Abstract
Fungal diversity in Antarctic seems to be greater than what is known and remains largely unexplored. In this study, we identified the endophytic symbiont yeasts associated with leaves of the angiosperms Deschampsia antarctica and Colobanthus quitensis living on King George Island, Antarctica using a culture-based approach. One hundred and twelve yeast isolates were obtained from the tissue of the different plants sampled. These yeasts were identified using sequencing of the D1/D2 domains of the LSU region of the rRNA gene as Cryptococcus victoriae, Cystobasidium laryngis, Rhodotorula mucilaginosa, Sporidiobolus ruineniae and Leucosporidium aff. golubevii. The psychrophilic yeast C. victoriae was the most abundant species associated with the two angiosperms. Cystobasidium laryngis occurs only in the leaves of D. antarctica. In contrast, R. mucilaginosa, S. ruineniae and L. aff. golubevii occurred only in C. quitensis. Phylogenetic analysis indicates the Antarctic endophytic yeast strains are closely related to taxa obtained from substrates located in different habitats of the world. However, the endophytic yeast C. victoriae was closely related to psychrophilic taxa isolated from Antarctica, but also from the Arctic, Alpine and Himalayan environments. The abundance of endophytic yeasts associated with Antarctic angiosperms suggests a possible symbiotic relationship with their plant hosts, which may provide shelter and growing conditions suitable for the yeasts’ survival, dispersal and colonization other Antarctic environments. In contrast, the endophytic yeasts might directly or indirectly promote the fitness of their host plants by producing metabolites beneficial to plant survival in the extreme environments of Antarctica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica is a relatively pristine region of planet Earth. The fungal presence and diversity of some Antarctic ecosystems have been investigated. However, fungal occurrence and diversity in Antarctic may be greater than what is known, mainly because the environments and substrates of continental and oceanic Antarctica remain largely unexplored. The diversity of the fungal communities in Antarctica varies with the studied substrate, examples of which have included samples of soils, rocks, freshwater, plants, macroalgae, permafrost, rocks, ice and historic wood (Ruisi et al. 2007). As symbionts of Antarctic plants, plant-associated fungi have previously been recovered from mosses (Bradner et al. 2000; Tosi et al. 2002; Zhang et al. 2013), as well as two vascular plants native to Antarctica: Deschampsia antarctica Desv. (Poaceae) and Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) (Rosa et al. 2009, 2010; Uspon et al. 2009).
Endophytic fungi, which live asymptomatically in plant tissues, have been obtained from every major plant lineage in the world, and these microorganisms represent an important part of the global mycobiome diversity. The majority of described endophytic fungi are filamentous fungi of the phylum Ascomycota, although some species are members of the phyla Basidiomycota and Zygomycota (Huang et al. 2001). According to Isaeva et al. (2010), yeasts are common epiphytic fungi that inhabit the surfaces of various plant parts, including leaves, flowers and fruits. However, reports of yeasts as endophytes are scarce when compared with the reports of filamentous fungi. Nassar et al. (2005), Gai et al. (2009), Xin et al. (2009), Khan et al. (2012) Solis et al. (2015) and Zhang and Yao (2015) reported endophytic yeasts associated with plants from different parts of the world, including those present in the Arctic ecosystem. Endophytic fungal communities have been reported as important symbionts for their host plants; however, their biological significance in polar ecosystems remains poorly characterized.
The plant diversity in Antarctica is restricted to several species of mosses and two angiosperms. D. antarctica (Antarctic hair grass), a monocot, and C. quitensis (Antarctic pearlwort), a dicot, are the only two plants able to grow in the Antarctic ecosystem that extends from the maritime Antarctic (Lewis-Smith and Poncet 1987). According to Fowbert and Smith (1994), Smith (1994), Grobe et al. (1997) and Convey (2003), the populations of D. antarctica and C. quitensis have been growing in the Argentine Island archipelago and in the western Antarctic Peninsula in recent years, which may be considered a response to the increasing summer air temperatures and local snow recession, possibly caused by global climate changes. For this reason, these Antarctic angiosperms represent interesting models to study the dispersal of their associated microbiome communities. Additionally, studies of the biology of fungal communities present in Antarctic environments are very important because they may clarify the ecological roles of the fungal species and other organisms, the complexity, evolution and speciation of the fungal webs under extreme conditions, as well as the influence of climate change on the Antarctic biota (Santiago et al. 2015). These two angiosperms have been investigated for filamentous fungal endophytes in their leaves (Rosa et al. 2009, 2010) and roots (Uspon et al. 2009), but the present study represents the first focused work on the endophytic yeasts associated with these species.
Materials and methods
Host plants and isolation of the endophytic yeasts
The study area is located at Admiralty Bay, King George Island, one of the South Shetland Islands, Antarctica. Leaves of D. antarctica and C. quitensis were sampled from six different sites (Botany Point, Ullman Point, Brazilian Refuge II, Macchu Picchu Station, Hennequin Point and Demay Point) forming a 25.5-km transect through Admiralty Bay (Fig. 1). The collection was performed during the austral summer in January and February of 2008. Healthy leaves were cut into pieces and stored in plastic bags at 10 °C for no more than 24 h before isolation of the endophytes. Five leaf fragments 1 cm in length were dipped in 70 % ethanol (1 min) and 2 % sodium hypochlorite (3 min), followed by one wash with sterile distilled water (2 min) (Rosa et al. 2009). The fragments were plated on Petri plates containing potato dextrose agar (PDA; Difco, USA) supplemented with chloramphenicol (100 µg mL−1). The plates were incubated at 15 °C for up to 60 days, and individual colonies were transferred to the PDA and stored at 4 °C. Yeasts were stored in cryotubes with 15 % sterilized glycerol at −80 °C for the long-term preservation and later deposited in the Culture Collection of Microorganisms and Cells at the Universidade Federal de Minas Gerais under UFMGCB codes.
Map of Admiralty Bay at King George Island, in Antarctica showing the sites and the transect where samples were collected for this study (Simões et al. 2004). Bp = Botany point (62°05′S, 58°19′W); Up = Ullman point (62°05′S, 58°20′W); Br = Brazilian Refuge II (62°04′S, 58°25′W); Mp = Macchu Picchu station (62°07′S, 58°23′W); Hp = Hennequin point (62°05′S, 58°24′W); and Dp = Demay point (62°12′S, 58°19′W). The distance among the sites was 2 km apart (Bp to Up), 4 km apart (Up to Br), 4 km apart (Br to Mp), 5 km apart (Mp to Hp) and 10.5 km apart (Hp to Dp)
Yeasts identification
The yeasts were grouped and identified according to protocols established by Kurtzmam et al. (2011). Yeast molecular identities were confirmed by sequencing the D1/D2 variable domains of the large-subunit rRNA gene using the primers NL1 and NL4 as described by Lachance et al. (1999). Yeast isolates with query coverage and identity ≥99 % were considered to represent the same taxon. To sequence the purified PCR products of all endophytic yeast, we used the BigDye reaction kit (Applied Biosystems, USA). Sequencing was performed using an ABI 3730 (Life Technologies, USA) automated sequencing system in the Fundação Oswaldo Cruz (FIOCRUZ, MS), Brazil. The consensus sequences (forward/reverse) of all yeasts were obtained and compared with those included in the GenBank database using the Basic Local Alignment Search Tool (BLAST at http://www.ncbi.nlm.nih.gov). Alignments were performed using MUSCLE (Edgar 2004). Representative consensus sequences of fungal taxa were deposited into GenBank (Table 1). To achieve species-rank identification based on the D1/D2 variable domain, the consensus sequence (four different sequences) was aligned with the nearest (≥99 % of query cover and ≥98 % of identity) sequences of type species retrieved from the NCBI GenBank database using BLAST (Altschul et al. 1997). The followed criteria were used to interpret the sequences from the GenBank database: For query coverage and sequence identities ≥99 %, the genus and species were accepted; for sequence identities showing ≤98 %, the genus and species were accepted, but the term ‘aff.’ (species affinis = species related to) was used to indicate that the specimen resembles the reference species, yet has certain minor features not found in the reference. The phylogenetic tree of the D1/D2 sequences was obtained by using the neighbor-joining method and the Kimura 2-P algorithm with 1,000 bootstrap replications according to Felsenstein (1985) performed with the program MEGA version 6.0 (Tamura et al. 2013).
Results
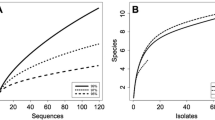
One hundred and twelve endophytic yeast isolates were obtained. Twenty-eight isolates originated from the leaves of 56 specimens of D. antarctica, and 80 isolates were from leaves of 60 specimens of C. quitensis. The endophytic yeasts were identified as Cryptococcus victoriae, Cystobasidium laryngies, Leucosporidium aff. golubevii, Rhodotorula mucilaginosa and Sporidiobolus ruineniae using the sequencing of D1/D2 rRNA (Table 1). The psychrophilic yeast C. victoriae was the most abundant species isolated from the two angiosperms. In contrast, C. laryngies was detected only in D. antarctica, and R. mucilaginosa, S. ruineniae and L. aff. golubevii were found only in C. quitensis. The phylogenetic analysis showed that the Antarctic endophytic yeasts and their closest relatives formed four clades (Fig. 2). The endophytic yeasts showed sequences with high similarity with taxa obtained from substrates located in different habitats of the world, including tropical, temperate and polar environments. Clade 4, formed by the sequences of C. victoriae UFMGANT 205 and 257 obtained from C. quitensis and D. antarctica, respectively, was closely related to taxa isolated from Antarctica, but also from the Arctic, Alpine and Himalayan environments. The species R. mucilaginosa UFMGANT 303 (endophyte of C. quitensis) and C. laryngies (endophyte of D. antarctica) sequences formed a clade (Clade 3) with sequences of taxa obtained from different habitats, environments and regions, which included leaves and fruits, sewage sludge, high-altitude freshwater lakes and alpine glaciers. The sequence of S. ruineniae UFMGANT 295 from C. quitensis formed Clade 2 and displayed similarities with related to taxa obtained from banana nectar in the tropical environment and from ant nests in temperate regions of the USA. The L. golubevii UFMGANT 273 sequence, obtained from leaves of C. quitensis, formed Clade 1 with sequences of Leucosporidiella, Rhodotorula and Leucosporidium taxa obtained from snow, glacial meltwater, soil and macroalgae present in different extreme habitats of the world.
Phylogenetic tree showing the nearest relatives and its origin (in gray) of endophytic yeasts (in bold) as well as sequences of type species (TS). The tree was constructed based on the rRNA gene sequences (D1/D2) using the maximum composite likelihood model. The tree was rooted with Debaryomyces hansenii CBS 1099 (EU816355) as out-group (OG). For detailed information about the origin and distribution of sequences, please access their GenBank codes
Discussion
Multiple studies have shown a high diversity of endophytic fungal communities associated with plants living in tropical, temperate, Arctic and Antarctic ecosystems (Higgins et al. 2007; Rosa et al. 2009). Few studies have reported the presence of endophytic yeasts associated with plants around the world (Doty 2013); even rarer reported is their association with polar plants (Zhang and Yao 2015). However, the increasing reports of endophytes from the next-generation sequencing studies seem to suggest that endophytic yeasts may not actually be rare, but instead overlooked, likely due to historic biases in culture methods that focus on filamentous fungi. In contrast, the molecular methods used here can not identify the uncultivable and/or slow endophyte communities. Our results corroborate Doty (2013), who states that yeasts are common endophytes, particularly the genera Cryptococcus, Debaromyces, Sporobolomyces and Rhodotorula.
The endophytic yeasts obtained from the Antarctic angiosperms were isolated at 15 °C after 60 days. For this reason, these yeasts can be classified as psychrotolerant fungi. According to Robinson (2001) and Ruisi et al. (2007), several fungi obtained from Antarctic environments appear to be psychrotolerant taxa when compared with those classified as psychrophilic. The frequent recovery of psychrotolerant microorganisms in Antarctica may be caused by local microclimate conditions of the Antarctic summer season, when the samples were obtained, as well as with the ability of the fungi to tolerate large variations in temperature (Vaca et al. 2013).
The yeast C. laryngies was detected as an endophyte only in D. antarctica (a monocot), and R. mucilaginosa, S. ruineniae and L. aff. golubevii were found only in C. quitensis (a dicot). The host specificity among endophytes and their hosts remains unknown. May (1991) suggests that strong host affinity is rare in communities containing a high diversity of host plants. However, Arnold and Lutzoni (2007) observed a lower host affinity among endophytes of angiosperms in tropical versus boreal sites. Our data provide additional preliminary evidence that polar endophytes may be specific to species or classes of plant hosts.
Cryptococcus victoriae was the most frequently isolated endophytic species of D. antarctica and C. quitensis. Cryptococcus (Basidiomycota) species have been found frequently in various Antarctic substrates. C. victoriae was originally described from Antarctica (Vishniac 2006) and was already described in penguin guano, soil, sediment, freshwater samples, the rhizosphere of D. antarctica (Vaz et al. 2011), macroalgae (Furbino et al. 2014), lichens (Santiago et al. 2015). However, C. victoriae has been characterized in environments outside of Antarctica, including soil and rhizosphere soil in Korea (Hong et al. 2002), sea water in Portugal (Gadanho et al. 2003), the gut of the insect Chrysoperla rufilabris in the USA (Woolfolk and Inglis 2004), an industrial malting area and indoor air in Finland (Laitila et al. 2006; Pitkäranta et al. 2008), in glacial ice from the Arctic (Butinar et al. 2007), in a dry meat processing factory in Norway (Asefa et al. 2009) and from the Italian Alps (Turchetti et al. 2008; Branda et al. 2010). According to Garcia et al. (2012), C. victoriae can be considered as a generalist species able to tolerate different stressful environments but not the most extreme conditions, which show strains with high physiology, morphology and molecular plasticity. Additionally, C. victoriae has been described in association with plants. Renker et al. (2004) and Wuczkowski and Prillinger (2004) reported C. victoriae in the roots, rhizosphere and seeds of different plants in Germany and Austria, and Zhang and Yao (2015) showed its presence as an endophyte of Arctic plants.
The genus Rhodotorula includes pigmented yeast species with a worldwide distribution. Rhodotorula mucilaginosa is a ubiquitous basidiomycetous yeast, which occurs in different habitats and substrates, including cold, extreme environments (Sampaio 2011a). In Antarctica, Rhodotorula has been isolated from terrestrial and marine substrates (Vaz et al. 2011; Godinho et al. 2013). Additionally, C. laryngis, also reported as R. laryngis, was isolated from natural environments in seawater and ice cores (Sampaio 2011a). However, R. mucilaginosa was reported as inhabiting the phylloplane and stems of living and dead parts of plants (Glushakova et al. 2015), as well as an endophyte of Citrus sinensis (Gai et al. 2009).
Although S. ruineniae has been isolated from foliage, rotten wood, soil, dung and air, its original habitat seems to be the phylloplane (Sampaio 2011b). L. aff. golubevii, obtained as an endophyte of C. quitensis, was isolated from the water of the river in the north of Portugal (Sampaio et al. 2003). To the best of our knowledge, our detection of S. ruineniae and L. aff. golubevii represents the first reports of these two species in Antarctica. The fact that we detected two species new to Antarctica in a relatively small and geographically limited study suggests that the Antarctic fungal diversity may be more extensive than is currently reported.
Conclusion
Endophytic fungi have been reported as able to increase the fitness of their host plants by conferring abiotic and biotic stress tolerance. Fracchia et al. (2003) showed the growth-promoting effects of R. mucilaginosa, a soil yeast, on the arbuscular mycorrhizal fungi (AMF) Glomus mosseae and Gigasora rosea. Those authors proposed that R. mucilaginosa produce exudates that stimulated the hyphal growth of AMF spores, increasing the chance of contact between AMF hyphae and plant roots and consequently increasing mycorrhizal establishment. Despite the yeasts’ occurrence in other substrates in Antarctica, their abundance in an endophytic association with the Antarctic angiosperms suggests a possible symbiotic relationship with their plant hosts, which may provide shelter and growing conditions suitable for the yeasts’ survival, dispersal and colonization of other Antarctic environments. In contrast, the endophytic yeasts might directly or indirectly promote the fitness of their host plants by producing metabolites beneficial for plant survival in the extreme environments of Antarctica, such as hormones, pigments, natural products and polysaccharides. However, our study only allows speculating on the nature of the interaction between the endophytic yeasts and their hosts. To elucidate details of the symbiosis between the endophytic yeasts and its hosts, further mesocosm experiments will be necessary in situ in Antarctica.
References
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88:541–549
Asefa DT, Moretro T, Gjerde RO, Langsrud S, Kure CF, Sidhu MS, Nesbakken T, Skaar I (2009) Yeasts diversity and dynamics in the production processes of Norwegian dry-cured meat products. Int J Food Microbiol 133:135–140
Bradner JR, Sidhu RK, Yee B, Skotnicki ML, Selkirk PM, Nevalainen KMH (2000) A new microfungal isolate, Embellisia sp., associated with the Antarctic moss Bryum argenteum. Polar Biol 23:730–732
Branda E, Turchetti B, Diolaiuti G, Pecci M, Smiraglia C, Buzzini P (2010) Yeast and yeast-like diversity in the southernmost glacier of Europe (Calderone Glacier, Apennines, Italy). FEMS Microbiol Ecol 72:354–369
Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007) Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie Van Leeuwenhoek 91:277–289
Convey P (2003) Maritime Antarctic climate change: signals from terrestrial biology. Antarct Res Ser 79:145–158
Doty SL (2013) Endophytic yeasts: biology and applications. In: Aroca R (ed) Symbiotic endophytes. Springer, Berlin, pp 335–343
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1795
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 36:783–791
Fowbert JA, Smith RIL (1994) Rapid population increases in native vascular plants in the Argentine Islands, Antarctic Peninsula. Arct Alp Res 26:290–296
Fracchia S, Godeas A, Scervino JM, Sampedro I, Ocampo JA, Garcıa-Romera I (2003) Interaction between the soil yeast Rhodotorula mucilaginosa and the arbuscular mycorrhizal fungi Glomus mosseae and Gigaspora rosea. Soil Biol Biochem 35:701–707
Furbino LE, Godinho VM, Santiago IF, Pellizari FM, Alves TMA, Zani CL et al (2014) Diversity patterns, ecology and biological activities of fungal communities associated with the endemic macroalgae across the Antarctic Peninsula. Microb Ecol 67:775–787
Gadanho M, Almeida JMF, Sampaio JP (2003) Assessment of yeast diversity in a marine environment in the south of Portugal by microsatellite-primed PCR. Antonie Van Leeuwenhoek 84:217–227
Gai CS, Lacava PT, Maccheroni Jr. W, Glienke C, Araújo WL, Miller TA, Azevedo JL (2009) Diversity of endophytic yeasts from sweet orange and their localization by scanning electron microscopy. J Basic Microbiol 49:441–451
Garcia V, Zalar P, Brizzio S, Gunde-Cimerman N, van Broock M (2012) Cryptococcus species (Tremellales) from glacial biomes in the southern (Patagonia) and northern (Svalbard) hemispheres. FEMS Microbiol Ecol 82:523–539
Glushakova AM, Kachalkin AV, Zheltikova TM, Chernov IY (2015) Resistance of various yeast ecological groups to prolonged storage in dry state. Microbiology 84:442–448
Godinho VM, Furbino L, Santiago IF, Pelizzari FM, Yokoya NS, Pupo D, Dicla A, Alves TM, Junior PA, Romanha AJ, Zani CL, Cantrell CL, Rosa CA, Rosa LH (2013) Diversity and bioprospecting of fungal communities associated with endemic and cold-adapted macroalgae in Antarctica. ISME 7:77–145
Grobe CW, Ruhland CT, Day TA (1997) A new population of Colobanthus quitensis near Arthur Harbor, Antarctica: correlating recruitment with warmer summer temperatures. Arct Alp Res 29:217–221
Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni F (2007) Phylogenetic relationship, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol Phyl Evol 42:543–555
Hong SG, Lee KH, Bae KS (2002) Diversity of yeasts associated with natural environments in Korea. J Microbiol 40:55–62
Huang Y, Wang J, Li G, Zheng Z, Su W (2001) Antitumor and antifungal activities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol Med Microbiol 31:163–167
Isaeva OV, Glushakova AM, Garbuz SA, Kachalkin AV, Chernov IY (2010) Endophytic yeast fungi in plant storage tissues. Biol Bull 37:26–34
Khan Z, Ahmad S, Al-Ghimlas F, Al-Mutairi S, Joseph L, Chandy R, Sutton DA, Guarro J (2012) Purpureocillium lilacinum as a cause of cavitary pulmonary disease: a new clinical presentation and observations on atypical morphologic characteristics of the isolate. J Clin Microbiol 50:1800–1804
Kurtzmam CP, Fell JW, Boekhout T (2011) The yeast, a taxonomic study, 5th edn. Elsevier, Amsterdam
Lachance MA, Bowles JM, Starmer WT, Barker JSF (1999) Kodamaea kakaduensis and Candida tolerans, two new yeast species from Australian Hibiscus flowers. Can J Microbiol 45:172–177
Laitila A, Wilhelmson A, Kotaviita E, Olkku J, Home S, Juvonen R (2006) Yeasts in an industrial malting ecosystem. J Microbiol Biotechnol 33:953–966
Lewis-Smith RI, Poncet S (1987) Deschampsia antarctica and Colobanthus quitensis in the Terra Firma Island. Br Antarct Surv Bull 74:31–35
May RM (1991) A fondness for fungi. Nature 352:475–476
Nassar AH, El-Tarabily KA, Sivasithamparam K (2005) Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. J Biol Fertil Soils 42:97–108
Pitkäranta M, Meklin T, Hyvärinen M, Pulin L, Auvinen P, Nevalainen A, Rintala H (2008) Analysis of fungal flora in indoor dust by ribosomal DNA sequences analysis, quantitative PCR, and culture. Appl Environ Microbiol 74:233–244
Renker C, Blanke V, Börstler B, Heinrichs J, Buscot F (2004) Diversity of Cryptococcus and Dioszegia yeasts (Basidiomycota) inhabiting arbuscular mycorrhizal roots or spores. FEMS Yeast Res 4:597–603
Robinson CH (2001) Cold adaptation in Arctic and Antarctic fungi. New Phytol 151:341–353
Rosa LH, Vaz ABM, Caligiorne RB, Campolina S, Rosa CA (2009) Endophytic fungi associated with the Antarctic Grass Deschampsia antarctica Desv. (Poaceae). Polar Biol 32:161–167
Rosa LH, Vieira MLA, Santiago IF, Rosa CA (2010) Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol Ecol 73:178–189
Ruisi S, Barreca D, Selbmann L, Zucconi L, Onofri S (2007) Fungi in Antarctica. Rev Environ Sci Biotechnol 6:127–141
Sampaio JP (2011a) Rhodotorula Harrison (1928). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts: a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 1873–1927
Sampaio JP (2011b) Sporidiobolus Nyland (1949). In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeasts: a taxonomic study, 5th edn. Elsevier, Amsterdam, pp 1549–1561
Sampaio JP, Gadanho M, Bauer R, Weiß M (2003) Taxonomic studies in the Microbotryomycetidae: Leucosporidium golubevii sp. nov., Leucosporidiella gen. nov. and the new orders Leucosporidiales and Sporidiobolales. Mycol Prog 2:53–68
Santiago IF, Rosa CA, Rosa LH (2015) Lichensphere: a protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles 19:1087–1097
Simões JC, Arigony-Neto J, Bremer UF (2004) O uso de mapas antárticos em publicações. Pesquisa Antartica Brasileira 4:191–197
Smith RIL (1994) Vascular plants as indicators of regional warming in Antarctica. Oecologia 99:322–328
Solis MJL, Yurkov A, Cruz TED, Unterseher M (2015) Leaf-inhabiting endophytic yeasts are abundant but unevenly distributed in three Ficus species from botanical garden. Mycol Progress 14:1019–1029
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tosi S, Casado B, Gerdol R, Caretta G (2002) Fungi isolated from Antarctic mosses. Polar Biol 25:262–268
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G, Agata D, Smiraglia C, Vaughan-Martini A (2008) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83
Uspon R, Newsham KK, Bridge PD, Pearce DA, Read DJ (2009) Taxonomic affinities of dark septate root endophytes of Colobanthus quitensis and Deschampsia antarctica, the two native Antarctic vascular plant species. Fungal Ecol 2:184–196
Vaca I, Faúndez C, Maza F, Paillavil B, Hernández V, Acosta F, Levicán G, Martínez C, Chávez R (2013) Cultivable psychrotolerant yeasts associated with Antarctic marine sponges. World J Microbiol Biotechnol 29:183–189
Vaz ABM, Rosa LH, Vieira MLA, Garcia V, Brandão LR, Teixeira LCRS, Moliné M, Libkind D, Maria VB, Rosa CA (2011) The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz J Microbiol 42:937–947
Vishniac HS (2006) Yeast biodiversity in the Antarctic. In: Rosa CA, Péter G (eds) Biodiversity and ecophysiology of yeasts. Springer, Berlin, pp 221–240
Woolfolk SW, Inglis GD (2004) Microorganisms associated with field-collected Chrysoperla rufilabris (Neuroptera: Chrysopidae) adults with emphasis on yeast symbionts. Biol Control 29:155–168
Wuczkowski M, Prillinger H (2004) Molecular identification of yeasts from soils of the alluvial forest national park along the river Danube downstream of Vienna, Austria (National Park Donauauen). Microbiol Res 159:263–275
Xin G, Glawe D, Doty SL (2009) Characterization of three endophytic, indole-3-acetic acid-producing yeasts occurring in Populus trees. Mycol Res 113:973–980
Zhang T, Yao YF (2015) Endophytic fungal communities associated with vascular plants in the high arctic zone are highly diverse and host-plant specific. PLoS ONE 10:e0130051
Zhang T, Zhang YQ, Liu HY, Wei YZ, Li HL, Su J, Zhao LX, Yu LY (2013) Diversity and cold adaptation of culturable endophytic fungi from bryophytes in the Fildes Region, King George Island, maritime Antarctica. FEMS Microbiol Lett 341:52–61
Acknowledgments
We acknowledge the financial support from CNPq PROANTAR 407230/2013-0, INCT Criosfera (CNPq) and FAPEMIG (0050-13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflict of interest among the authors.
Rights and permissions
About this article
Cite this article
Santiago, I.F., Rosa, C.A. & Rosa, L.H. Endophytic symbiont yeasts associated with the Antarctic angiosperms Deschampsia antarctica and Colobanthus quitensis . Polar Biol 40, 177–183 (2017). https://doi.org/10.1007/s00300-016-1940-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-016-1940-z