Abstract
This study documents horizontal distribution and demography of Antarctic krill (Euphausia superba) from the Southern Ocean during January–March 2008. The cruise predominantly occurred in CCAMLR Subarea 48.6, where knowledge about the ecosystem is limited. E. superba were not found north of 52°S. The biomass, estimated from trawl catches, was highest (63.09 g/m2) at a station 680 km southeast of Bouvetøya and at two stations 1,400 and 600 km southeast and southwest of Bouvetøya, 54.67 and 61.38 g/m2, respectively. Body length ranged from 19 to 61 mm (N = 8,538), with a mean of 42.0 ± 6.4 mm (SD). The overall sex ratio was 1:1, 46.2% males (13.2% adults and 33.0% subadults), 46.1% females (33.6% adults and 12.5% subadults), while 7.5% were juveniles. Trawl stations dominated by adults were found west and north of Bouvetøya. Stations with high proportions of subadults and juveniles were mainly found southeast of the island. Four cluster groups were differentiated: analyzing data on krill sex proportions, maturity stages, hydrography, nutrients and chlorophyll concentrations. Two groups represented stations located in the northern part of the study area, where E. superba were absent; water temperatures were higher and the nutrient concentrations lower compared to the groups where E. superba were present. This study shows that bathymetric features like the North Weddell Ridge including Bouvetøya are important for concentrating krill probably due to water mass characteristics and advective processes which influence regional krill demography. The southern regions of CCAMLR sector 48.6 are essential for understanding regional krill recruitment and production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antarctic krill (Euphausia superba) have a circumpolar distribution, predominantly inhabiting the seasonal sea-ice zone and have been recorded to form the largest biomass aggregations among marine life (Macaulay et al. 1984). They are plankton feeders and an important direct food source to apex predators in the Antarctic marine ecosystem such as whales, seals, squid, fish, penguins, and other seabirds (Everson 2000). Krill are rich in omega-3 fatty acids, antioxidants, and high-quality proteins, which also make them an attractive resource for both human consumption and for the animal-feed industry (Tou et al. 2007).
Krill have been harvested in the Southern Ocean since the early 1970s. For management purposes of krill and other marine life in the Southern Ocean, the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) came into force in 1982. Knowledge about distribution and population parameters is fundamental for managers to set catch limits or to generally assess the well-being of a particular animal group in a geographic area. Population parameters are not static, but change over time, and assessed in a comparative manner allow more in depth assessments of how the population is likely to change in the future.
Monitoring of the global climate shows that the Southern Ocean has experienced a rapid warming during the second half of the twentieth century (e.g. Levitus et al. 2000; Gille 2002), and there are concerns that the mean krill recruitment and even the overall biomass of E. superba have declined (Siegel et al. 1997; Loeb et al. 1997; Atkinson et al. 2004). E. superba is a stenothermal, cold-water species and especially vulnerable to small changes in temperature during the early stages of development. The warming trend is likely to favor other Macro- and Meso-zooplankton species that occupy the more northerly parts of the Antarctic Circumpolar Current (ACC) (Whitehouse et al. 2008). Increased scientific interest has been devoted to the pelagic tunicate Salpa thompsoni, which has the potential to undergo explosive development and outcompete E. superba (Loeb et al. 1997; Walsh et al. 2001; Wiebe et al. 2010). Recent studies suggest that the salp distribution has changed over the past century by shifting southward (Pakhomov et al. 2002; Atkinson et al. 2004).
Over the past decades, much work has been carried out to monitor E. superba, particularly along the Antarctic Peninsula and in the western Scotia Sea (Ross et al. 1996; Hofmann et al. 2004; Watkins et al. 2004) as well as in the Indian Ocean area (Nicol 2000) and the western Ross Sea (Faranda et al. 2000). Other areas like the Bellingshausen, Amundsen and Weddell Seas, and also the East Atlantic sector have received less attention (Mackintosh 1973), mainly due to logistic inaccessibility.
The purpose of this particular study was to generate basic distributional information of E. superba in the largely unstudied CCAMLR area 48.6, which is in the Southeast Atlantic sector of the Southern Ocean. Trawl catches along with concurrent station data on hydrography, nutrients and chlorophyll are here used to describe spatial patterns of demography and occurrences of E. superba in relation to its environment.
Materials and methods
The Norwegian research vessel G.O. Sars was used for the AKES (Antarctic Krill and Ecosystem Studies) survey, as part of the IPY (International Polar Year) 2008 program, in the Southeast Atlantic Ocean conducted from 4 January to 28 March 2008. The first part of the cruise started around South Georgia and proceeded toward Bouvetøya, following a predetermined route centered ~55°S. Further, the second part consisted of a parallel-transect starting north of the Antarctic Polar Front (APF) and following the ~15°E meridian southwards to 67°07′S, then returning on a northerly route following the ~5°E meridian passing east of Bouvetøya (see Iversen et al. 2008 for a detailed survey map).
Trawling for Macrozooplankton was made at predetermined sampling stations when weather conditions permitted use of fishing gear and electronic equipment. Three different trawls were used: the preferred trawl was the “Macroplankton trawl”; a fine-meshed plankton trawl having a 38 m2 mouth-opening and a mesh size of 3 mm from the trawl-opening to the rear end. Towing speed was normally 2.5–3 knots. A MultiSampler unit (see Engås et al. 1997) with five separate cod-ends (mesh size 3 mm) was attached to the rear end of the trawl enabling sampling of up to five different depth strata during the same tow. The approximate sampling-depths for standardized hauls were 750–500, 500–300, 300–200, 200–10, and 10–0 m. Krill biomass was calculated from depth stratified hauls (N = 21) obtaining net velocity data by a Scanmar acoustic speed sensor (type: HC4-TSS) attached to the headline of the trawl. Opening and closing of the MultiSampler nets were achieved using a manual acoustic release system at the predetermined depths (Engås et al. 1997). The depth of the Macroplankton trawl was determined using a standard Scanmar depth trawl sensor. A larger and more conventionally designed trawl, the “Micronekton trawl”, with a vertical opening of 14 m and variable mesh sizes in the front part of the trawl, but 6-mm stretched meshes in the rear section, was also used, particularly to identify acoustic scatterers. The maximum sampling-depths with the Micronekton trawl ranged from surface waters to 765 m, but the average maximum depth was ~150 m. In the present work, the Micronekton trawl was only used as a supplement to the Macrozooplankton trawl with respect to the presence–absence and demographic patterns of E. superba. Additionally, the “Åkratrawl”, a medium-sized midwater trawl originally equipped with a 22-mm-meshed cod-end was used to catch pelagic fish (see Fernø and Olsen 1994; Wenneck et al. 2008).
When a trawl-catch was landed on deck, Macrozooplankton and Micronekton were sorted, identified to species (as for E. superba) or to the nearest possible taxonomic group, and weights were determined. For this study, a subsample of E. superba was taken, and the length of the individual krill was measured (±1 mm) from the anterior margin of the eye to tip of telson excluding the setae, according to the “Discovery method” used in Marr (1962). Additional subsamples were preserved on borax-buffered formalin (4%), in 96% alcohol, or kept in a freezer at −80°C for later analyses. Body length was measured for a total of 8,538 animals.
Sex and maturity stages of E. superba in the formalin-preserved samples were determined for a total of 6,609 animals using the classification methods outlined by Makarov and Denys (1981).
Vertical casts with a Seabird 911 CTD were performed along the cruise track, recording temperature, salinity, and sigma-t profiles from the surface to a standard depth of 1,500 m or to 5 m above bottom in shallower waters.
Water samples (20 ml, scintillation vials PE) for nutrient analyzes (nitrite, nitrate, phosphate, and silicate) were collected from standardized depths between 1,500 m and the surface, or from between 5 m above bottom and the surface in shallower waters, by means of rosette-mounted Niskin-bottles on the CTD. The samples were fixed with chloroform (1%) and kept at 4°C until subsequent analyses on shore. The samples were analyzed, following the methods of Bendschneider and Robinson (1952) and Grasshoff (1965), on a SKALAR auto-analyzer equipped with matrix detectors and manifolds developed at the Norwegian Institute of Marine Research. For chlorophyll a and phaeopigment measurements, water was collected (263 ml) at standardized depths between 200–5 m. The water was filtered using a 0.7-μm filtration system (Munktell glass fiber filters Grade: MGF, vacuum 400 mm Hg) and stored at −20°C until analysis on shore. The assay was performed by extraction with 90% acetone followed by centrifugation, and the measurements were taken with a fluorometer (model 10 AU, Turner Designs Inc., Sunnyvale, Ca., USA), according to Welshmeyer (1994) and Jeffrey and Humphrey (1975), giving concentrations in mg/m3.
Due to the vast area covered and duration of the survey, sampling at the predetermined stations was made independently of time of day. The survey covered regions exhibiting different physical and biological properties, also including varying phases of the seasonal production cycle. Since the Macroplankton and Micronekton trawls have different designs and were mainly deployed for different purposes and used during different weather conditions; a comparison of length measurements of E. superba from the two types of gear was made in order to evaluate the possibility of combining these data to obtain an increased geographic coverage for krill demography. We noticed that the shapes of the distributions are similar, both having a tail of smaller individuals on their left side and a near identical slope on the right side of the distributions. The ranges of lengths are nearly identical (19–60 vs. 21–61 mm), as are also the means (42.6 vs. 41.7 mm), modes (both 40 mm), medians (both 42 mm), and SD (6.2 vs. 6.5 mm) for the Macroplankton and Micronekton trawls, respectively.
For the Micronekton trawl, we noticed a somewhat larger proportion of the smallest individuals compared to the Macroplankton trawl. For lengths <35 mm, the proportions were 12.9 and 7.4%, while for lengths <30 mm, the corresponding proportions were 3.4 and 0.7% for the Micronekton and Macroplankton trawls, respectively.
The higher proportions of smaller individuals are particularly evident from Micronekton hauls undertaken in the south-eastern regions of the study area. However, when both trawl types were used in vicinity of each other, similar demographic patterns were found. We believe that the apparent geographical differences in demographic properties through the study area have a sound biological basis and are not merely artefacts caused by differences in sampling gear and methodology. Thus, we chose to use the data from both sources in the analysis and presentations of krill demography.
An objective multivariate approach including principal component analysis (PCA) and cluster analysis was used to identify potential horizontal patterns of krill demography and environmental parameters. Input variables for the multivariate statistics included 32 trawl stations with a complete set of physical parameters: average nitrite, nitrate, phosphate, and silicate concentrations above 200 m (μmol/l); average temperature above 200 m (°C); average salinity above 200 m (Practical Salinity Unit: PSU); average Sigma-t above 200 m (mg/m3); average chlorophyll a and phaeopigment concentrations above 200 m (mg/m3); and krill proportions for different maturity stages. This comprises a total of 21 krill and environmental variables.
None of the variables were normally distributed (assessed visually as well as by a modified Kolmogorov–Smirnov test). Attempting to meet the assumption of normality, all variables were log10 + 1 transformed to avoid zero-values (van Tongeren 1995; Zar 1996). This approach did not result in normalization. However, PCA is not sensitive to non-normality, and cluster analysis is not a statistical interference technique and departure from normality may affect it only by diminishing the observed correlation (Hair et al. 1998). Because clustering methods are highly sensitive to discordant scales (Hair et al. 1998), all data were standardized to a mean of 0 and a variance of 1 (Zar 1996).
To avoid the problem of multicollinearity, we first introduced the variables into a principal components analysis (PCA) and used the resulting uncorrelated principal components in the subsequent cluster analysis. The appropriate number of factors to extract was selected using the latent root criterion (Hair et al. 1998; Norman and Streiner 2008) (Table 2). The uncorrelated components from the PCA were entered into a complete-linkage hierarchical cluster analysis. The cluster level with the highest percent change in the agglomeration coefficient was used to identify the appropriate number of clusters (Hair et al. 1998).
The number of clusters and the initial seed points identified by the procedure described previously were used to perform a non-hierarchical k-means cluster analysis. The results from the k-means cluster analysis provided information about the trawl stations cluster-type membership, which was then merged with the raw data for further analysis. Cluster analysis groups objects into relatively few classes (Fowler et al. 1998), and during the first run, the procedure recognized two groups. One cluster group contained stations with complete absence of E. superba in the catch (cluster group 1) while the other group had E. superba in some of the catches (cluster group 2). To obtain a finer differentiation; cluster group 2 was used as new input and the whole PCA and cluster procedure was run a second time, once again revealing two groups: one group lacking E. superba (cluster group 2A) and one group with E. superba in some of the catches (cluster group 2B). The PCA and cluster procedure was finally run a third time on the group with E. superba in the catch, revealing the two groups 2B1 (E. superba in some of the catches) and 2B2 (E. superba in all of the catches).
Results
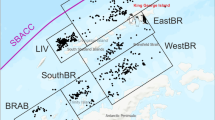
The northern limit of E. superba was found to be 52°S during this survey. From a total of 61 sampling stations, 41 stations contained specimens of E. superba (Fig. 1). E. superba were absent from the catch in 9 of a total of 31 Macroplankton trawl stations; on two occasions, this was due to net failure, 2 stations were dominated by salps (Salpa thompsoni, Ihlea racovitzai or Isais zonaria) and in 5 of the cases, where E. superba was absent, the net catches were dominated by other krill species (E. vallentini, E. triacantha, E. frigida, Thysanoessa sp.), amphipods (e.g., Themisto sp., T. gaudichaudii) or fish (mainly myctophids). When using the Micronekton trawl, E. superba was absent from the catch in 6 out of 24 stations. Only one of six Åkratrawl hauls contained E. superba in the catch. These hauls were mainly dominated by fish species. For a more detailed description of the catch of other species than E. superba; see Iversen et al. (2008).
Positions for macrozooplankton stations with presence and absence of E. superba in the trawl catch, during the AKES 2008. The CCAMLR Statistical Reporting Subareas are presented with their respective convention number. Positions for PBF (Antarctic Polar Front), SACCF (South Antarctic Circumpolar Current Front), and SBF (South Boundary Front) are based on data from Orsi et al. (1995)
Biomass calculations based on the 21 Macroplankton trawl stations displayed highest concentrations (63.09 g/m2) at a station 680 km southeast of Bouvetøya and at two stations 1,400 and 600 km southeast and southwest of Bouvetøya, 54.67 and 61.38 g/m2, respectively (Fig. 2). The average body length was 42.0 ± 6.4 (SD) mm, and the range was 19–61 mm.
Biomass estimations of E. superba (wet weight/m2) based on data from Macroplankton trawls (N = 21) with complete datasets, performed during the AKES 2008. The CCAMLR Statistical Reporting Subareas are presented with their respective convention number. Positions for PBF (Antarctic Polar Front), SACCF (South Antarctic Circumpolar Current Front) and SBF (South Boundary Front) are based on data from Orsi et al. (1995)
From the animals collected throughout the study area, 7.5% were juveniles, 45.5% were subadults, and 46.8% were adults (Table 1; Fig. 3). The corresponding sex ratio was 1:1 (46.2% males and 46.1% females) and this sex ratio with moderate variation was represented throughout the study area (Fig. 4). The samples were roughly dominated by subadult males at stage MIIA2 (25%) and adult females stage FIIIA (21%) (Table 1).
Distribution of the maturity stages throughout the study area from Macrozooplankton stations during AKES 2008. Black lines from the male and female icons represent leader lines to the stations positions. The CCAMLR Statistical Reporting Subareas are presented with their respective convention number. Positions for PBF (Antarctic Polar Front), SACCF (South Antarctic Circumpolar Current Front), and SBF (South Boundary Front) are based on data from Orsi et al. (1995)
Distribution and proportion of E. superba males and females from the Macrozooplankton stations performed during the AKES, 2008. Black lines from the histograms represent leader lines to the stations positions. The CCAMLR Statistical Reporting Subareas are presented with their respective convention number. Positions for PBF (Antarctic Polar Front), SACCF (South Antarctic Circumpolar Current Front), and SBF (South Boundary Front) are based on data from Orsi et al. (1995)
The PCA run the first time on the 21 selected variables suggested that four factors had sufficient explanatory power (79.9%) to be retained, while during the second PCA run five factors explaining 82.9% variance were retained, and in the third and final run five factors explaining 82.0% of the cumulative variance were retained (Table 2). During the hierarchical clustering procedure, the percentage change in the agglomeration coefficient from one classification level to the next indicated that four cluster groups were present (Table 3). Cluster group 1 includes only 2 trawl stations, E. superba was absent in the catch, the concentrations of nitrite, nitrate, phosphate, and silicate were lower compared to all other cluster groups. Moreover, the water temperature was higher and the stations belonging to cluster group 1 where geographically further to the north compared to the other cluster groups (Fig. 5). Cluster group 2A consisted of 6 stations, and also here, E. superba was absent in the catch. In addition, this group differed by higher concentrations of silicate and higher water temperature and these stations were also located further north compared with the other members of cluster group 2. Ten of the investigated stations belonged to cluster group 2B1. E. superba was present at all stations belonging to this group. Group 2B1 was located further to the north and differed from cluster group 2B2 by its lower concentration of silicate and ~1°C warmer water temperature. In addition, cluster group 2B1 differed from 2B2 by lower proportions of juveniles, higher proportions of males at stage MIIIB, lower proportions of females in stage FIIA, and higher proportions of females in stage FIIIA. Cluster group 2B2 consisted of a total of 14 stations and E. superba was absent from 3 of these stations. The trawl stations in cluster group 2B2 were generally located further south compared to the trawl stations in the other cluster groups.
Distribution of the cluster groups 1, 2A, 2B1, and 2B2 identified from variables containing nutrients, oceanographic properties, and sex and sexual maturity stages of E. superba collected in the Southern Ocean during the austral summer of 2008. The CCAMLR Statistical Reporting Subareas are presented with their respective convention number. Positions for PBF (Antarctic Polar Front), SACCF (South Antarctic Circumpolar Current Front), and SBF (South Boundary Front) are based on data from Orsi et al. (1995)
Discussion
This paper focuses on horizontal demographic patterns of E. superba from the Southeast Atlantic sector of the Southern Ocean. It is estimated that between 50 and 70% of the global stock of krill is concentrated in the Atlantic sector, mainly around the Antarctic Peninsula, the Scotia Sea, and South-Georgia Island (Atkinson et al. 2008). Our survey covered parts of the CCAMLR statistical area 48.6, the largest ocean area in the Atlantic sector. In this area, only a restricted number of studies from which there exists quantitative information on the E. superba populations (Marr 1962; Fevolden 1979; Pakhomov 2004; Siegel 2006; Siegel et al. 2007) have previously been conducted. Hence the knowledge of the ecosystem of this region is at best fragmentary.
The northernmost distribution limit of E. superba during our survey was 52°S (Fig. 1), which is in accordance with the distribution found during the Discovery voyages as early as in the 1920s (Marr 1962; Mackintosh 1973). A north–south gradient emerged when plotting the geographic positions for the four cluster groups (Fig. 5). The two cluster groups lacking krill in the catch, cluster groups 1 and 2A, were found north of the two cluster groups where krill were caught (cluster groups 2B1 and 2B2). E. superba is stenothermic and will avoid the gradually warmer water when approaching the Antarctic Polar Front (APF) from south (Fig. 1). Cluster groups 1 and 2A were located north of, or in proximity to the APF, and the mean water temperatures in the upper 200 m were higher in these areas compared to areas of cluster groups 2B1 and 2B2 which were located south of the APF. The lower nutrient concentrations found in the region where E. superba was absent, at least for the area of cluster group 1, indicate conditions were a considerable amount of nutrients had been consumed by primary producers, although this is not reflected from the chlorophyll a levels at the time the area was visited. However, high water temperature is a likely reason for the absence of E. superba in these areas.
In the Southern Ocean, horizontal advection is the most important physical mechanism that controls population dynamics of its resident zooplankton (Huntley and Niiler 1995). Using ocean circulation models, simulation studies suggest that possible pathways of krill advection into the area 48.6 are mainly from the Antarctic Peninsula and the South Sandwich Islands via the eastward flow of the Antarctic Circumpolar Current (ACC) and from the Weddell Sea via the northern eastward flow of the Weddell Gyre (Thorpe et al. 2007). The ACC eastward flow is driven by strong westerly winds, extends from the sea surface to depths of 2,000–4,000 m, and the typical current speed is 10 cm−1 (Knauss 2005). It would theoretically take ~360 days to drift in a straight horizontal line from the tip of the Antarctic Peninsula to the longitude of Bouvetøya (>3,100 km) and ~490 days to the eastern boarder of area 48.6 (>4,200 km). Transport patterns on a finer scale are more complex (Deacon 1984). Local environmental factors within this large area will influence krill growth, feeding, maturation, and mortality and are therefore important in establishing the demographic characteristics, including size of organisms and population abundance. Thus, separating the effects of advective processes from population specific and environmentally driven processes is not straightforward. According to Huntley and Niiler (1995), the intensity of advection might destroy the demographic integrity of plankton populations in the Southern Ocean. This is particularly important with respect to the expected life span of organisms. As the life duration of E. superba can be more than 5 years with several year classes drifting at the same time, this effect is vital in understanding population development and in particular maturation.
In this study, the stations close to South Georgia displayed high proportions of both subadults and adults, but very few juveniles (Fig. 3). Normally, high concentrations of adult E. superba are found around South Georgia, as a result of immigration and not local recruitment (Tarling et al. 2007). The E. superba population from South Georgia that was sampled in our study is reasonably homogeneous with respect to its state of maturation. This is interesting, as it is not unusual to find large variations in size and maturity stages of E. superba between sampling stations (Mauchline and Fisher 1969; Witek et al. 1981; Fevolden and George 1984; Siegel 1986). Our between-station similarity regarding level of maturity might be ascribed to the animals being collected within a limited time span (6 days, 18–23 January 2008) and within a limited region on the north side of the island but could equally well represent the actual state of the South-Georgia krill population at this time. In addition to the general demography outlined elsewhere, we also noticed that no spent females (FIIIE) and very few females with swollen ovaries (FIIID) were recorded in the samples from this region.
Moving eastwards from South Georgia, we observe a change in the demographic characteristics of E. superba. Figure 3 displays that trawl stations dominated by adults were found close to, and in areas west and north of Bouvetøya. This is in accordance with Fevolden (1979), who found a “total predominance” of mature specimens off Bouvetøya. The situation at Bouvetøya seems quite different from what was observed at South Georgia in late January. However, the sampling west of Bouvetøya and north of the North Weddell Ridge was conducted somewhat later in the season (February/March) and might therefore reflect a more mature population, although it is not evident whether the population is in a pre- or a post-spawning state. The stations situated in this central region of the study area comprised the highest proportions of adults (Fig. 3), and this is also where the highest number of females in advanced maturity stages FIIID and FIIIE was identified. Retrospectively, it can be concluded that the South-Georgia population at the time of sampling was in a pre-spawning state. It is further noted that the stations belonging to cluster group 2B1 were mainly located in the area west of Bouvetøya and slightly north of the North Weddell Ridge between the location of the South Boundary Front (SBF) and the South Antarctic Circumpolar Current Front (SACCF). The same cluster group also holds the only station from South Georgia that was included in the cluster analysis.
Stations belonging to cluster group 2B2, showing high proportions of subadults and juveniles, were mainly found in areas southeast of Bouvetøya, in general south of the North Weddell Ridge and SBF.This pattern might reflect a north–south gradient with prevalence of younger krill stages further south. The maturation and length frequency data of E. superba in the Bouvetøya waters collected during the January–February season in 1977 (Fevolden 1979) are in accordance with our current 2008 observations. It is also interesting to note that Fevolden (1979, 1980) reported high percentages of “immature” E. superba with a total length <30 mm at the southernmost sampling stations although his stations were in general situated further west and south, from the South Orkney Islands to the eastern Weddell Sea waters.
We speculate that the overall demographic patterns displayed in Fig. 3, for stations situated mainly south of the North Weddell Ridge and roughly coinciding with the stations belonging to cluster group 2B2, could reflect a population in a post-spawning state, given that these stations were sampled early and mid March, and as such relatively late in the season for krill spawning activity (Marr 1962; Siegel 2005). The presence of adult females with both swollen ovaries and with spent ovaries (Table 3) in cluster group 2B2 might also support such an interpretation. Further, Siegel (2006) found a dominance of pre-spawning females (FIIIA), but also spermatophore bearing and even spent females in the Lazarev Sea during December 2005 in an area not very distant from our study region. The high proportion of juveniles and subadults at stations belonging to cluster group 2B2 is a conspicuous feature that leads us to believe that the area south of the North Weddell Ridge might be important regarding the recruitment of E. superba for area 48.6. This is supported by findings in the Lazarev Sea of a high proportion of juvenile krill along the southern part of 6°E meridian (Pakhomov 2004), while Siegel (2006) working somewhat further west, report a multimodal size distribution of E. superba showing a significant proportion of juveniles. Thus, local recruitment and production seem to be an important component of the ecosystem in the southern part of the CCAMLR 48.6 sector, although its contribution to the total krill production and recruitment cannot be quantified at this stage. Evidence from previous studies (Siegel 2006; Siegel et al. 2007) seems, however, to indicate that interannual variability in recruitment is large, possibly affecting whole year classes and hence regional population abundance.
The most common overall sex ratio found in E. superba populations is 1:1 (Marr 1962). However, large variation in sex composition between swarms and sampling stations are described in previous studies; Marr (1962) reported that 10% of the samples had a sex ratio more extreme than 1:3 and the same was the case in more than 25% of the samples in Watkins et al. (1992). The results from this study, with an overall sex ratio of 1:1 and approximately 7% of the stations displaying a sex ratio more than 1:3 (with the most extreme composition of 78% males vs. 22% females and 29% males vs. 71% females) (Fig. 4), are in accordance with previous studies investigating sex ratios from E. superba populations.
Dense krill swarm aggregations are often found to be associated with shelf areas and ice edges. This is where growth rates are high and also where the predation pressure from seabirds and marine mammals are substantial (Atkinson et al. 2008). However, 87% of the E. superba biomass is found in regions of less productive deep oceanic waters >2,000 m due to the comparatively much larger size of such areas. This distribution reflects a balance between advection, migration, top-down, and bottom-up processes (Atkinson et al. 2008). In the present study, E. superba was regularly found over deep ocean waters, along or south of the deep North Weddell Ridge stretching between South Sandwich Islands and Bouvetøya, and in the vicinity of Bouvetøya (Fig. 2). Except for the coastline of the Antarctic continent, Bouvetøya is the only landmass within area 48.6. Based on chlorophyll a measurements, Atkinson et al. (2008) have shown that the region surrounding Bouvetøya includes large areas with high-predicted growth potential for krill. There seems to be a general perception that krill are present in reasonably large numbers in this area (Marr 1962; Fevolden 1979, 1980; Pakhomov 2004; Atkinson et al. 2008). Our results, although sparse, suggest that high krill biomasses do occur over deep waters (see Fig. 2). The numerical densities and krill biomass recorded during our study range from 0 to 81.4 ind m−2 and from 0 to 63.09 g m−2, respectively. As such our results with regard to abundance are well within the range computed for the same region by Atkinson et al. (2008). The upper limits given above also compare in order of magnitude to values appearing from acoustic estimates in the Antarctic Peninsula region and the Indian Ocean but are also higher than many net derived estimates from a variety of regions around the Antarctic (Hewitt et al. 2004; Siegel 2005). Despite limited sampling on the shelf surrounding Bouvetøya as well as lack of sampling of shelf and ice edge areas close to the Antarctic continent in our study, the possibility that the central part of CCAMLR area 48.6 including Bouvetøya and the North Weddell Ridge waters can support high krill concentrations and high densities of swarms seems likely (c.f. Atkinson et al. 2008). Acoustic integrated krill biomass from the present survey is presented in Skaret et al. (2009) and shows a patchy distribution of krill biomass (on average 4.42 g m−2), with a tendency of the distributed biomass to be affiliated with regional bathymetric and frontal features. This is particularly evident from the acoustic recordings along the North Weddell Ridge around Bouvetøya and closer to the Astrid Ridge in the southern part of our study area. Likewise the considerably lower acoustic krill biomass recorded over an extended deep water region between the Astrid Ridge and the North Weddell Ridge is a particular feature from the current survey. Some of our highest net estimates are obtained from regions that coincide with the high acoustics estimates in Skaret et al. (2009), for instance when crossing the North Weddell Ridge southwest of Bouvetøya.
In conclusion, this study documents that the distribution of E. superba within CCAMLR ocean sector 48.6 was limited northwards to 52°S during our cruise. The populations comprised an equal mixture of male and female krill, and the observed demographic patterns suggest that the krill populations sampled during the present study are in a viable biological state. Defined bathymetric features including the Bouvetøya waters seem to be important for concentrating krill, sometimes with abundances comparable to other high abundance regions in the western Atlantic sector. The demography of krill in the visited south-eastern regions demonstrates a considerable proportion of juvenile krill that might suggest production of young krill in the southern areas within the CCAMLR ocean sector 48.6. Advective production cannot be ruled out, but its pathway and relative importance is difficult to determine at this stage.
References
Atkinson A, Siegel V, Pakhomov EA, Rothery P (2004) Long term decline in krill stock and increase in salps within the Southern Ocean. Nature 432:100–103
Atkinson A, Siegel V, Pakhomov EA, Rothery P, Loeb V, Ross RM, Quetin LB, Schmidt K, Fretwell P, Murphy EJ, Tarling GA, Fleming AH (2008) Oceanic circumpolar habitats of Antarctic krill. Mar Ecol Prog Ser 362:1–23
Bendschneider K, Robinson RI (1952) A new spectrophotometric method for the determination of nitrite in seawater. J Mar Res 2:87–96
Deacon G (1984) The Antarctic circumpolar ocean. Cambridge University Press, Cambridge
Engås A, Skeide R, West CW (1997) The MultiSampler: a system for remotely opening and closing multiple codends on a sampling trawl. Fish Res 29:295–298
Everson I (2000) Role of krill in marine food webs 7.3 the Southern Ocean. In: Everson I (ed) Krill: biology, ecology and fisheries. Blackwell, Oxford, pp 194–201
Faranda FM, Guglielmo L, Ianora A (2000) Ross sea ecology. Springer, Berlin
Fernø A, Olsen S (1994) Marine fish behavior in capture and abundance estimation. Fishing news books, London
Fevolden SE (1979) Investigations on krill (Euphausiacea) sampled during the Norwegian Antarctic research expedition 1976–77. Sarsia 64:189–198
Fevolden SE (1980) Krill off Bouvetøya and in the southern Weddell Sea with a description of larval stages of Euphausia crystallorophias. Sarsia 65:149–162
Fevolden SE, George RY (1984) Size frequency pattern of Euphausia superba in the Antarctic Peninsula waters in the austral summer of 1983. J Crust Biol 4:107–122
Fowler J, Cohen L, Jarvis P (1998) Practical statistics for field biology. Wiley, Chichester
Gille ST (2002) Warming of the Southern Ocean since the 1950s. Science 295:1275–1277
Grasshoff K (1965) On the automatic determination of phosphate, silicate and fluoride in sea water. Coun Met Int Coun Explor Sea 129:1–3
Hair JF, Anderson RE, Tatham RL, Black WC (1998) Multivariate data analysis: with readings, 5th edn. Prentice Hall, NJ
Hewitt RP, Watkins J, Naganobu M, Sushin V, Brierley AS, Demer D, Kasakina S, Takao Y, Goss C, Malyshko A, Brandon M, Kawaguchi S, Siegel V, Trathan P, Emery J, Everson I, Miller D (2004) Biomass of Antarctic krill in the Scotia Sea in January/February 2000 and its use in revising an estimate of precautionary yield. Deep Sea Res II 51:1215–1236
Hofmann EE, Wiebe PH, Costa DP, Torres JJ (2004) Integrated ecosystem studies of western Antarctic Peninsula continental shelf waters and related Southern Ocean Regions. Deep Sea Res II 51:1921–2344
Huntley ME, Niiler PP (1995) Physical control of population dynamics in the Southern Ocean. ICES J Mar Sci 52:457–468
Iversen SA, Melle W, Bagøien E, Chu D, Edvardsen B, Ellertsen B, Grønningsæter E, Jørstad K, Karslbakk E, Klevjer T, Knutsen T, Korneliussen R, Kowall, H, Krafft BA, Kaartvedt S, Lona PB, Murray S, Naustvoll L, Nøttestad L, Ostrowski M, Siegel V, Skagseth Ø, Skaret G, Søiland H, Zhao X, Årnes CB (2008) The Antarctic krill and ecosystem studies with R/V “G.O. Sars” in 2008. WG-EMM CCAMLR 2008 21 pp
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Plantzen 167:191–194
Knauss JA (2005) Introduction to physical oceanography. Waveland Pr Inc, IL
Levitus S, Antonov JI, Boyer TP, Stephens C (2000) Warming of the world oceans. Science 287:2225–2229
Loeb V, Siegel V, Holm-Hansen O, Hewitt R, Fraser W, Trivelpiece W, Trivelpiece S (1997) Effects of sea-ice extent and krill or salp dominance on the Antarctic food web. Nature 387:897–900
Macaulay MC, English TS, Mathisen OE (1984) Acoustic characterization of swarms of Antarctic krill (Euphausia superba) from Elephant Island and Bransfield Strait. J Crust Biol 4:16–44
Mackintosh NA (1973) Distribution of post-larval krill in the Antarctic. Discov Rep 36:95–156
Makarov RR, Denys CJI (1981) Stages of sexual maturity of Euphausia superba. BIOMASS Handbook No. 11, pp 1–13
Marr J (1962) The natural history and geography of the Antarctic krill (Euphausia superba Dana). In: Discovery reports vol 32. National Institute of Oceanography, Cambridge University Press, Cambridge pp 33–464
Mauchline J, Fisher LR (1969) The biology of euphausiids. Adv Mar Biol 18:1–677
Nicol S (2000) “BROKE”: a multidisciplinary survey of the waters off east Antarctica (80–150°E). Deep Sea Res II 47:2281–2613
Norman GR, Streiner DL (2008) Biostatistics: the bare essentials, 3rd edn. People’s Medical Publishing House, Beijing
Orsi AH, Whitworth T III, Nowlin WD (1995) On the meridional extent and fronts of the Antarctic circumpolar current. Deep Sea Res I 42:641–673
Pakhomov EA (2004) Salp/krill interactions in the Eastern Atlantic sector of the Southern Ocean. Deep Sea Res II 51:2645–2660
Pakhomov EA, Froneman PW, Perissinotto R (2002) Salp/krill interactions in the Southern Ocean: spatial segregation and implications for the carbon flux. Deep Sea Res II 49:1881–1907
Ross RM, Quetin LB, Lascara CM (1996) Distribution of Antarctic krill and zooplankton west of the Antarctic Peninsula. In: Ross RM, Hofmann EE, Quetin LB (eds) Foundations for ecological research west of the Antarctic Peninsula. AGU, Washington, pp 199–217
Siegel V (1986) Structure and composition of the Antarctic krill stock in the Bransfield strait (Antarctic Peninsula) during the second international BIOMASS experiment (SIBEX). Arch Fish Wiss 37:51–72
Siegel V (2005) Distribution and population dynamics of Euphausia superba: summary of recent findings. Polar Biol 29:1–22
Siegel V (2006) Demograohy of Antarctic krill in the Lazarev Sea (Subarea 48.6) in the 2005/06 season. WG-EMM CCAMLR 2006, 14 pp
Siegel V, de la Mare WK, Loeb V (1997) Long-term monitoring of krill recruitment and abundance indicies in the Elephant Island area (Antarctic Peninsula). CCAMLR Sci 4:19–35
Siegel V, Haraldsson M, Vortkamp M, Würzberg L, Schöling S (2007) Demography of Antarctic krill and other Euphausiacea in the Lazarev Sea in winter 2006. WG-EMM CCAMLR 2007, 13 pp
Skaret G, Krafft BA, Korneliussen R (2009) Krill density estimates in CCAMLR subarea 48.6 based on acoustic data collected during January-March 2008. WG-EMM CCAMLR 2009, 11 pp
Tarling GA, Cuzin-Roudy J, Thorpe SE, Shreeve RS, Ward P, Murphy EJ (2007) Recruitment of Antarctic krill Euphausia superba in the South Georgia region: adult fecundity and the fate of larvae. Mar Ecol Prog Ser 331:161–179
Thorpe SE, Murphy EJ, Watkins JL (2007) Circumpolar connections between Antarctic krill (Euphausia Superba Dana) populations: investigating the roles of ocean and sea ice transport. Deep Sea Res I 54:792–810
Tou JC, Jaczynski J, Chen YC (2007) Krill for human consumption: nutritional value and potential health benefits. Nut Rev 65:63–77
van Tongeren OFR (1995) Cluster analysis. In: Jongmann RHG, ter Braak CJF, van Tongeren OFR (eds) Data analysis in community and landscape ecology. Cambridge University Press, Cambridge, pp 174–203
Walsh JJ, Dieterle DA, Lenes J (2001) A numerical analysis of carbon dynamics of the Southern Ocean phytoplankton community: the roles of light and grazing in effecting both sequestration of atmospheric CO2 and food availability to larval krill. Deep Sea Res I 48:1–48
Watkins JL, Buchholz F, Priddle J, Morris DJ, Ricketts C (1992) Variation in reproductive status of Antarctic krill swarms; evidence for a size-related sorting mechanism? Mar Ecol Prog Ser 82:163–174
Watkins JL, Hewitt R, Naganobu M, Sushin V (2004) The CCAMLR 2000 survey: a multinational, multi-ship biological oceanography survey of the Atlantic sector of the Southern Ocean. Deep Sea Res II 51:1205–1456
Welshmeyer NA (1994) Fluorometric analysis of chlorophyll-α in the presence of chlorophyll b and phaeopigments. Limnol Oceanogr 39:1985–1992
Wenneck TL, Falkenhaug T, Bergstad OA (2008) Strategies, methods and technologies adopted on the R.V.G.O.Sars MAR-ECO expedition to the mid-Atlantic Ridge in 2004. Deep Sea Res Part II 1–2:6–28
Whitehouse MJ, Meredith MP, Rothery P, Atkinson A, Ward P, Korb RE (2008) Rapid warming of the ocean around South Georgia, Southern Ocean, during the 20th century: forcing, characteristics and implications for lower trophic levels. Deep Sea Res I 55:1218–1228
Wiebe PH, Chu D, Kaartvedt S, Hundt A, Melle W, Ona E, Batta-Lona P (2009) The acoustic properties of Salpa thompsoni. ICES JMS 67. doi:10.1093/icesjms/fsp263
Witek Z, Kalinowski J, Grelowski A, Wolnomiejski N (1981) Studies of aggregations of krill (Euphausia superba). Meeresforsch 28:228–243
Zar JH (1996) Biostatistical analysis. Prentice-Hall, Englewood Cliffs
Acknowledgments
AKES (Antarctic Krill and Ecosystem Studies) was supported by the Royal Norwegian Ministry of Fisheries and Coastal affairs, the Institute of Marine Research, the University of Bergen, the Norwegian Antarctic Research Expeditions (NARE), the Norwegian Research Council, StatoilHydro, and the Norwegian Petroleum Directorate. Dr V. Siegel was supported by the German LAKRIS Project (BMBF MGS 03F046A). We thank Dr P. Wiebe, Department of Biology, Wood Hole Oceanographic Institution, USA, for help and advice during the survey and Dr. G. A. Tarling and the British Antarctic Survey for valuable help with species determination both prior to and after the cruise. We would also like to extend our gratitude to the crew of the RV “G. O. Sars”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krafft, B.A., Melle, W., Knutsen, T. et al. Distribution and demography of Antarctic krill in the Southeast Atlantic sector of the Southern Ocean during the austral summer 2008. Polar Biol 33, 957–968 (2010). https://doi.org/10.1007/s00300-010-0774-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0774-3