Abstract
We report on the first record of interstitial cnidarians in sea ice. Ice core samples were collected during eight field periods between February 2003 and June 2006 in the coastal fast ice off Barrow, Alaska (71°N, 156°W) at four locations. A total of 194 solitary, small (0.2–1.1 mm) elongated specimens of a previously unknown interstitial hydroid taxon were found. By cnidome composition and the occurrence of a highly retractable pedal disc formed by epidermal tissue only, the specimens are tentatively assigned to representatives of the family Protohydridae, subclass Anthomedusae. The hydroids were found almost exclusively in the bottom 10 cm-layer (at the ice–water interface) of 118 ice cores, with abundances ranging from 0 to 27 individuals per core section (0–4,244 ind m−2) and a grand mean of 269 ind m−2 in bottom 10 cm-layer sections. Abundances were lower in December and late May than in months in between with considerable site variability. A factor analysis using 12 variables showed that hydroid abundance correlated highest with abundances of copepod nauplii and polychaete juveniles suggesting a trophic relationship.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The 3D brine pocket and channel system in sea ice (Weissenberger et al. 1992) provides a habitat for a multitude of unicellular and multicellular organisms (Carey 1992; Gradinger 2002). These interconnected channels and pockets have previously been called an upside-down-benthos (Mohr and Tibbs 1963 in Carey 1985), because of their similarity to the 3D structure of the interstitial spaces within sandy sediments. Vertical environmental gradients can be dramatic within both the sea ice and the sediment interstitial systems, although they are partly of a different nature. Within sea ice, practical salinity increases from near sea water salinity at the water–ice interface to over 200 in winter sea ice near the ice–snow interface as ice temperatures can drop from near freezing at the sea ice–water interface to less than −20°C in winter sea ice near the ice–snow interface (Junge et al. 2003; Gradinger and Bluhm 2005). Inhabitable brine volume increases with increasing temperature and sea ice bulk salinities and can reach values above 20% of the overall ice volume in the bottom 10 cm of the sea ice (Eicken 2003). Within the sediment, biochemical gradients driven by slow pore water exchange can be very steep, e.g., for pH, oxygen and/or hydrogen sulfide (Vanaverbeke et al. 1997), and interstitial space is mainly a function of vertical and horizontal grain size distribution (Gayraud and Philippe 2003). Both systems have strong vertical gradients in organic matter and algal pigment concentrations with generally higher values at the water–substrate interface than in the ice/sediment interior (Gradinger 1999a; Schewe 2001).
The physical habitat similarities between the brine channel system and the sediment interstitial are likely responsible for a range of meiofaunal taxa that are common in the two systems, but uncommon in the water column in between. This is true for nearshore fast ice and coastal sediments (Feder and Paul 1980; Carey 1992) as well as offshore pack ice and shelf or deep-sea sediments (Schewe 2001; Gradinger et al. 2005). Taxa common in both systems in the Arctic include nematodes, harpacticoid copepods, and turbellarians (Pfannkuche and Thiel 1987; Schewe 2001; Gradinger et al. 2005; Schuenemann and Werner 2005). This study reports on another taxon to be added to the list of shared fauna: hydroids (Hydrozoa, Cnidaria), previously unrecorded from Arctic and Antarctic sea ice to the best of our knowledge.
Less than 50 meiobenthic cnidarian species have been described from around the world’s benthic sediments with most of them characterized by a reduction of diagnostic features and a small set of taxonomic characters for species identification (Giere 1993). Most of these cnidarians belong to the Hydrozoa, but Scyphozoa, Anthozoa and Cubozoa are also represented (Thiel 1988). Early studies recorded meiobenthic cnidaria predominantly in shallow waters (2–60 m; summarized in Bozhenova et al. 1989) and relatively coarse sediments (Thiel 1988), but recent records also documented the distribution in the deep sea (Sommer and Pfannkuche 2000; Schewe 2001) which tends to be dominated by finer sediments (Lampitt et al. 1986). Interstitial cnidarians are now known to be widely distributed with distribution records from, e.g., the Baltic and North Seas (Remane 1927; Salvini-Plawen 1987), the south Atlantic (Bouillon and Grohmann 1990) and the North Pacific (Feder and Paul 1980; Norenburg and Morse 1983). Published records of hydroids from Arctic sediments include the White Sea (Bozhenova et al. 1989), the central Arctic Ocean (Schewe 2001) and the northern Barents Sea shelf (Pfannkuche and Thiel 1987). The few studies investigating the role of meiobenthic hydroids in sediments suggest they are predators of other meiofaunal organisms such as copepods and nematodes and are, hence, high up in the food web (Schultz 1950a, b; Heip 1971).
This article describes the first record of cnidarians, namely solitary hydrozoans, in sea ice, observed in Arctic coastal fast ice off Barrow, Alaska. The observation is discussed with regard to comparisons between the sea ice brine channel and sediment interstitial habitats.
Methods
Study area
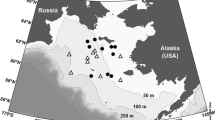
Samples were collected during eight field trips to Barrow, Alaska: 12–17 February, 1–5 April and 27–30 May 2003; 7–12 December 2005; 30 January–4 February, 13–18 March, 21–26 April and 27 May–1 June 2006. During each of the 2003 trips, two study sites were sampled (Fig. 1): Site “BASC” was located in the Chukchi Sea just off the Barrow Arctic Science Consortium facilities (71°19′N, 156°41′W) and site “Beaufort” was located just past Barrow Point in the Beaufort Sea (71°22′N, 156°24′W). During the December 2005–June 2006 field periods, three study sites were sampled during each trip (Fig. 1): site “BASC” (sea above); site “Hanger”, which was situated a few miles further northeast in the Chukchi Sea (71°20′N, 156°39′W); site “Elson” was located at 71°21′N, 156°28′W in shallow Elson Lagoon. Water depth at these sites ranged from 2.0 to 6.3 m. All sites were <0.5 miles offshore. The sampling periods covered almost the complete seasonal ice cycle from the time of early sea ice formation (December), intense ice growth (January–March), onset of the ice algal bloom (March), maximum ice algal biomass (April/May) to onset of ice melt (late May, June).
Field sampling
For faunal counts, at least three replicate ice core bottom sections (0–10 cm from ice–water interface) per site and sampling period were collected with a Kovacs-type ice auger (9 cm diameter). Additionally in 2005 and 2006, one complete core per site and sampling period was collected. A total of 118 cores were studied for hydroid abundance of which 15 were studied over the entire ice thickness. Densities are provided as the number of specimens per individual melted segment (10 cm segment thick and 9 cm in diameter) and, for comparability with other studies, were also calculated per 10 cm2 and 1 m2 surface area for a 10 cm layer. Additional parameters were determined as part of a broader study and, in the context of this work, were used to identify potential factors related to hydroid abundance. For algal pigment and particulate organic nitrogen (PON) and particulate organic carbon (POC) concentration measurements, a second set of cores with at least three replicates for the bottom 10 cm sections was collected at the same sites during the same periods using the same sampling scheme. Ice temperature of one ice core per site was measured in 10 cm intervals over the entire ice thickness with a Traceable thermometer immediately after coring. Bulk salinity was measured on melted core sections with a YSI 85 sensor and is reported as Practical Salinity.
Sample processing
For faunal studies, segments were melted in the dark with the addition of 1 l of 0.2 μm-filtered seawater per 10 cm segment to avoid osmotic stress for the biota (Garrison and Buck 1986). Melted samples were concentrated over 20 μm gauze and hydroids and other meiofauna were counted alive with Wild MZ3 and Leica MZ12 dissecting scopes at 10–100× magnification. Hydroids and other meiofauna were subsequently fixed with 1% buffered formaldehyde–seawater solution (final concentration) and hydroids were shipped to the lab at the University of Salento (Lecce, Italy) for morphological study using dissecting and compound microscopes. Hydroid size measurements (n = 40) were conducted with Image-J software from digital images taken with a Canon Rebel camera attached to a Zeiss inverted compound microscope and 10–40× objective lenses.
Chlorophyll and POC/PON cores were melted in the dark and sub-samples were filtered onto GF/F filters which were stored at −18°C (Arar and Collins 1992) and shipped to the lab in Fairbanks. For Chl a determination, filters were extracted with 7 ml of 90% (v:v) acetone for 24 h in the dark at −18°C (Karl et al. 1990). Chl a concentration was determined with a Turner TD-700 fluorometer (Arar and Collins 1992). POC and PON concentrations were measured with a Costech elemental analyzer connected to a Thermo Finnigan Delta Plus isotope radio mass-spectrometer at the Alaska Stable Isotope Facility.
Data analysis
To identify potential correlations of hydroid abundance with physical and biological variables, we conducted a factor analysis using ©Systat software (version 11) with Equimax rotation mode in which the number of variables that load highly on a factor and the number of factors needed to explain a variable are minimized. Variables used in the factor analysis included: Chl a, POC and PON concentrations and C/N ratio of ice organic matter; temperature and bulk salinity of the sea ice; and abundances of six ice meiofauna taxa (hydroids, turbellarians, nematodes, nauplii, polychaete larvae/juveniles and copepods). All values used were from 0 to 10 cm bottom sections only, where the overwhelming majority of hydroids occurred.
Results
A total of 194 hydroid specimens were found in 33.9% of the investigated bottom sections (and in 20.8% of all investigated sections). Around 97.9% of the specimens occurred in the bottom 10 cm of the cores. Abundances in individual cores ranged from 0 to 27 individuals (ind) per 10 cm core section (0–4,244 ind m−2). Mean abundances per site and sampling period ranged from 0 to 2,012 (SD = 1,553) ind m−2 with a grand mean of 269 (SD = 630) ind m−2 (in bottoms sections). Overall, abundance was lowest early and late in the season (December and late May) and highest in January/February–April with maximum values occurring at the BASC site in both 2003 and 2006. Variability between replicate cores, sites and sampling periods was large (Fig. 2).
Abundance of sea ice hydroids in Arctic coastal fast ice off Barrow, Alaska during eight sampling periods (2003 and 2005/2006) at a total of four sites. N = 3–8 ice cores per sampling period and site. Means and standard deviations (error bars) are given per m2 for a 10 cm thick ice–water interface layer
The hydroid collected is a solitary, naked polyp, with three to four oral tentacles. It is almost spherical when contracted and tubular when relaxed, nearly doubling the body length by means of a highly extensible aboral side, forming a tubular foot-like structure made by epidermal tissue only (Fig. 3). Body shrinkage is accomplished by the inward folding of a hollow column of epidermal cells from the aboral end of the polyp towards the oral side. Body length ranged from 207 to 1,141 μm with a mean of 411 μm (SD 160 μm); body width ranged from 69 to 433 μm with a mean of 215 (SD 79 μm); tentacle length ranged from 54 to 252 μm with a mean of 121 μm (SD 46 μm). Part of the variability was explained by the variable degree of contraction. The tentacles, the foot-like structure, and the cnidocyst types (stenoteles, macrobasic mastigophores, desmonemes) and distribution label this taxon as a new hydroid species which we preliminarily assign here to the class Hydroidomedusa, subclass Anthomedusae, order Capitata, family Protohydridae. Detailed morphological and taxonomic description will appear elsewhere (Piraino et al. in preperation).
The factor analysis identified three factors with eigenvalues >1. After rotation, factors 1–3 explained 34.5, 23.6, and 15.5%, respectively (sum 73.6%), of the total variance. Based on the factor loading matrix (Table 1), factor 1 represented correlations >0.5 between Chl a, POC, and PON concentrations, sea ice temperature and nematode abundances; abundances of hydroids, nauplii, and polychaete larvae/juveniles loaded highest on factor 2; factor 3 had an inverse relationship between C/N ratios and turbellarian and copepod densities as well as bulk salinity. The range of values at which hydroids occurred did not greatly differ from the total range measured for any variable (Table 1).
Discussion
To our knowledge, this study reports on the first finding of hydroids in sea ice, specifically in Arctic coastal fast ice off Barrow, Alaska. Despite a considerable volume of publications on Arctic sea ice meiofauna (for reviews see, e.g., Carey 1985; Melnikov 1997; Gradinger 1999b) records on cnidarians in sea ice have not previously been published. The underlying reasons are likely several. First, a large fraction of sea ice meiofauna studies is based on the analysis of preserved and/or directly melted samples (for details see, e.g., Gradinger 1999b), in which several meiofaunal taxa are known to preserve poorly, e.g., ciliates (Karayanni 2004) and cnidarians (Thiel 1988). Hence, cnidarians, which may have been collected previously from sea ice, may have remained unrecognized. Secondly, the cnidarians observed in this study were small (on average 400 μm) relative to the more common turbellarians and nematodes etc., and furthermore were very slow moving, not very numerous relative to other more common taxa, nearly transparent and inconspicuous in color. Lastly, most sea ice studies which focused on meiofauna (as well as other meiobenthos studies) only mention common higher taxonomic groups and cluster rarer taxa into an “other” category (e.g., Nozais et al. 2001; Gradinger et al. 2005) rather than listing individual rare phyla or orders. Hence, even if cnidarians were found, they were not reported as such.
Published abundances of meiobenthic hydroid densities are also scarce (Table 2), but overall low densities are suggested, with some variability between and within locations. Sea ice hydroid densities in this study were similar to densities found in sediments of the deep Arabian Sea, on the Antarctic shelf, in the Barents Sea, in the Håkon Mosby Mud Volcano, and in the shallow brackish Mediterranean Sea, while they were more than an order of magnitude lower than densities found in intertidal sediments in the Gulf of Alaska (Table 2). A second group of meiobenthic studies mention the occurrence of cnidarians or hydroids, but report their abundance clustered together within the “other” group, suggesting low hydroid abundances relative to the other meiobenthic taxa such as nematodes and copepods (Pfannkuche and Thiel 1987; Schewe 2001). The relative contribution of hydroids to total benthic meiofauna abundance (0.01–0.25% for studies referenced in Table 2) overlaps with reported values of hydroid abundance relative to total sea ice meiofauna abundance (0.01–>5%), with an order of magnitude higher contributions in individual ice core sections that had low abundances of other fauna.
We suggest that the overall low abundances of hydroids in sea ice and benthic sediments were likely related to the typically predatory feeding mode of hydroids. Prey items of meiobenthic hydroids included a wide range of other meiobenthic fauna such as nematodes, copepods, ostracods, gastrotrichs, oligochaetes (Schulz 1950a) as well as copepod nauplii (Schultz 1950b). A common interstitial hydroid of the North European coastal benthic community, Protohydra leuckarti, for example is known to heavily prey on harpacticoid copepods and nematodes (Heip 1971; Heip and Smol 1976). The ratio between hydroid abundances and the other meiofauna taxa suggests that the ice hydroid may exert a comparably heavy predatory impact within its sympagic community as Protohydra leuckarti. Hydroid stomach contents were not identified in this study, but the prey spectrum available included turbellarians, harpacticoid and cyclopid copepods, nematodes, polychaete larvae, and copepod nauplii besides a few rarer taxa (Gradinger and Bluhm 2005 and unpublished data). Hydroid densities were most closely correlated with densities of polychaete larvae/juveniles and copepod nauplii. This relationship suggests a possible trophic connection, although stomach content analysis is required for confirmation. Hydroid abundance was not strongly correlated with chlorophyll or POC/PON concentrations (Table 1) suggesting that hydroids are more independent of the ice algal bloom than other sea ice meiofauna taxa (Grainger and Hsiao 1990).
Increasing hydroid densities from December to January and February could be related to re-suspension of benthic sediment and its subsequent incorporation into the growing ice sheet, a likely mechanism for transporting benthic meiofauna into the growing ice sheet. We interpret the decreasing abundance in May and June as a result of the onset of ice melt, which coincides with a substantial release of organic matter from the ice into the water column and/or sea floor (Michel et al. 2002; Gradinger and Bluhm 2005).
The living space for ice cnidarians and other ice biota typically ranges in diameter from 0 to 1.2 mm (Krembs et al. 2000; Light et al. 2003). At the ice–water interface, in situ ice temperatures in this study measured −1.3 to −5.4°C. At these temperatures, about 10–15% of the brine channels in columnar and granular ice are >200 μm in diameter (Krembs et al. 2000) and are, therefore, available to most hydroids, although their flexible bodies may be able to penetrate smaller-diameter channels. The extensible foot-like structure may allow the hydroid to move within the brine channel as well as within sediments by acting like a bivalve foot. How basal epidermal cells are stretched out or condensed in the hydroid column merits further investigation. Like several other ice meiofauna taxa, hydroids were largely restricted to the bottom layer, likely because (1) it harbors the highest potential prey density (Horner 1985; Gradinger et al. 2005), (2) environmental stress is lowest in terms of temperature and salinity (Table 1), and/or (3) brine channels are wider than deeper inside the ice.
In conclusion, hydroid cnidarians appear to be a rare, but possibly regularly occurring component of the Arctic fast ice meiofauna community, adding a previously ignored diversity component and potential top predator to the sea ice-based food web. Similarities in the sea ice and benthic sediment habitats with regard to the 3D interstitial and the faunal community composition likely resulted in overall similar densities, trophic relationships and size ranges of meiobenthic and sea ice hydroids.
References
Arar EJ, Collins GB (1992) In vitro determination of chlorophyll a and phaeophytin a in marine and freshwater by fluorescence. EPA Method 445.0
Arlt G (1973) On the importance of biological production of the meiofauna in coastal waters. Wissensch Zeitschrift Univ Rostock 22:1141–1145 (in German)
Bouillon J, Grohmann PA (1990) Pinushydra chiquitita gen. et sp. nov. (Cnidaria, Hydrozoa, Athecata), a solitary marine mesopsammic polyp. Cah Biol Mar 31:291–305
Bozhenova OV, Stepanjants SD, Sheremetevsky AM (1989) The first finding of the meiobenthic cnidaria Boreohydra simplex (Hydrozoa, Athecata) in the White Sea. Zool Zh 68:11–16 (in Russian)
Carey AG (1985) Marine ice fauna: Arctic. In: Horner R (ed) Sea ice biota. CRC, Boca Raton, pp 173–190
Carey AG Jr (1992) The ice fauna in the shallow southwestern Beaufort Sea, Arctic Ocean. J Mar Syst 3:225–236
Eicken H (2003) From the microscopic, to the macroscopic, to the regional scale: growth, microstructure and properties of sea ice. In: Thomas DN, Dieckmann GS (eds) Sea ice: an introduction to its physics, biology, chemistry, and geology. Blackwell, Oxford, pp 22–81
Feder HM, Paul AJ (1980) Seasonal trends in meiofaunal abundance on two beaches in Port Valdez, Alaska. Syesis 13:27–36
van Gaever S, Moodley L, de Beer D, Vanreusel A (2006) Meiobenthos at the Arctic Håkon Mosby Mud Vulcano, with a parental-caring nematode thriving in sulphide-rich sediments. Mar Ecol Prog Ser 321:143–155
Garrison DL, Buck KR (1986) Organism losses during ice melting: a serious bias in sea ice community studies. Polar Biol 6:237–239
Gayraud S, Philippe M (2003) Influence of bed-sediment features on the interstitial habitat available for macroinvertebrates in 15 French streams. Internat Rev Hydrobiol 88:77–93
Giere O (1993) Meiobenthology: the microscopic fauna in aquatic sediments. Springer, Berlin
Gradinger R (1999a) Vertical fine structure of algal biomass and composition in Arctic pack ice. Mar Biol 133:745–754
Gradinger R (1999b) Integrated abundances and biomass of sympagic meiofauna from Arctic and Antarctic pack ice. Polar Biol 22:169–177
Gradinger R (2002) Sea ice microorganisms. In: Bitten G (ed) Encyclopedia of environmental microbiology. Wiley, New York, pp 2833–2844
Gradinger R, Bluhm BA (2005) Susceptibility of sea ice biota to disturbances in the shallow Beaufort Sea. Phase 1: Biological coupling of sea ice with the pelagic and benthic realms. Final report OCS study MMS 2005–062
Gradinger R, Meiners K, Plumley G, Zhang Q, Bluhm BA (2005) Abundance and composition of the sea ice meiofauna in off-shore pack ice of the Beaufort Gyre in summer 2002 and 2003. Polar Biol 28:171–181
Grainger EH, Hsiao SIC (1990) Trophic relationships of the sea ice meiofauna in Frobisher Bay, Arctic Canada. Polar Biol 10:283–292
Heip C (1971) The succession of benthic micrometazoans in a brackish water habitat. Biologisch Jaarboek 39:191–196
Heip C, Smol N (1976) On the importance of Protohydra leuckerti as a predator of meiobenthic populations. In: Persoone G, Jaspers E (eds) Proceedings of the 10th european marine biology symposium, 2: population dynamics of marine organisms in relation with nutrient cycling in shallow waters. University Press, Wetteren, Belgium, pp 285–296
Horner R (1985) Sea ice biota. CRC, Boca Raton
Junge K, Eicken H, Deming JW (2003) Bacterial activity at −2 to −20°C in Arctic wintertime sea ice. Appl Env Microbiol 70:550–557
Karayanni H (2004) Evaluation of double formalin-Lugol’s fixation in assessing number and biomass of ciliates: an example of estimations at mesoscale in NE Atlantic. J Microbiol Methods 56:349–358
Karl DM, Winn CD, Hebel DVW, Letelier R (1990) JGOFS Hawaii Ocean Time-series program field and laboratory protocols. http://www.hahana.soest.hawaii.edu/hot/protocols/protocols.html, Hawaii
Krembs C, Gradinger R, Spindler M (2000) Implications of brine channel geometry and surface area for the interaction of sympagic organisms in Arctic sea ice. J Exp Mar Biol Ecol 243:55–80
Lampitt RS, Billett DSM, Rice AL (1986) Biomass of the invertebrate megabenthos from 500–4100 m in the northeast Atlantic Ocean. Mar Biol 93:69–81
Lee HJ, Gerdes D, Vanhove S, Vincx M (2001) Meiofauna response to iceberg disturbance on the Antarctic continental shelf at Kapp Norvegia (Weddell Sea). Polar Biol 24:926–933
Light B, Maykut GA, Grenfell TC (2003) Effects of temperature on the microstructure of first-year Arctic sea ice. J Geophys Res 108. doi:10.1029/2001JC000887
Melnikov I (1997) The Arctic sea ice ecosystem. Gordon and Breach Science, Australia
Michel C, Nielsen TG, Nozais C, Gosselin M (2002) Significance of sedimentation and grazing by ice micro- and meiofauna for carbon cycling in annual sea ice (northern Baffin Bay). Aquat Microb Ecol 30:57–68
Norenburg JL, Morse MP (1983) Systematic implications of Euphysa ruthae n. sp. (Athecata: Corymorphidae), a psammophilic solitary hydroid with unusual morphogensis. Trans Am Microsc Soc 102:1–17
Nozais C, Gosselin M, Michel C, Tita G (2001) Abundance, biomass, composition and grazing impact of the sea-ice meiofauna in the North Water, northern Baffin Bay. Mar Ecol Prog Ser 217:235–250
Pati AC, Belmonte G, Ceccherelli VU, Boero F (1999) The inactive temporary component: an unexplored fraction of meiobenthos. Mar Biol 134:410–427
Pfannkuche O, Thiel H (1987) Meiobenthic stocks and benthic activity on the NE-Svalbard shelf and in the Nansen Basin. Polar Biol 7:253–266
Remane A (1927) Halammohydra, ein eigenartiges Hydrozoon der Nord- und Ostsee. Z Morph Oekol Tiere 7:82–92 (in German)
Salvini-Plawen Lv (1987) Mesopsammic cnidaria from plymouth (with systematic notes). J Mar Biol Assoc UK 67:623–637
Schewe I (2001) Small-sized benthic organisms of the Alpha Ridge, central Arctic Ocean. Internat Rev Hydrobiol 86:317–335
Schuenemann H, Werner I (2005) Seasonal variations in distribution patterns of sympagic meiofauna in Arctic pack ice. Mar Biol 146:1091–1102
Schultz E (1950a) Psammohydra nanna, ein neues solitäres Hyrozoon der westlichen Beltsee. Kieler Meeresforsch 7:122–127 (in German)
Schultz E (1950b) Zur Ökologie von Protohydra leuckarti Greef. Kieler Meeresforsch 7:128–136 (in German)
Sommer S, Pfannkuche O (2000) Metazoan meiofauna of the deep Arabian Sea: standing stocks, size spectra and regional variability in relation to monsoon induced enhanced sedimentation regimes of particulate organic matter. Deep-Sea Res II 47:2957–2977
Thiel H (1988) Chapter 19: Cnidaria. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington DC, pp 266–272
Vanaverbeke J, Soetaert K, Heip C, Vanreusel A (1997) The metazoan meiobenthos along the continental slope of the Globan Spur (NE Atlantic). J Sea Res 38:93–107
Weissenberger J, Dieckmann G, Gradinger R, Spindler M (1992) Sea ice: a cast technique to examine and analyze brine pockets and channel structure. Limnol Oceanogr 37:179–183
Acknowledgments
The results reported here are part of studies supported by the National Science Foundation (OPP 0520566) and the Coastal Marine Institute (Task Order 85242). SP’s work on hydroid biodiversity is supported by the Italian Ministry for University and Research, the MarBEF network and the Province Administration of Lecce. We thank the director and logistics staff of the Barrow Arctic Science Consortium for their continuous logistical support during field operations. We are grateful to S. Story-Manes and M. Nielson-Kaufman for their support while in Barrow. Three reviewers are thanked for improving a previous manuscript draft. This article contributes to the Arctic Ocean Diversity Census of Marine Life project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bluhm, B.A., Gradinger, R. & Piraino, S. First record of sympagic hydroids (Hydrozoa, Cnidaria) in Arctic coastal fast ice. Polar Biol 30, 1557–1563 (2007). https://doi.org/10.1007/s00300-007-0316-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0316-9