Abstract
The effect of salinity, pH, and dissolved inorganic carbon (TCO2) on growth and survival of three Arctic sea ice algal species, two diatoms (Fragilariopsis nana and Fragilariopsis sp.), and one species of chlorophyte (Chlamydomonas sp.) was assessed in controlled laboratory experiments. Our results suggest that the chlorophyte and the two diatoms have different tolerance to fluctuations in salinity and pH. The two species of diatoms exhibited maximum growth rates at a salinity of 33, and growth rates at a salinity of 100 were reduced by 50% compared to at a salinity of 33. Growth ceased at a salinity of 150. The chlorophyte species was more sensitive to high salinities than the two diatom species. Growth rate of the chlorophyte was greatly reduced already at a salinity of 50 and it could not grow at salinities above 100. At salinity 33 and constant TCO2 concentration, all species exhibited maximal growth rate at pH 8.0 and/or 8.5. The two diatom species stopped growing at pH > 9.5, while the chlorophyte species still was able to grow at a rate which was 1/3 of its maximum growth rate at pH 10. Thus, Chlamydomonas sp. was able to grow at high pH levels in the succession experiment and therefore outcompeted the two diatom species. Complementary experiments indicated that growth was mainly limited by pH, while inorganic carbon limitation only played an important role at very high pH levels and low TCO2 concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sea ice is permeated with pores and brine channels, which host a unique microbial community. The total brine channel volume of sea ice typically ranges between 1 and 30% depending on salinity, temperature, and ionic composition of the brine fluid (Weeks and Ackley 1986). When the temperature decreases, the thermodynamic phase equilibrium drives the sea ice toward a lower brine volume with higher salinities (Cox and Weeks 1983). Thus, the temperature and brine of sea ice are interrelated. At brine temperature between −1.9 and −6.7°C, the brine salinity may range from 34 to 108 (Gleitz et al. 1995). However, when sea ice is exposed to temperatures below −20°C, the brine salinity can be well above 200 (Cox and Weeks 1983). In the summer when sea ice melts, the salinity of the brine can be as low as one-third of normal sea water, e.g., salinity <10 (Ryan et al. 2004). Brine salinity also fluctuates vertically within the sea ice, with the lowest salinities usually encountered in the bottom sea ice layers (Gradinger 1999; Lizotte 2003; Ryan et al. 2004). Thus, sea ice algae must cope with severe physicochemical stress factors caused by natural variations in salinity. Only a few studies include investigations into the effect of salinity stress on growth rates of sea ice algae (Grant and Horner 1976; Arrigo and Sullivan 1992; Thiel et al. 1996; Ryan et al. 2004). A study on the sea ice diatoms (Amphiprora kufferathii, Nitzschia, and Thalassiosira Antarctica) isolated from ice cores from Weddell Sea revealed growth at salinities up to 90 at −5.5°C, and the diatoms were found to survive for 20 days at salinities up to 145 (Thiel et al. 1996). Other studies have shown that the growth of different sea ice diatoms from the Weddell Sea ceased at salinities above 50 (Grant and Horner 1976). Furthermore, studies have shown that most flagellate species of algae e.g., chlorophytes, dinoflagellates, and chrysophytes have been reported in the sea ice (Arrigo et al. 2010). These flagellate species is especially found in the top sea ice layer, where the highest salinity and lowest temperatures is encountered.
The differences in tolerance between sea ice algal species have been ascribed to various abilities for osmotic acclimations, e.g., production of osmolytes (such as proline), which balances the ionic pressure during changes in salinity (Gleitz and Thomas 1992). Moreover, changes in sea ice salinity and associate factors may be the key drivers for microbial succession in sea ice communities (Mikkelsen et al. 2008). The sea ice algal species that are capable to cope with a broad range of salinity may have an advantage and become dominant in the sea ice community. An understanding of the effect of fluctuating salinities in sea ice brine indicates which factors drive the distribution and succession of sea ice algae and might give important information that can be used to modeling sea ice species succession and carbon dynamics within the brine.
Variation in seawater pH levels can also have a marked effect on the growth and survival of sea ice algae. In sea ice, a number of biological and physical processes influence pH. Studies of sea ice have shown that in regions characterized by high primary production, the sea ice brine has considerably reduced concentrations of dissolved inorganic carbon (TCO2) and elevated pH levels as high as 10.0 (Gleitz et al. 1995; Thomas et al. 2001). Furthermore, changes in carbon chemistry alone can result in significant changes in pH of the sea ice brine. One mechanism behind this is CaCO3 precipitation that can occur at low temperatures (Rysgaard et al. 2007; Dieckmann et al. 2008). Carbonate precipitation will initially lead to a buildup of CO2 in the brine system leading to a decrease in pH. With time, CO2 can be transported to the water column through brine drainage. This net export of CO2 out of the sea ice brine will lead to increased pH in the brine, especially when sea ice starts to melt. In sea water, changes in pH influence the equilibrium of the carbonate system and therefore the inter-speciation of TCO2, i.e., CO2 (aq), HCO3 −, CO −23 , which may influence microalgae species succession and distribution (Hansen 2002; Rost et al. 2003; Trimborn et al. 2008). Limitation in the supply of CO2 due to elevated pH levels may restrict photosynthesis and growth of some algal species (Hansen 2002; Rost et al. 2003; Hansen et al. 2007) and favor species that utilize HCO3 − as an inorganic carbon source (Korb et al. 1997; Huertas et al. 2000; Hansen 2002). Diatoms have been found to actively take up HCO3 − and convert it into intracellular CO2 by extracellular enzymes (e.g., Korb et al. 1997; Tortell et al. 1997). Additionally, diatoms can utilize HCO3 − directly for carbon fixation through C4 photosynthesis (Tortell et al. 1997; Reinfelder et al. 2000). However, a previous study has shown that the ability to tolerate high pH is not related to particulate algal groups, but rather is species specific (Hansen 2002). In this study, we investigated the upper limit for growth with respect to salinity, pH, and TCO2 for three common Arctic sea ice algal species; the diatoms Fragilariopsis nana, Fragilariopsis sp. and the chlorophyte Chlamydomonas sp. The physiological response toward these stress factors are evaluated and discussed in relation to in situ succession patterns. This study is important to understand the factors controlling the growth, survival, composition, and distribution of the sea ice algal communities within the brine and can be used to accurately model the species succession and the productivity of this complex system.
Materials and methods
Algae species and maintenance of sea ice algae cultures
Three sea ice algae were selected for the study (Arrigo et al. 2010). The diatom Fragilariopsis sp. (CCMP2297) and the chlorophyte Chlamydomonas sp. (CCMP2294) originated from sea ice from Baffin Bay and were provided by Guillard National Center for Culture of Marine Phytoplankton (CCMP), and the diatom Fragilariopsis nana (SCCAP K-0637) was isolated from the Labrador Sea and provided by the Scandinavian Culture Collection of Algae and Protozoa, Department of Phycology, University of Copenhagen. The two species of diatoms were selected as representatives for pennate diatoms, which are very common in sea ice (Arrigo et al. 2010). We deliberately chose Fragilariopsis nana because it is a relatively small species (length 8.0–9.4 μm; width 1.9–2.0 μm) and Fragilariopsis sp. because it is somewhat larger diatom species (length 12–16 μm; width 6–10 μm). The chlorophyte Chlamydomonas sp. (length 8–10 μm; width 4.0–6.0 μm) was selected because chlorophytes are common in sea ice as well (Arrigo et al. 2010).
Algal cultures were grown in L1 growth medium (Guillard and Hargraves 1993) based on autoclaved seawater with a salinity of 33. The stock cultures were maintained at 3 ± 1°C and 50 μE m−2 s−1 following a light:dark cycle of 16:8 h. Illumination was provided by cool fluorescent lamps, and irradiance was measured using a LiCor 1400 (Li-Cor, NE, USA).
Experimental conditions
All experiments were carried out at 3 ± 1°C and at an irradiance of 50 μE m−2 s−1 following a light:dark cycle of 16:8 h. Only cells from exponentially growing cultures were used for inoculation of the experiments. However, the first 6–10 days were considered as an acclimation period; therefore, cell counts from these samplings were not included in the calculations of growth rates. All experiments were carried out in 62-ml polystylene bottles, except for the pH-drift experiments that were carried out in gas-tight laminated NEN/PE plastic bag (Hansen et al. 2000) fitted with a gas-tight Tygon tube and valve for sampling. All experiments were carried out in triplicates, i.e., each experiment was carried out in three separate bottles.
Cultures were kept suspended through the use of a plankton wheel, and an external cooling system was used to prevent heating associated with radiation absorption. The L1 growth medium was selected to make sure that algal cultures were not nutrient limited at anytime during the experiment.
Enumeration of cells was carried out using subsamples fixed in acidic Lugol’s iodine (2.5% final concentration), and cells were counted in a Sedgewick-Rafter chamber. Each count was based on at least 400 cells.
Growth rates (μ) were measured as increase in cell number and were calculated assuming exponential growth:
where N 0 and N 1 are number of cells at time t 0 and t 1, and t is the difference in time (d) between t 0 and t 1 samples (Hansen 2002). We determined the exponential phase of growth (straight line). Two points, N 0 and N 1, at the extremes of this linear phase was taken and substituted into the equation (same approach was used for determining the two points, t 0 and t 1). All experiments were carried out in triplicates; thus, this was done for each replicate and the mean of the three maximum growth rates was determined. The calculations of the growth rates were corrected for any dilutions. pH values were measured using a Sentron® 2001 pH-meter equipped with a Red Line electrode, which is an ISFET® sensor (Semi-conductor Ion Field Effect Transistor) with detection limit of 0.01. The pH sensor was calibrated (2 point) using Sentron buffers of pH 7.0 and 10.0. The concentration of dissolved inorganic carbon (TCO2) was measured in the growth medium by transferring samples (12 ml) to Exetainer tubes (12 ml Exetainer®, Labco High Wycombe, UK) spiked with 20 μl HgCl2 (saturated solution, 5% w/v) and was measured using a CO2 analyzer (CM5012 CO2 Coulometer).
Experimental setup
Growth rate of sea ice algae at different salinities
In the first set of experiments, growth rates of the three sea ice algae Fragilariopsis nana, Fragilariopsis sp, and Chlamydomonas sp. were measured at different salinities ranging from 5 to 150 (i.e., salinity of 5, 20, 33, 50, 75, 100, and 150). The salinity was adjusted from a salinity of 33 by addition of artificial seawater based on Red Sea salt with known TCO2 concentrations to the L1 medium. The pH value was kept constant at 8.0 throughout the experiment. If the pH differed by more than 0.03 from the set point, it was adjusted by the aliquot addition of 0.1 M NaOH or HCl. The experiment was initiated with an inoculation of 1,000 cells ml−1 and was allowed to run for minimum 18 d and maximum 20 d. Every second day, pH was measured, and subsamples (1 ml) were taken for enumeration of algae cells. After subsampling, the bottles were refilled to capacity with L1 growth medium (1 ml). The L1 growth medium was at each event adjusted to the correct salinity to prevent salinity in the experimental bottles to drift. Salinity and TCO2 concentrations were measured initially and at the termination of the experiment.
To test the effect of lowered salinity on the growth of the three species of sea ice algae, a second set of experiments was conducted. The algal cultures were grown in L1 growth medium (Guillard and Hargraves 1993) based on autoclaved seawater with a salinity of 75 and a known TCO2 concentration for a month. The salinity was adjusted from a salinity of 75 to different salinities of 5, 20, and 33 to mimic the transition from cold to melting sea ice. The pH value was kept constant at 8.0 throughout the experiment. If the pH differed by more than 0.03 from the set point, it was adjusted by the aliquot addition of 0.1 M NaOH or HCl. The experiment was initiated with an inoculation of 1,000 cells ml−1 and was allowed to run for a minimum of 18 d and a maximum of 20 d.
Growth rate of the sea ice algae at different pH and TCO2
In the first set of pH experiments, growth rates of the three species of sea ice algae: Fragilariopsis nana, Fragilariopsis sp., and Chlamydomonas sp. were measured at different pH values ranging from 8.0 to 10.0 (i.e., pH 8.0, 8.5, 9.0, 9.5, and 10.0). The salinity was 33 throughout the experiment. The pH was adjusted by addition of 0.1 M HCl or NaOH to the medium. The experiment was initiated by inoculating 1,000 cells ml−1 and was allowed to run for 20 d. The TCO2 concentration was, in all instances, 2.4 mM. Every second day, pH of the culture media was measured, and subsamples (1 ml) were taken for enumeration of algae cells. After subsampling, the bottles were refilled to capacity with pH adjusted-L1 growth medium (i.e., pH 8.0, 8.5, 9.0, 9.5, 10.0), and the bottles were remounted on the plankton wheel. If the pH differed by more than 0.03 from the set point, it was adjusted by addition of aliquots of 0.1 M HCl or NaOH.
In the pH-drift experiment, Fragilariopsis sp. and Chlamydomonas sp. were inoculated (1,000 cells ml−1) in media with a pH of 8.0 and initial TCO2 concentrations of c. 1.2 or 2.4 mM and were allowed to grow into stationary growth phase (up to 26 d). The 1.2 mM TCO2 concentration medium was obtained by mixing the 2.4 mM L1 growth medium with a very low TCO2 concentration medium (<0.5 mM). The very low TCO2 medium was prepared by acidifying the growth medium (to pH < 3), followed by heating to 110°C for 30 min and aerating the medium. The pH was then adjusted to 8.0 by the addition of 0.1 or 1.0 M NaOH or HCl (Hansen et al. 2007). The experiment was carried out in gas-tight laminated NEN/PE plastic bags (Hansen et al. 2000). Every second day, the pH of the culture medium was measured, and subsamples were withdrawn for enumeration of algae cell concentration (3 ml) and for measurements of the TCO2 concentration (36 ml). The NEN/PE plastic bags were not refilled after each sampling. The [CO2 + HCO3 −] concentrations were calculated from measurements of TCO2, temperature, salinity, and pH (Lewis and Wallace 1998).
Succession experiment
The three sea ice algal species were inoculated in a mixed culture (i.e., 1,000 cells ml−1) at a pH of 8.0 and a salinity of 33 and an initial TCO2 concentration of 2.4 mM. The three sea ice algal species were allowed to grow well into stationary growth phase (up to 22 d). Every second day, pH of the culture media was measured, and subsamples (1 ml) were taken for enumeration of the mixed algae cells. After subsampling, the bottles were refilled to capacity with pH adjusted-L1 growth medium and the bottles were remounted on the plankton wheel. TCO2 concentration was measured at the initiation and the termination of the experiment to ensure that the concentration was sufficient for algae growth during the experiments.
Results
Effect of salinity on the growth rates of three Arctic sea ice algae
The two sea ice diatoms exhibited similar growth rates as a function of salinity, and no significant differences were observed between acclimation salinities (33 or 75) used in the salinity experiments (Student’s t-test, P > 0.05). Maximum growth rates were obtained at a salinity of 33 (Fig. 1a). At salinities above 33, growth rates gradually decreased with salinity. However, growth rates at a salinity of 100 were reduced by 50%, and none of the diatoms could grow at a salinity of 150. At salinities below 33, growth rates of the two diatoms decreased only slightly and they were still quite high at a salinity of 5 (Fig. 1a, b). The two diatom species showed significantly more reduced growth rates at high salinities than at low salinities (Fragilariopsis nana OLS, P = 0.005, one-sided and Fragilariopsis sp., P = 0.005). The growth response of the chlorophyte as a function of salinity was similar to that of the two diatoms showing more reduced growth rates at high salinities (Chlamydomonas sp. OLS, P = 0.009, one-sided) (Fig. 1a). Growth rates of the chlorophyte was greatly reduced already at a salinity of 50 and it could not grow at salinities above 100 (Fig. 1a, b). Effect of pH and TCO 2 limitation on the growth rate of the three sea ice algae.
A very profound effect of the pH was observed on the growth rates of all three species (Fig. 2). All species exhibited maximum growth rates at a pH of 8.0–8.5 (Fig. 2). Above a pH of 8.5, a negative effect of increasing pH was observed on the growth rate of all species. However, all species were still able to grow at half the maximum growth rate at pH 9.5. The diatom species could not grow at pH 10, while the chlorophyte species demonstrated a growth rate of one-third its maximum. The growth rates of the two diatoms were significantly reduced at pH > 9.0 (Student’s t-test Fragilariopsis nana, P = 0.0066; Fragilariopsis sp., P = 0.0061).
Fragilariopsis nana, Fragilariopsis sp., and Chlamydomonas sp. Growth rates of the three sea ice algae as a function of different fixed pH levels. Dissolved inorganic carbon (TCO2) concentration was initially between 2.2 and 2.4 mM in the experiment flasks. Data points represent treatment means SE ± (n = 3)
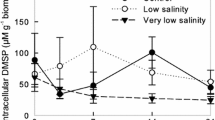
In the pH-drift experiments of Fragilariopsis sp., the pH reached a maximum of 9.5 and 9.7 in the experiments initiated at a TCO2 concentration of 1.4 and 2.4 mM, respectively (Fig. 3). Final TCO2 concentrations in these experiments were 1.0 and 1.5 mM, respectively.
Fragilariopsis sp. and Chlamydomonas sp. Cell concentration in 105, pH, dissolved inorganic carbon (TCO2) and available inorganic carbon [CO2 + HCO3 −] as a function of time for pH-drift experiments at initial TCO2 concentration for the two sea ice algae. Initial TCO2: (a, b) 2.4 mM and (c–d) 1.4 mM. Data points represent treatment means ± (n = 3)
For the pH-tolerant species, Chlamydomonas sp., the pH reached 9.8, when grown at initially high and low TCO2 concentrations (Fig. 3). The final TCO2 concentration was 1.0 mM in the experiments initiated at a high TCO2 concentration, whereas the concentrations decreased from 1.4 to 1.0 mM for this species in experiments initiated at a low TCO2 concentration (Fig. 3).
Succession experiment
The importance of pH in succession of sea ice algae species was studied using mixed cultures of three sea ice algal species (Fragilariopsis nana, Fragilariopsis sp., and Chlamydomonas sp.) with an initial pH of 8.0 (Fig. 4). All three species grew until pH reached 9.4 to 9.5 on Day 18. At Day 20, the pH had increased to above 9.6, and the two diatoms stopped growing, while the chlorophyte species maintained a positive growth rate.
Succession experiment. a Change in cell concentration in 105 of the three sea ice algae species. Fragilariopsis nana, Fragilariopsis sp., and Chlamydomonas sp. as a function of time (d) from inoculation at pH 8.0. b pH as a function of time from inoculation. Data points represent treatment means SE ± (n = 3)
Discussion
Growth of sea ice algae at different salinities
The ability of sea ice algae to grow within the physiochemical gradient found in the sea ice suggests that the algae are well adapted to cope with fluctuations in light, temperature, salinity, pH, and TCO2 concentrations. However, salinity has a pronounced effect on growth, photosynthetic efficiency, and metabolism (Misra et al. 2001). Some microalgae are considered euryhaline, since they can adapt to varying external salinities (Hellebust 1985). However, the salinity range over which active growth takes place differs greatly among species, and the physiochemical conditions in the sea ice will provide a selection pressure that influences the final community composition (Ryan et al. 2004). A previous study has indicated that most sea ice algae are more tolerant to reduced, rather than elevated salinities (Bates and Cota 1986). The present study supports the results of Bates and Cota (1986), as the three sea ice algae showed more reduced growth rates at high salinity levels than at low salinity levels. The experiments suggest that the two diatoms have a competitive advantage in sea ice, where brine salinity is greater than 50. These salinity conditions are typically encountered where the sea ice temperature is between −1.9 and −6.7°C (Gleitz et al. 1995).
When sea ice melts, the algae are exposed to altered salinities and subsequently the algal growth may be influenced. A previous study showed that diatom species are only slightly affected by decreasing salinities, whereas decreasing salinities may result in substantial losses of ciliates and flagellate species (Garrison and Buck 1986; Ryan et al. 2004; Mikkelsen and Witkowski 2010). In the present study, the three sea ice algal species were exposed to changes in salinity conditions with different initial salinities of 33 or 75 to test the effect of rapid shifts in salinity from high to low on the growth rates. The salinity stress had the smallest effect on the growth rate of the two diatoms compared to the effect on the chlorophyte. This suggests that sea ice diatoms are less affected by decreasing salinities and thus may have a competitive advantage during summer and spring thaw when sea ice salinity becomes low. This result compares with previous studies showing that sea ice diatoms dominates during sea ice summer and spring thaw (Palmisano and Garrison 1993; Ikävalko and Thomsen 1997; Mikkelsen et al. 2008). Furthermore, the present study shows that some sea ice algal species are better adapted to changes in salinity than other algal species, and thus, the changes in sea ice salinity may drive species succession of sea ice algae.
All the experiments were conducted at higher temperatures (3 ± 1°C) than the in situ temperatures observed in sea ice (Søgaard et al. 2010). Previous studies have shown that photosynthetic rates in sea ice algae are influenced by temperature (Palmisano et al. 1987; Ralph et al. 2005). This suggests that the growth rates of the three sea ice algae might be overestimated compared to growth rates at in situ temperatures. However, it is a nontrivial task to incubate samples at low bulk salinity at 0 or sub-zero temperature without introducing freezing and thaw artifacts.
Tolerance of sea ice algae to elevated pH
The effect of high pH on the growth rates of marine planktonic algae is well established. Some species are very sensitive to elevated pH and cannot grow when pH exceeds 8.8, while others still grow at pH above 10 (e.g., Hansen 2002; Lundholm et al. 2004). Several studies have also shown that the tolerance to high pH is species specific, and large differences exist within important marine algal groups, such as diatoms and dinoflagellates (Hansen 2002; Lundholm et al. 2004; Søderberg and Hansen 2007).
The knowledge of the effect of high pH on growth rates of sea ice algae is however, very limited. High pH is observed in sea ice with high primary production (Gleitz et al. 1995; Thomas et al. 2001) and thus prevails during spring when irradiance increase (Cota and Horne 1989; Kühl et al. 2001). In the present study, influence of high pH levels on the growth rate of the three species of sea ice algae was studied at pH levels ranging from pH 8.0 to 10.0 in nutrient-rich growth media. We did not measure the nutrient concentrations in the nutrient rich media during all the experiments, but the amount of nutrients left at the termination of the experiments assuming Redfield stoichiometry documents that nutrients were not limiting at any point during the experiments (see Table 1).
In the present study, our results clearly demonstrate that all species were restricted by high pH even at a high initial TCO2 concentration. Growth rates were significantly reduced for both diatom species at pH > 9.0 (Fig. 2). Above pH 9.5, the two sea ice diatoms stopped growing irrespective of TCO2, showing that pH had a direct effect on algal growth. Only a limited number of diatoms have been studied with respect to effect of pH on growth; however, among those studied the effect of pH on growth varied (Lundholm et al. 2004). Lundholm et al. (2004) found that smaller diatoms have a higher upper pH limit for growth than larger diatom. In present study, we deliberately chose Fragilariopsis nana because it is a relatively small diatom species and Fragilariopsis sp. because it is somewhat larger diatom species. Despite the difference in cell volume, the two diatom species showed the same upper pH limit. The sea ice chlorophyte showed an extreme pH tolerance as only a small reduction in growth rate was observed above this pH level. The results suggest that these sea ice algal species are not limited by inorganic carbon at pH 8.0–9.0 (Fig. 3), a pH level close to that found in sea ice brine (Papadimitriou et al. 2007). However, pH increases in colonized sea ice because of a decline in TCO2 as a result of photosynthetic carbon assimilation (Thomas et al. 2010). This may affect species such as Fragilariopsis nana and Fragilariopsis sp (Figs. 2, 3 and 4). Other species such as Chlamydomonas sp. can tolerate much higher pH levels and thus will have a competitive advantage in sea ice with high pH levels (Figs. 2, 3 and 4). However, this species was limited by low TCO2 concentrations and thus may be outcompeted by algal species in the sea ice, which are able to grow at high pH levels and very low TCO2 concentrations. Algae can only utilize CO2 and HCO3 − for photosynthesis (e.g., Stumm and Morgan 1996; Korb et al. 1997), and it is well known that the speciation of inorganic carbon species depends upon pH. For instance, at pH 9.3, only half of the TCO2 is available in the form of [CO2 and HCO3 −]. In the pH-drift experiments initiated at a low TCO2, the algae were able to deplete the available inorganic carbon [CO2 and HCO3 −] to a lower limit of 0.45 mM for Fragilariopsis sp. and 0.25 mM for Chlamydomonas sp., assuming equilibrium in the carbonate system (Fig. 3). At those low concentrations of [CO2 and HCO3 −], growth rates of the algal species may have become restricted by carbon, as has been shown previously for dinoflagellates (Hansen et al. 2007).
For plankton communities, pH changes have been shown to drive species succession, because many planktonic algae appear to be quite sensitive to high pH (Hansen 2002; Pedersen and Hansen 2003). However, possible role of elevated pH in the succession of Arctic sea ice algae has received little attention. The succession experiment carried out in the present study suggested that elevated pH may well drive species succession, as the pH-tolerant species (Chlamydomonas sp.) out-grew the two sea ice diatoms (Fig. 4). However, how can we be sure that the observed succession pattern in the study is due to pH changes and not due to for instance production of toxic substances (allelochemicals) that affect the growth of the two diatoms? Well, we cannot completely out rule that the chlorophyte exudes allelochemicals, as we did not test this specifically. However, no marine chlorophytes have yet been convincingly shown to produce allelochemicals (see review by Granéli and Hansen 2006). Secondly, the growth of the two diatom species in the mixed culture experiment can be explained by pH changes alone, and there are no indications in our data set which suggest that allelochemicals were produced. Our study has only dealt with a few species of ice algae. Thus, much more attention is required in this topic. It would be particularly interesting to study how elevated pH and therefore decreasing TCO2 affects in situ succession pattern and the growth rates of the algal species in the sea ice.
Conclusions
Our results suggest that the three sea ice algal species have different tolerance to fluctuations in salinity and pH. The results suggest that the three sea ice algal species were mainly limited by pH, whereas TCO2 concentrations only played a role at high pH levels and low TCO2 concentrations. The salinity stress had the smallest effect on the growth rate of the two diatoms compared to the effect on the chlorophyte. This suggests that sea ice diatoms are less affected by salinities changes and thus may have a competitive advantage compared to the chlorophyte in sea ice with rapid fluctuations in salinity. Finally, the fluctuations in pH levels may drive species succession of sea ice algae. The chlorophyte was able to tolerate much higher pH levels than the two diatom species. Thus, Chlamydomonas sp. was able to grow even at high pH levels in the succession experiment and therefore outcompeted the two diatom species.
Consequently, sea ice algal species, which are able to grow at fluctuating pH and salinity conditions, may have an advantage in surviving in the harsh environment of forming and melting sea ice.
References
Arrigo KR, Sullivan CW (1992) The influence of salinity and temperature covariation on the photophysiological characteristics of Antarctic sea ice microalgae. J Phycol 28:746–756
Arrigo KR, Mock T, Lizotte MP (2010) Primary producers and sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Blackwell Science, Oxford, pp 283–325
Bates SS, Cota GF (1986) Flurorescence induction and photosynthetic responses of arctic ice algae to sample treatment and salinity. J Phycol 22:421–429
Cota GF, Horne EPW (1989) Physical control of arctic ice algal production. Mar Ecol Prog Ser 52:111–121
Cox GFN, Weeks WF (1983) Equations for determining the gas and brine volume in sea ice samples. J Glaciol 29:306–316
Dieckmann GS. Nehrke G, Papadimitriou S, Göttlicher J, Steininger R, Kennedy H, Wolf-Gladrow D, Thomas DN (2008) Calcium carbonate as ikaite crystals in Antarctic sea ice. Geophys Res Lett 35:L08501. doi:10.1029/2008GL033540
Garrison DL, Buck KR (1986) Organisms losses during melting: a serious bias in sea ice community studies. Polar Biol 6:237–239
Gleitz M, Thomas DN (1992) Physiological responses of a small Antarctic diatom (Chaetocerros sp.) to simulated environmental constraints associated with sea ice formation. Mar Ecol Prog Ser 88:271–278
Gleitz G, Rutgers VD, Thomas DN, Dieckmann GS, Millero FJ (1995) Comparison of summer and winter inorganic carbon, oxygen and nutrient concentrations in Antarctic sea ice brine. Mar Chem 51:81–91
Gradinger R (1999) Vertical fine structure of the biomass and composition of algal communities in Arctic pack ice. Mar Biol 133:745–754
Granéli E, Hansen PJ (2006) Allelopathy in harmful algae: a mechanism to compete for resources? In: Granéli E, Turner JT (eds) Ecology of Harmful Algae, Ecological Studies series of Springer-Verlag, vol 189. Springer, Chap 15 pp 189–201
Grant WS, Horner RA (1976) Growth responses to salinity variation in four Arctic ice diatoms. J Phycol 12:180–185
Guillard RRL, Hargraves PE (1993) Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 32:234–236
Hansen PJ (2002) The effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquat Microb Ecol 28:279–288
Hansen JW, Thamdrup B, Jørgensen BB (2000) Anoxic incubation of sediment in gas-tight plastic bags: a method for biogeochemical process studies. Mar Ecol Prog Ser 208:273–282
Hansen PJ, Lundholm N, Rost B (2007) Growth limitation in marine red-tide dinoflagellates: effects of pH versus inorganic carbon availability. Mar Ecol Prog Ser 334:63–71
Hellebust JA (1985) Mechanisms of response to salinity in halotolerant microalgae. Plant Soil 89:69–81
Huertas IE, Colman B, Espie GS, Lubian LM (2000) Active transport of CO2 by the three species of marine microalgae. J Phycol 36:314–320
Ikävalko J, Thomsen HA (1997) The Baltic Sea ice biota (March 1994): a study of the protistan community. Eur J Protistol 33:229–243
Korb RE, Saville PJ, Johnston AM, Raven J (1997) Sources of inorganic carbon for photosynthesis by three species of marine diatoms. J Phycol 33:433–440
Kühl M, Glud RN, Borum J, Roberts R, Rygaard S (2001) Photosynthetic performance of surface-associated algae below sea ice as measured with a pulse-amplitude-modulated (PAM) fluorometer and O2 microsensors. Mar Ecol Prog Ser 223:1–14
Lewis E, Wallace D (1998) Program developed for CO2 system calculations. Environmental Science Division. No. 4735
Lizotte MP (2003) The microbiology of sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice an introduction to its physics, chemistry, biology and geology. Blackwell Science, Oxford, pp 184–210
Lundholm N, Hansen PJ, Kotaki Y (2004) Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar Ecol Prog Ser 273:1–15
Mikkelsen DM, Witkowski A (2010) Melting sea ice for taxonomic analysis: a comparison of four melting procedures. Polar Res. doi:10.1111/j.1751-8369.2010.00162.x
Mikkelsen DM, Rysgaard S, Glud RN (2008) Microalgal composition and primary production in Arctic sea ice: a seasonal study from Kobbefjord (Kangerluarsunnguaq), West Greenland. Mar Ecol Prog Ser 368:65–74
Misra AN, Srivastava A, Strasser RJ (2001) Utilization of fast chlorophyll a fluorescence techniques in assessing salt/ion sensitivity of mung bean and Brassica seedlings. J Plant Physiol 158:1173–1181
Palmisano AC, Garrison DL (1993) Microorganisms in Antarctic sea ice. In: Friedmann EI (ed) Antarctic microbiology. Wiley-Liss, New York, pp 167–218
Palmisano AC, SooHoo JB, Sullivan CW (1987) Effects of four environmental variables on photosynthesis-irradiance relationships in Antarctic sea ice microalgae. Mar Biol 94:299–306
Papadimitriou S, Thomas DN, Kennedy H, Hass C, Kuosa H, Krell A, Dieckmann GS (2007) Biochemical composition of natural sea ice brines from the Weddell Sea during early austral summer. Limnol Oceanogr 52:1809–1823
Pedersen MF, Hansen PJ (2003) Effects of high pH on a natural marine planktonic community. Mar Ecol Prog Ser 260:19–31
Ralph PJ, McMinn A, Ryan KG, Ashworth C (2005) Short-term effect of temperature on the photokinetics of microalgae from the surface layers of Antarctic pack ice. J Phycol 41:763–769
Reinfelder JR, Kraepiel AM, Morel FM (2000) Unicellular C4 photosynthesis in a marine diatom. Nature 407:996–999
Rost B, Riebesell U, Burkhardt S, Sültermeyer D (2003) Carbon acquisition of bloom-forming marine phytoplankton. Limnol Oceanogr 48:55–67
Ryan KG, Ralph PJ, McMinn A (2004) Acclimation of Antarctic bottom-ice algal communities to lowered salinities during melting. Polar Biol 27:679–686
Rysgaard S, Glud RN, Sejr MK, Bendtsen J, Christensen PB (2007) Inorganic carbon transport during sea ice growth and decay: A carbon pump in polar seas. J Geophys Res 112:C03016. doi:10.1029/2006JC003572
Søderberg LM, Hansen PJ (2007) Growth limitation due to high pH and low inorganic carbon concentrations in temperate species of the dinoflagellate genus Ceratium. Mar Ecol Prog Ser 351:105–112
Søgaard DH, Kristensen M, Rysgaard S, Glud RN, Hansen PJ, Hilligsøe KM (2010) Autotrophic and heterotrophic activity in Arctic first-year sea ice: seasonal study from Malene Bight, SW Greenland. Mar Ecol Prog Ser 419:31–45
Stumm W, Morgan J (1996) Chemical equilibria and rates in natural waters. Aquatic chemistry. Wiley, New York
Thiel H, Pörtner HO, Arntz WE (1996) Marine life at low temperatures–a comparison of polar and deep-sea characteristics. Biosys Ecol Ser 11:183–219
Thomas DN, Kennedy H, Kattner G, Gerdes D, Gough C, Dieckmann GS (2001) Biogeochemistry of platelet ice: its influence on particle flux under fast ice in the Weddell Sea, Antarctica. Polar Biol 24:486–496
Thomas DN, Papadimitriou S, Michel C (2010) Biogeochemistry of sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice, 2nd edn. Blackwell Science, Oxford, p 432
Tortell PD, Reinfelder JR, Mortel FMM (1997) Active uptake of bicarbonate by diatoms. Nature 390:243–244
Trimborn S, Lundholm N, Thoms S, Richter K-U, Krock B, Hansen PJ, Rost B (2008) Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in the seawater carbonate chemistry. Physiol Plant 133:92–105
Weeks WF, Ackley SF (1986) The growth, structure and properties of sea ice. In: Understeiner N (ed) The geophysics of sea ice. Martinus Nijhoff Publisher, Dordrecht
Acknowledgments
We thank Anna Haxen and Michael R. Schrøder for assistance in field and laboratory and Thomas Juul-Pedersen, Kristine Arendt and Paul Batty for valuable comments. Furthermore, we want to thank Nina Lundholm from the Scandinavian Culture Collection of Algae and Protozoa, Department of phycology, University of Copenhagen for providing the diatom Fragilariopsis nana. The study received financial support from the Danish Agency for Science, Technology and Innovation, KVUK Commission for Scientific Research in Greenland and is a part of the Greenland Climate Research Centre (GCRC 6507) and a contribution to the Nuuk Basic and Zackenberg Basic programmes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Søgaard, D.H., Hansen, P.J., Rysgaard, S. et al. Growth limitation of three Arctic sea ice algal species: effects of salinity, pH, and inorganic carbon availability. Polar Biol 34, 1157–1165 (2011). https://doi.org/10.1007/s00300-011-0976-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-0976-3