Abstract
We investigated the renal morphology, histology and ultrastructure of Harpagifer bispinis, as a first step toward understanding the morpho-functional basis of its adaptation to potentially freezing brackish seawater. Fish were separated into two groups of ten individuals each, and acclimated to 2‰ and 38‰ salinity. A study of complete serial sections of the kidney revealed that the nephrons were aglomerular. At the highly convoluted proximal segment two different regions were evident, a feature that has not been previously reported for other aglomerular species. In electron photomicrographs we distinguished light and dark cells in the proximal tubule epithelium, with highly infolded basolateral membranes and closely associated mitochondria. The dark cells also had a large number of mitochondria in the apical region. The intercellular spaces at the epithelium of the proximal tubule were larger in fish acclimated at 2‰ salinity, a modification that might facilitate urine secretion, thus contributing to the survival of an aglomerular fish in a hyposmotic medium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The kidneys of many marine teleost fishes have reduced or absent renal corpuscles (Elger et al. 2000) since extensive glomerular filtration is not essential in an osmotically stable seawater environment. The lack of glomeruli (aglomerularism) has an adaptive value for fishes that inhabit waters with sub-zero temperatures: the small antifreeze glycopeptides (AFGPs) present in the blood of these species cannot be lost by ultrafiltration. In this type of nephron urine is formed by tubular secretion rather than by ultrafiltration (Hickman and Trump 1969; Schmidt Nielsen 1976; Eastman 1993).
Notothenioid species are distributed in the Antarctic, sub-Antarctic and temperate waters of the Southern Hemisphere (Fischer and Hureau 1985). There is a clear tendency toward glomerular reduction in species inhabiting higher latitudes: the kidneys of Antarctic notothenioids possess aglomerular nephrons (Dobbs et al. 1974; Dobbs and DeVries 1975a, 1975b; Eastman and DeVries 1986a; Eastman 1993), whereas most of the few non-Antarctic species of this group studied to date for possess glomeruli (Eastman 1993; Pérez et al. 2001).
Harpagifer bispinis is a small notothenioid species that inhabits coastal zones of Tierra del Fuego Island and the Magellan region in southern South America. During low tide this fish can be found in the medium level of the intertidal zone, in tide pools. In this habitat they are often exposed to reduced salinity caused by the flow of small freshwater streams (locally called “chorrillos”) especially during spring and summer, when salinity in these pools ranges from 0 to 38‰ (Calvo, unpublished data). In addition, these tide pools are usually frozen during winter (Calvo, unpublished data). Under these conditions H. bispinis faces possible osmotic water gain and salt loss, and also the risk of freezing.
Although there are no studies on the morphology of the kidney of H. bispinis, it is worth noting that the closely related Antarctic species Harpagifer antarcticus possesses aglomerular nephrons (Eastman 1993). If it shares this characteristic, H. bispinis may have morphological or physiological adaptation that enable it to deal with the osmotic problems in hyposmotic media. For example, the aglomerular teleost Opsanus tau which is able to survive for up to 3 weeks in 5% seawater (ca. 2‰ salinity) in the laboratory. Some of the mechanisms described for O. tau are regulation of urine production and reabsorption of Cl- through the bladder (Lahlou et al. 1969). However, this species is not able to produce a dilute urine, and thus excretes large quantities of Na+ following transfer to dilute media (Baustein et al. 1997).
The aim of this investigation was to study the renal morphology, histology and ultrastructure of H. bispinis acclimated to different salinities, as a first step toward understanding the morpho-functional basis of its adaptation to potentially freezing dilute seawater.
Materials and methods
Animals
Harpagifer bispinis were hand-caught in the intertidal fringe of Ensenada Bay, Beagle Channel (54°35′S, between 66°30′E and 70°W) during the summer of 2000 and 2001. At the laboratory, the fish were randomly separated into two groups of ten individuals each, and progressively acclimated in aquaria with aerated seawater reduced by 5‰ salinity per day until reaching 2‰ and 38‰ salinity respectively. Each group was maintained in its final salinity level for at least 20 days. Water temperature (7.5°C) and pH (7.6) were maintained at similar values to those found in the Beagle Channel (Fernández 2000).
Light microscopy
Fishes were anaesthetized with benzocaine. After cutting the spinal cord, we opened the fish body cavity from anus to isthmus, moved aside the viscera and excised the dorsally located kidneys after removing the overlying peritoneum. Kidneys were immersed in Bouin’s fixative for 2 h, dehydrated and embedded in Paraplast. The whole organ was serially sectioned at 5–7 μm thickness, and sections were stained using the following techniques: Masson’s trichrome, periodic acid-Schiff (PAS) and Alcian Blue (pH 0.5, 2.5 and 3.5). Photomicrographs were taken with a digital camera attached to a Zeiss Axiostar microscope.
Transmission electron microscopy
Small tissue blocks from kidneys of H. bispinis acclimated to the different salinities were fixed in 2.5% glutaraldehyde in phosphate buffer (pH 7.75). Samples were then post-fixed in ice-cold 1% osmic tetroxide in the same buffer solution for 1 h. The tissue samples were then rinsed in buffer, dehydrated in a graded ethanol series and embedded in Spurr resin. Thin sections (750–900 Å) were cut with a diamond knife in a Sorvall Porter-Blum MT2-B ultramicrotome, double-stained with 1% aqueous uranyl acetate for 45 min at room temperature and lead citrate for 3 min at room temperature, and examined with a Zeiss EM T109 electron microscope operated at 80 kV.
Results
Survival at low and high salinity
Animals from both groups tolerated the acclimation period well. Mortality was low and similar in both groups. All animals were sacrificed between 20 and 35 days after transfer to the final salinity. These animals did not show any sign of stress or illness.
Gross renal morphology
The external shape of the H. bispinis kidney corresponds to type III in Ogawa’s (1961) classification of gross structure of marine teleostean kidneys. The kidneys of H. bispinis respond to the general description made by Dobbs and DeVries (1975b) for other notothenioid species.
Light microscopy
Study of complete serial sections of H. bispinis kidneys revealed that the nephrons have no renal corpuscles (Fig. 1). The nephron consisted of a blind end and a proximal tubule, several of which drained into a common collecting tubule. Many of these tubules drained into the same collecting duct, which led to the opisthonephric duct (see Dobbs and DeVries 1975b for a general description). No difference between acclimation salinities was evident at this level of resolution.
Harpagifer bispinis. Detail of the blind end of the nephron. Notice the difference in shape between the blind end (round) and the loops of the proximal tubule (oval) sited to the right and left. L Lumen, PC peritubular capillary, (*) blind end; scale bar 15 μm
H. bispinis. Detail of the proximal tubule, near the blind end. The diameter of the lumen and the high brush border at the IPT distinguish it from the rest of the proximal tubule. L Lumen, ( ►) brush border, IPT initial segment of the proximal tubule, PT proximal tubule; scale bar 15 μm
H. bispinis. Detail of the proximal tubule. DC Dark cell, LC light cell, L lumen, PC peritubular capillary, scale bar 15 μm
The proximal tubule (also called “principal segment” or “proximal tube II” by Dobbs and DeVries 1975b) was highly convoluted and was the longest segment of the nephron. Near the blind end, the tubule had a smaller diameter than in the rest of the segment. In this zone, cells were small and the luminal brush border was higher than in the rest of the segment (Fig. 2). As far as we know, this zone has not yet been described in nephrons of aglomerular fish.
The main section of the proximal tubule was made up of cuboidal to low columnar epithelial cells, with strongly acidophilic cytoplasm. Nuclei were apical, and darkly stained. There were two types of epithelial cells in this region. The difference was based on the intensity of the cytoplasmic staining: light and dark cells were clearly evident (Fig. 3). Among the renal tubules there were large numbers of small capillaries and some connective tissue, but no lymphoid or hemopoietic tissues. Between the proximal tubule and the collecting tubule there was a small transition zone, made up of cuboidal cells with acidophilic cytoplasm. The collecting tubule, collecting duct and opisthonephric ducts were very similar to those described by Dobbs and DeVries (1975b) for other notothenioids.
Histochemical localization of acid and neutral glycoconjugates
An apical reaction to both PAS and Alcian Blue was detected along the renal tubule, which may have been due to the presence of a glycocalix associated with microvilli in the proximal tubule and with acid and neutral mucus in the rest of the renal tubule. At the opisthonephric duct, the apical region was also stained intensely. The basal membrane and the connective tissue surrounding the renal tubules were stained intensely with PAS, whereas the cytoplasm of the tubular epithelium was only faintly stained. The intensity of the staining with Alcian Blue at all pH levels was higher in the animals acclimated to low salinity, both in the cytoplasm and apical region of the cells. The Alcian Blue and PAS techniques did not enable identification of the different segments of the nephron.
Electron microscopy
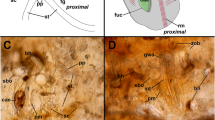
We studied the ultrastructural characteristics of the epithelium forming the proximal tubule. The apical membrane showed a poorly developed brush border with several clusters of microvilli, together with at least one cilium per cell. Beneath this membrane there was a large vesicular apparatus and Golgi complexes were observed in this zone. Tight junctions and desmosomes were present all along the proximal tubule (Figs. 4, 5).
H. bispinis . Proximal tubule. L Lumen, Mv microvilli, C cilia, (→) tight junction, (►) desmosome, N nucleus; scale bar 4 μm
H. bispinis . Detail of the apical zone of the proximal tubule epithelium. Lm Lumen with mucus, DC dark cell, with numerous supranuclear mitochondria, LC light cell, with sparse supranuclear mitochondria, (→) tight junction, (►) desmosome, M mitochondria, N nucleus; scale bar 4 μm
H. bispinis . Detail of the basal zone. Notice the close association between the basolateral membranes and the mitochondria. BL Basal lamina, MI membrane interdigitations, M mitochondria, N nucleus; scale bar 4 μm
H. bispinis . Intercellular spaces at the proximal tubule epithelium of the nephron of H. bispinis acclimated to (a) low and (b) high salinity. Note the difference in size between the intercellular spaces under different acclimations. L Lumen, BL basal lamina, N nucleus, IS intercellular space, MI membrane interdigitations; scale bar 4 μm
Light and dark cells were clearly identified in preparations from both acclimation groups. In addition to the intensity in the cytoplasmic staining, these cells differed in the aspect and distribution of mitochondria. Dark cells showed large numbers of mitochondria both in the apical and in the basal regions. Most of these mitochondria were oval, with numerous transverse cristae, and were often tightly clustered. These cells also comprised many long mitochondria that were at times complexly ramified. However, light cells possessed almost no mitochondria in the apical region, where the vesicular apparatus prevailed instead. The mitochondria of light cells comprised tubular cristae and also appeared oval, but shorter and wider than those in dark cells. In both light and dark cells the basolateral membranes were highly infolded in the form of a complex tubular system, which was closely associated with mitochondria (Figs. 5, 6).
The principal difference between fish held at different acclimation salinities was evident in the intercellular spaces of the proximal tubule epithelium. These spaces were much larger in fish acclimated to low salinity than in those acclimated to high salinity (Fig. 7a, b). There was no difference with respect to mitochondrial characteristics between the proximal tubule light and dark cells from both salinity acclimation groups.
Discussion
Notothenioids are widespread in Antarctic and sub-Antarctic seas, and in the Antarctic, the temperature is often lower than 0°C (DeVries 1982). As far as we know the kidneys of Antarctic notothenioids are aglomerular, while the sub-Antarctic members of this group are glomerular (Dobbs and DeVries 1975b; Eastman 1993; Pérez et al. 2001). According to Hickman and Trump (1969), for marine fishes, the reduction or loss of renal corpuscles may be a mechanism associated with energy conservation, because since the urine is formed by secretion rather than ultrafiltration, there is no requirement for reabsorption of useful solutes. In the particular case of Antarctic species the lack of glomeruli might be critical for conserving small blood glycopeptides with antifreeze properties, essential for survival in these environments (Dobbs et al. 1974; Eastman and DeVries 1986a, 1986b; Eastman 1993). H. bispinis is a notothenioid fish that is often hidden under rocks in the intertidal zone of sub-Antarctic coasts during low tide (Calvo unpublished data).
In this work we have studied the renal morphology of H. bispinis by means of light and electron microscopy. Histological details described in the Results section, are discussed here from a functional morphology point of view.
The most noteworthy feature of the H. bispinis nephrons is the absence of renal corpuscles. A short segment with a small lumen and a high brush border originates from the blind end of the nephron. The most recent review on fish renal morphology, which describes the structure of an aglomerular nephron (Elger et al. 2000), does not mention this kind of structure. Our study provides the first description of this initial segment, whose function is still unknown. This segment transitions into a “proximal tubule II” or “principal segment”, typical of aglomerular teleosts (Hickman and Trump 1969; Dobbs and DeVries 1975b; Elger et al. 2000).
Since H. bispinis is exposed to sub-zero temperatures in frozen tide pools during the winter, aglomerularism may be adaptive in terms of AFGPs conservation. Although, the presence of these compounds in the blood of this species has not been yet demonstrated, the occurrence of AFGPs is a common characteristic of all notothenioids, with the exception of the Bovichtidae (Clarke and Johnston 1996).
In aglomerular fishes urine is formed by secretion of cations through the proximal tubular epithelium (Bulger 1965; Bulger and Trump 1968; Olsen and Ericsson 1968; Trump and Bulger 1971; Wendelaar Bonga 1973; Elger et al. 2000). As our results show, the kidney of H. bispinis has several structural arrangements that seem to be associated with urine formation, most of which are located within the proximal tubule. There is a close relation between nephrons and peritubular capillaries in the trunk kidney, a circulatory pattern that might optimize solute secretion, and thus water elimination.
The proximal tubule in the kidneys of H. bispinis is highly convoluted and is the longest segment of the nephron. In the epithelium of this segment the basolateral interdigitations are so well developed that the nucleus is displaced to the apical zone. In both kinds of proximal tubule cells, light and dark cells, great numbers of mitochondria are packed between the interdigitating membranes even though they are differently organized. These mitochondria are possibly related to the presence of Na+/K+-ATPase, which is located at the basolateral membrane of a variety of secretory and absorptive epithelia (see Ernst and Mills 1977 for references). In this respect, abundant Na+/K+-ATPase and Na+/K+/Cl- co-transport proteins have been reported for this segment in two nototheniod species indicating a great secretory activity (Massini et al. 2001). Mitochondrial density in the H. bispinis proximal tubule is higher than in the same segment of other notothenioids such as Trematomus bernacchii and Gymnodraco acuticeps (Dobbs and DeVries 1975b) and also compared with glomerular fishes (Elger et al. 2000), suggesting an elevated ion transport capacity related to urine formation activity. A distinctive feature of the dark cells to the proximal tubule is that they also have numerous mitochondria in the apical zone, which might provide energy for an apical enzyme involved in ion transport, such as the V-H+ATPase (Perry and Fryer 1997).
Aglomerular fishes are typically found in stable marine habitats (Hickman and Trump 1969); in this medium both glomerular and aglomerular fishes compensate for osmotic water loss by drinking seawater and excreting the salt excess through the gills. As H. bispinis is often found in low-salinity environments, it must also be able to cope with water influx. In this medium the absence of glomeruli becomes an important challenge: they cannot produce hyposmotic urine by glomerular ultrafiltration tubular reabsorption as do glomerular freshwater fishes (Schmidt-Nielsen 1976). It has been previously reported that an aglomerular notothenioid, Notothenia neglecta, regulates serum ion levels in diluted medium (17‰)(Romão et al. 2001). These authors have concluded that the observed reduction of Na+/K+-ATPase activity may reflect either a reduction in NaCl and water reabsorption in the renal tubules or a decrease in ionic secretory processes. It has also been shown that the osmoregulatory capacity of notothenioid fishes can be augmented by modulation of Na+/K+-ATPase activity in osmoregulatory tissues including kidneys (Gonzalez-Cabrera et al. 1995). H. bispinis can probably tolerate some dilution of the blood when exposed to a hyposmotic medium for short periods like the aglomerular O. tau (Lahlou et al. 1969). Morphological adaptations such as possessing an integument impermeable to water and solutes, plus increased resistance of tight junctions at the gill and gut epithelia, could contribute to lowering the water intake. However, after long-term exposure to low salinity excessive water intake would be unavoidable. The kidneys of H. bispinis may possess adaptations to eliminate this water excess, since all the individuals acclimated to 2‰ salinity were as active as those acclimated to seawater after more than one month. In this respect, we have observed that the intercellular space between adjacent cells of the proximal tubule is wider in those animals acclimated to low salinity, a morphological modification that could facilitate water flux intercellularly. The great development of basolateral interdigitations and abundance of mitochondria support the hypothesis of ion reabsorption from this fluid. However, more detailed studies on the structure and nature of these spaces as well as on ion transport proteins are necessary in order to propose a mechanism explaining how an aglomerular fish eliminates the excess water during exposure to a hyposmotic medium.
References
Baustein MD, Wang SQ, Beyenbach KW (1997) Adaptive responses of aglomerular toadfish to dilute seawater. J Comp Physiol B 167:61–69
Bulger RE (1965) The fine structure of the aglomerular nephron of the toadfish, Opsanus tau. Am J Anat 117:171–192
Bulger RE, Trump BF (1968) Renal morphology of the English sole (Parophrys vetulus). Am J Anat 123:195–226
Clarke A, Johnston IA (1996) Evolution and adaptative radiation of Antarctic fishes. Trends Evol Ecol 11:212–218
DeVries AL (1982) Biological antifreeze agents in coldwater fishes. Comp Biochem Physiol A73:627–266
Dobbs GH, DeVries AL (1975a) Renal function in Antarctic teleost fish: serum and urine composition. Mar Biol 29:59–70
Dobbs GH, DeVries AL (1975b) The aglomerular nephron of Antarctic teleost: a light and electron microscopic study. Tissue Cell 7:159-170
Dobbs GH, Lin Y, DeVries AL (1974) Aglomerularism in Antarctic fish. Science 185:793–794
Eastman JT (1993) Antarctic fish biology. Evolution in a unique environment. Academic, San Diego
Eastman JT, DeVries A (1986a) Renal glomerular evolution in Antartic notothenioid fishes. J Fish Biol 29:649–662
Eastman JT, DeVries A (1986b) Antarctic fish. Sci Am 254:106–114
Elger M, Hentschel H, Dawson M, Renfro JL (2000) Microscopic functional anatomy: urinary tract. In: Ostrander G (ed) The laboratory fish. Academic, New York, pp 385–413
Ernst SA, Mills JW (1977) Basolateral plasma membrane localization of ouabain-sensitive sodium transport sites in the secretory epithelium of the avian salt gland. J Cell Biol 75(1):74–94
Fernández D (2000) Histoquímica, distribución y crecimiento de las fibras musculares en nototénidos subantárticos. Análisis inicial de dos factores relacionados: flotabilidad y temperatura. Universidad de Buenos Aires. Graduate thesis
Fischer W, Hureau JC (1985) FAO species identification sheets for fishery purposes. Southern Ocean (Fishing areas 48,58 and 88) (CCAMLR Convention Area). Prepared and published with the support of the Commission for the Conservation of Antarctic Marine Living Resources. FAO, Rome, vol 2, pp 233–470
Gonzalez-Cabrera PJ, Dowd F, Pedibhotla VK, Rosario R, Stanley-Samuelson D, Petzel D (1995) Enhanced hypo-osmoregulation induced by warm-acclimation in Antarctic fish is mediated by increased gill and kidney Na+/K+-ATPase activities. J Exp Biol 198:2279–2291
Hickman CP, Trump BF (1969) The kidney. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 1. Academic, New York, pp 91–239
Lahlou B, Henderson W, Sawyer WH (1969) Renal adaptations by Opsanus tau, a euryhaline aglomerular teleost, to dilute media. Am J Physiol 216:1266–1272
Massini MA, Sturla M, Prato P, Uva B (2001) Ion transport systems in the kidney and urinary bladder of two Antarctic teleosts, Chionodraco hamatus and Trematomus bernacchii. Polar Biol 24:440–446
Ogawa M (1961) Comparative study of the external shape of the teleostean kidney with relation to phylogeny. Sci Rep Tokyo Kyoiku Daigaku Sect B10:61–69
Olsen S, Ericsson JLE (1968) Ultrastructure of the tubule of the aglomerular teleost Nerophis ophidion. Z Zellforsch 87:17–30
Pérez A, Luquet C, Calvo J (2001) Morfología renal de los nototenoideos subantárticos Harpagifer bispinis y Patagonotothen tessellata. Aglomerulismo en un pez eurihalino. Congreso Brasileño de Ictiología. San Leopoldo, Rio Grande Do Sul, Brazil
Perry SF, Fryer JN (1997) Proton pumps in the fish gill and kidney. Fish Physiol Biochem 17:363–369
Romão S, Freire CA, Fanta E (2001) Ionic regulation and Na+, K+-ATPase activity in gills and kidney of the Antarctic aglomerular cod icefish exposed to dilute sea water. J Fish Biol 59:463–468
Schmidt-Nielsen K (1976) Regulación del agua y regulación osmótica. In: Fisiología animal. Adaptación y medio ambiente. Ediciones Omega, Barcelona, pp 263–312
Trump BF, Bulger RE (1971) Experimental modification of lateral and basilar plasma membranes and extracellular compartments in the flounder nephron. Fed Proc Fed Am Soc Exp Biol 30:22–41
Wendelaar Bonga SE (1973) Morphometrical analysis with the light and electron microscope of the kidney of the anadromous three-spined stickleback Gasterosteus aculeatus, form trachurus, from fresh water and from sea water. Z Zellforsch Mikrosk Anat 137:563–588
Acknowledgements
We wish to thank Martin Ansaldo, Elba Morriconi, Daniel Aureliano, Fabian Vanella, Griselda Genovese and Julia Halperin for their kind help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez, A.F., Calvo, J., Tresguerres, M. et al. Aglomerularism in Harpagifer bispinis: a subantarctic notothenioid fish living at reduced salinity. Polar Biol 26, 800–805 (2003). https://doi.org/10.1007/s00300-003-0551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-003-0551-7