, Larson E, Vacchi M (2004) Age and growth of Scotia Sea icefish, Chaenocephalus aceratus, from the South Shetland Islands. Antarct Sci 16:253–262
La Mesa M, Riginella E, Mazzoldi C, Ashford J (2015) Reproductive resilience of ice-dependent Antarctic silverfish in a rapidly changing system along the Western Antarctic Peninsula. Mar Ecol 36:235–245
Munro JL, Pauly D (1983) A simple method for comparing growth of fishes and invertebrates. ICLARM Fishbyte 1:5–6
Near TJ, Kendrick BJ, Detrich HW III, Jones CD (2007) Confirmation of neutral buoyancy in Aethotaxis mitopteryx DeWitt (Notothenioidei: Nototheniidae). Polar Biol 30:443–447
Near TJ, Dornburg A, Kuhn KL, Eastman JT, Pennington JN, Patarnello T, Zane L, Fernandez DA, Jones CD (2012) Ancient climate change, antifreeze, and the evolutionary diversification of Antarctic fishes. PNAS 109:3434–3439
North AW (1988) Age of Antarctic fish: validation of the timing of annuli formation in otoliths and scales. Cybium 12:107–114
Pearse AGE (1985) Histochemistry. Theoretical and applied analytical technology. Churchill Livingstone, Edinburgh
Sanchez S, Dettaï A, Bonillo C, Ozouf-Costaz C, Detrich HW III, Lecointre G (2007) Molecular and morphological phylogenies of the Antarctic teleostean family Nototheniidae, with emphasis on the Trematominae. Polar Biol 30:155–166
Schröder M (2016) The expedition PS96 of the research vessel Polarstern to the southern Weddell Sea in 2015/2016. Ber Polar- Meeresforsch 700:1–142
Takahashi M, Iwami T (1997) The summer diet of demersal fish at the South Shetland Islands. Antarct Sci 9:407–413
Vacchi M, La Mesa M, Dalu M (2004) Early life stages in the life cycle of Antarctic silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarct Sci 16:299–305
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool 21:325–343
White MG (1991) Age determination in Antarctic fish. In: Di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic fish. Springer, Berlin, pp 87–100
White MG, Piatkowski U (1993) Abundance, horizontal and vertical distribution of fish in eastern Weddell Sea micronekton. Polar Biol 13:41–53
Wöhrmann APA (1998) Aspects of eco-physiological adaptations in Antarctic fish. In: Di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Milan, pp 119–128
Zimmermann C (1997) On the demersal fish fauna of the Lazarev Sea (Antarctica): composition and community structure. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 26–32
Acknowledgements
We are much indebted to the Alfred-Wegener-Institut, Helmholtz-Zentrum für Polar- und Meeresforschung for providing the opportunity to collect samples during two RV Polarstern expeditions PS82 (ANT-XXIX/9) and PS96 (ANT-XXXI/2). We wish to thank for their invaluable contribution all the crew members and scientific staff aboard of the R/V Polarstern during the field activities. Finally, we would like to sincerely thank P. Cziko, M. Landaeta and M. Militelli for their valuable comments on the early version of the manuscript. This study was supported by the Progetto Nazionale di Ricerche in Antartide (PNRA) and by the Ministero dell’Istruzione, dell’Università`e della Ricerca (MIUR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no conflicts of interest and that all applicable institutional, national or international guidelines for the use and care of animals were strictly followed in the study.
Rights and permissions
About this article
Cite this article
La Mesa, M., Donato, F., Riginella, E. et al. Life history traits of a poorly known pelagic fish, Aethotaxis mitopteryx (Perciformes, Notothenioidei) from the Weddell Sea. Polar Biol 41, 1777–1788 (2018). https://doi.org/10.1007/s00300-018-2318-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-018-2318-1