Abstract
Key message
ZmMYC2 was identified as the key regulator of JA signaling in maize and exhibited diverse functions through binding to many gene promoters as well as enhanced JA signaling in transgenic Arabidopsis.
The plant hormone jasmonate (JA) extensively coordinates plant growth, development and defensive responses. MYC2 is the master regulator of JA signaling and has been widely studied in many plant species. However, little is known about this transcription factor in maize. Here, we identified one maize transcription factor with amino acid identity of 47% to the well-studied Arabidopsis AtMYC2, named as ZmMYC2. Gene expression analysis demonstrated inducible expression patterns of ZmMYC2 in response to multiple plant hormone treatments, as well as biotic and abiotic stresses. The yeast two-hybrid assay indicated physical interaction among ZmMYC2 and JA signal repressors ZmJAZ14, ZmJAZ17, AtJAZ1 and AtJAZ9. ZmMYC2 overexpression in Arabidopsis myc2myc3myc4 restored the sensitivity to JA treatment, resulting in shorter root growth and inducible anthocyanin accumulation. Furthermore, overexpression of ZmMYC2 in Arabidopsis elevated resistance to Botrytis cinerea. Further ChIP-Seq analysis revealed diverse regulatory roles of ZmMYC2 in maize, especially in the signaling crosstalk between JA and auxin. Hence, we identified ZmMYC2 and characterized its roles in regulating JA-mediated growth, development and defense responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants exquisitely balance growth, development and defensive capacities in response to the constantly changing environment by orchestrating multiple biological processes to reach the optimal growing state (Browse 2009; Wasternack and Hause 2013). Plant hormones are the most critical factors regulating plant growth and environmental adaptation. Among these hormones, jasmonate (JA) is employed to mediate both biotic and abiotic stress responses to enhance plant resistance against various adverse environments, such as pathogen infection, herbivore attack, wounding and drought (Campos et al. 2014; Verma et al. 2016; Hu et al. 2017). In addition to its role in mediating defense response, JA is also necessary for plant developmental processes, including lateral root formation, leaf senescence, anthocyanin accumulation, seed germination, and stamen maturation (Yuan and Zhang 2015; Huang et al. 2017).
In past decades, the molecular mechanisms of JA signaling and cross-talk with other signaling pathways have been investigated comprehensively (Santner et al. 2009). The core component of JA signaling pathway is the Skp-Cullin-F-box-type E3 ubiquitin ligase complex SCFCOI1, which consisted of an F-box protein coronatine insensitive 1 (COI1), Arabidopsis SKP1-like 1 (ASK1), together with a CULLIN1 (CUL1) and a RING BOX protein 1 (RBX1) (Xie et al. 1998; Devoto et al. 2002; Xu et al. 2002). The perception of JA signal is dependent on JA receptor COI1. After perception of jasmonoyl-l-isoleucine (JA-Ile), an active form of jasmonate, the SCFCOI1 complex subsequently directly binds to the JASMONATE ZIM-domain (JAZ) proteins, which function as transcriptional repressors, to facilitate protein degradation of JAZ proteins by the ubiquitin–proteasome system (Thines et al. 2007). JAZ functions together with the corepressor TOPLESS (TPL) and the NOVEL INTERACTOR OF JAZ (NINJA) adaptor protein in the absence of JA-Ile by binding directly to a series of transcription factors (TFs), thereby suppressing their transactivation to downstream JA-responsive genes (Pauwels et al. 2010). One of the best documented TFs is MYC2, which is known to be a key regulator of a subset of JA-responsive genes. The perception of JA leads to derepression of MYC2 from JAZ proteins, subsequently activating JA-responsive gene expression (Dombrecht et al. 2007; Kazan and Manners 2013).
MYC2 belongs to the basic helix–loop–helix (bHLH) TF family containing a conserved bHLH domain at the C terminus (Kazan and Manners 2013). This domain is responsible for forming homodimer with itself or heterodimer with other proteins, such as AtMYC3 and AtMYC4, which are closely related proteins of AtMYC2, playing not only partial redundant but also specific roles in JA signaling (Fernandez-Calvo et al. 2011; Schweizer et al. 2013). The basic region of MYC2 consisted of 15–20 basic amino acids, which is responsible for binding to G-boxes in target gene promoters (Toledo-Ortiz et al. 2003; Amoutzias et al. 2008; Carretero-Paulet et al. 2010). Decades of studies have uncovered diverse functions of MYC2 as the master regulator acting in many JA-mediated processes. The most comprehensive investigation was conducted in Arabidopsis and characterized AtMYC2, as well as three homolog genes (AtMYC3-5) (Fernandez-Calvo et al. 2011; Schweizer et al. 2013). In tomato, SlMYC2 regulated JA-mediated plant immunity (Du et al. 2017). In rice, OsMYC2 was identified as an essential factor for JA-inductive sakuranetin production (Ogawa et al. 2017). In tobacco, two homologue genes of AtMYC2, the NtMYC2a and NtMYC2b have been demonstrated to mediate JA-induced nicotine biosynthesis by forming nuclear complexes with the NtJAZ1 (Shoji and Hashimoto 2011). The JA-activated MdMYC2 has been identified to promote ethylene biosynthesis during apple fruit ripening (Li et al. 2017a).
Hormone crosstalk has been reported to extensively regulate stress and developmental processes. As the master regulator of JA signaling, MYC2 also functions in integrating the signaling pathways between JA and other plant hormones, such as abscisic acid (ABA), salicylic acid (SA), gibberellins (GAs), ethylene and auxins (Dombrecht et al. 2007; Kazan and Manners 2013). In Arabidopsis, MYC2 suppressed the expression of pathogen defense-related gene PDF1.2 through negative regulation of ERF1 and ORA59 in both JA and ABA signaling (Anderson et al. 2004; Dombrecht et al. 2007). JA and SA signaling are generally considered to operate antagonistically. In tga2 tga5 tga6 myc2 quadruple mutant, the negative regulation of SA on JA-induced PDF1.2 was abolished, suggesting involvement of MYC2 in JA and SA signaling crosstalk (Zander et al. 2010). The DELLA proteins are known as repressors of GA signaling, which interact with JAZ proteins, the repressor of JA signaling, therefore, release the inhibition of MYC2 by JAZ to activate JA response (Hou et al. 2010). JA signal also interacts with GA to regulate sesquiterpene biosynthesis in an MYC2-dependent manner (Hong et al. 2012). Ethylene has been proved to function synergistically with JA signaling during necrotrophic pathogen infection and this process is also partly dependent on MYC2 (Glazebrook 2005).
Auxins are considered to promote plant growth, while JA is most related to plant defense. Some complicated connections have been uncovered between these two hormones to fine-tune growth, development and defense responses. For example, two auxin response factors, ARF6 and ARF8, promote JA production and regulate JA-mediated flower maturation (Nagpal et al. 2005). Some auxin biosynthetic genes, such as ANTHRANILATE SYNTHASE a1 (ASA1), YUCCA8 and YUCCA9, can be induced by JA signaling (Sun et al. 2009; Hentrich et al. 2013). A recent study demonstrated that JA and auxins coordinately activate ERF115 to promote tissue regeneration (Zhou et al. 2019). These findings suggest the synergy effect between auxins and JA. However, auxins and JA also were reported to act antagonistically. Some ARFs up-regulate the expression of JAZ proteins, thereby compromise JA-responsive gene expression (Grunewald et al. 2009). Moreover, MYC2 has been found to negatively regulate auxin-induced adventitious root formation and root growth (Gutierrez et al. 2012). Hence, the signaling crosstalk between auxins and JA still remain unclear.
One maize putative bHLH TF was proposed to play roles in response to insect elicitors (Engelberth et al. 2012). However, whether this transcription factor is the functional ortholog of Arabidopsis MYC2 and its function still need to be elucidated. In this study, we identified this bHLH transcription factor as ZmMYC2 and characterized its function preliminarily through Arabidopsis transformation. Additionally, in vitro ChIP-seq assay confirmed the roles of ZmMYC2 in modulation of JA signaling as well as the crosstalk between JA and other hormone signaling in maize. Notably, the regulator of auxin signaling, including AUX/IAAs and ARFs, is indicated as directed target of ZmMYC2, suggesting a role for ZmMYC2 in fine-tuning plant growth and development.
Materials and methods
Plants, fungi and treatments
Maize inbred line B73 was grown in soil at 28 °C with 16 h light/8 h dark period. The seedlings at the three-leaf stage were treated with different plant hormones separately. These treatments included 100 μM methyl jasmonate (MeJA), combined application of 100 μM MeJA with 50 μM ethephon (EP), 100 μM GA3, or 100 μM SA. Meanwhile, the spores of Fusarium graminearum with concentration of 1 × 106 mL−1 were also used to infect the leaves as described previously (Fu et al. 2018). Leaves scratched lightly with fresh blade were used as wound treatment. The sterile water was applied as the control treatment. The hydroponic cultured seedling growing in 40% Hoagland solution were used for treatment with 100 μM ABA, 250 mM NaCl or 20% PEG6000, respectively. Aerial parts or roots were collected at 1, 3, 6, 12, and 24 h after treatments for further analysis.
The seeds of Arabidopsis thaliana (Col-0) were germinated and grown in nutrient soil under the condition of 22 °C for 14 h light and 20 °C for 10 h dark period. For the root growth assays and hormone treatment, the seeds were sterilized and sown on 1/2 Murashige and Skoog medium with or without 25 μM MeJA. The root growth and anthocyanin accumulation were observed and measured 7 days after germination.
Botrytis cinerea was grown on potato dextrose agar medium at 25 °C in dark for 5 days. For plant infection, 4-week-old Arabidopsis leaves were cut and placed into sterile water with 1 mg/L 6-BA to keep alive. Mycelium pellets (2 mm × 2 mm) of B. cinerea was inoculated on Arabidopsis leaves, which were kept in Arabidopsis growth chamber. Pathogen infection was monitored with photographing and leaf infection percentage was calculated with the Image J software.
The hypha of F. graminearum was cultured on potato dextrose agar medium at 28 °C in dark for 7 days to produce spores.
Phylogenetic analysis and sequence alignment
The sequences of maize whole bHLH family transcription factor were downloaded from MaizeGDB (http://archive.maizegdb.org). The sequences of other MYC2 genes were obtained from NCBI (http://www.ncbi.nlm.nih.gov) or Phytozome (http://phytozome.jgi.doe.gov/pz/portal.html), including AtMYC2 (AT1G32640), AtMYC3 (At5g46760), AtMYC4 (At4g17880) and AtMYC5 (At5g46830) from A. thaliana; AlMYC2 (XM_002893686) from Arabidopsis lyrata; CrMYC2 (AF283507.2) from Catharanthus roseus; NaMYC2 (KC832837.1) and NaMYCl (KC906192.1) from Nicotiana attenuata, NtMYC1b (GQ859159.1), NtMYC2a (GQ859160.1) and NtMYC2c (GQ859158.1) from Nicotiana tabacum; SlMYC2 (KF428776.1) from Solanum lycopersicum; TcJAMYC1 (FJ608574.1), TcJAMYC2 (JX519289) and TcJAMYC4 (JX519290) Taxus cuspidata, OsMYC2 (AK288082) from Oryza sativa; MdMYC2 (MDP0000136498) from Malus domestica; StJAMYC (CAF74710) from Solanum tuberosum and MtMYC2 (XM_003628772.1) from Medicago truncatula. The CLC Sequence Viewer 7.0 (CLC bio) software was used for phylogenetic analysis by the neighbor-joining method. Amino acid sequence of ZmMYC2 was aligned with AtMYC2, AtMYC3 and AtMYC4 using Clustal W and DNAMAN software.
RNA isolation, gene cloning and expression analysis
Maize total RNA was isolated using TRNzol reagents (Tiangen, Beijing) according to the manufacturer’s instructions. cDNA was synthesized using the M-MLV reverse transcriptase from Takara. The coding sequence (CDS) of ZmMYC2 (GRMZM2G001930) was cloned from maize leaves and ligated into pMD19-T (Takara) for sequencing. The expression patterns of ZmMYC2 were determined by quantitative real-time PCR (qRT-PCR), which was performed on the Bio-Rad CFX96 using the SsoFast EvaGreen Supermix (Bio-Rad). The maize elongation factor Ef1a was used as the endogenous control according to previous studies (Fu et al. 2016). The primer sequences are listed in Table S1. Maize and Arabidopsis leaves were used for cloning of JAZ genes, including ZmJAZ3 (GRMZM2G117513), ZmJAZ8 (GRMZM2G086920), ZmJAZ14 (GRMZM2G064775), ZmJAZ17 (GRMZM2G126507), and ZmJAZ23 (GRMZM2G143402).
Yeast two-hybrid analysis
The full-length CDS of ZmMYC2 was cloned into pGBKT-7 and used for self-transactivation assay by co-transforming with empty pGADT-7 into yeast strain AH109. Positive clones were screened on the selective medium SD/Trp-Leu-His-Ade with X-α-Gal (4 mg mL−1). For the MYC2-JAZ interaction assays, the truncated ZmMYC2 (1–139 aa) containing the JID domain was ligated into pGADT-7 and full-length JAZs were constructed into pGBKT-7. ZmMYC2 and each JAZ were co-transformed into yeast strain AH109. The positive clones were selected as mentioned above. All the primers used for Y2H constructs are listed in Tables S2 and S3.
Generation of transgenic plants
To further analyze the biological functions of ZmMYC2, we generated transgenic Arabidopsis overexpressing ZmMYC2 by the floral dipping method under the control of 35S promoter (Zhang et al. 2006). The CDS of ZmMYC2 was subcloned into pCAMBIA3301 and further transformed into Agrobacterium tumefaciens strain GV3101. Additionally, the complementation lines of ZmMYC2 in myc2myc3myc4 (myc234) triple mutant was also generated (Fernandez-Calvo et al. 2011). The seeds of myc234 were obtained from Prof. Roberto Solano at Universidad Autónoma de Madrid and Prof. Benke Kuai at Fudan University. Transgenic plants were selected by 0.15% Basta and identified by PCR amplification with Arabidopsis gDNA or cDNA as the templates. At least two independent transgenic lines for each transformation were selected to grow until T3 generation for further analysis. The specific electrophoretic bands were further sequenced to validate successful transformation. Primers used to generate transgenic plants are listed in Table S4.
In vitro ChIP-Seq assay and gene expression analysis
In vitro ChIP was performed with the method as described previously (Li et al. 2017b; Fu et al. 2018). Two-week-old maize seedlings were used for gDNA extraction. The gDNA were sheared to fragments mainly enriched in 200–500 bp by ultrasonication. The recombinant pET28-ZmMYC2 protein was purified on Ni-NTA Agarose beads and desalted by ultrafiltration. ZmMYC2 protein and DNA fragments were co-incubated for 4 h (4 °C, 7 rpm) with the incubation buffer (100 mM KCl, 50 mM Tris, 1 mM EDTA, 5% glycerol, 0.1% Triton X-100, 1 mM DTT, pH 7.0). After co-incubation, the beads were washed three times using the incubation buffer. The cross-linking product was then eluted from the beads with the elution buffer (1% SDS, 0.1 M NaHCO3) at room temperature and 0.2 M NaCl was added to break down cross-linked protein and DNA fragments at 65 °C for 2 h. The target DNA fragments were extracted with chloroform–isoamylol (24:1, v/v). Sequencing was performed with the Illumina HiSeq 2500 (Novogene, Beijing). The ChIP-Seq raw data are listed in Supplementary dataset 1–3.
ZmMYC2 was subcloned into pBI221 under the control of the maize ubiquitin promoter for transient overexpression in maize protoplasts as described previously (Fu et al. 2018). qRT-PCR was performed to analyze the expression of ZmMYC2-targeted genes in maize protoplasts with ZmMYC2 transient overexpression. All Primers were verified for specificity by amplicon sequencing and are listed in Supplementary Table 5.
Analysis of ChIP-seq data
The ZmMYC2 binding DNA obtained from in vitro ChIP assay was used for sequencing (Park 2009; Pepke et al. 2009). By mapping the sequencing reads to maize genome using BWA (Burrows Wheeler Aligner) (Li and Durbin 2009), 4 million (4M) mapped reads were detected in ZmMYC2 binding sample. Subsequently, the binding peaks were obtained by model-based analysis of ChIP-seq (MACS) with the q value < 0.05 (Zhang et al. 2008; Liu 2014). The peak-related genes were screened based on the number, the width and the distribution of the peaks. The transcription start site (TSS) of each peak-related gene was detected using Peak Annotator (Salmon-Divon et al. 2010). The peaks were identified within the gene regions (including 2 kb upstream of the TSS and 2 kb downstream of the stop codon) or intergenic regions (other regions excluding gene regions). Peaks within 2 kb upstream region to TSS were considered to be MYC2-binding promoters. To understand the biological functions of those putative ZmMYC2 target genes, we performed Gene Ontology (GO) enrichment analysis to find related GO terms with different categories based on functions of molecular functions, biological processes and cell components (Kanehisa et al. 2008). Then Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were used to further determine the biochemical pathways and signal transduction pathway (Kanehisa et al. 2008).
Results
Identification of ZmMYC2
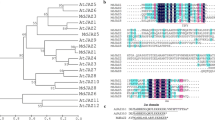
Plants recruit JA signaling to defend necrotrophic fungi invasion (Glazebrook 2005; Robert-Seilaniantz et al. 2011). MYC2, belonging to the bHLH TF family, has been proved as a master regulator of JA signaling and involved in defense against necrotrophic fungi(Dombrecht et al. 2007; Kazan and Manners 2013). However, little is known about this TF in maize. Therefore, we first performed the phylogenetic analysis of maize bHLH genes and identified one bHLH TF with highest sesquence identity (47%) to AtMYC2 in Arabidopsis (Fig. S1), thereby name it as ZmMYC2 putatively. Further analysis revealed that ZmMYC2 felled into the same clade with OsMYC2, AtMYC2, AtMYC3, AtMYC4 and AtMYC5, suggesting the potential role of ZmMYC2 in JA signaling (Fig. 1).
The full CDS of ZmMYC2 was cloned from maize leaves with 2118 bp encoding a putative protein of 705 amino acids. Sequence alignment revealed that ZmMYC2 contained the typical conserved functional domains (Fig. 2). The JID domain is necessary for interaction with JAZ protein, and TAD domain directly binds to MED25 for activating downstream target genes (Chini et al. 2007; Fernandez-Calvo et al. 2011; Cevik et al. 2012). In addition, MYC2 forms homo- or heterodimer mediated by the bHLH domain and leucine zipper domain (Toledo-Ortiz et al. 2003; Amoutzias et al. 2008; Carretero-Paulet et al. 2010). ZmMYC2 was also detected to contain these two domains, suggesting potential interactions with itself or other MYC2-like proteins. The basic region of bHLH domain is also responsible for binding activities of MYC2 to target gene promoters through G-box (5′-CACGTG-3′) and its variants (Kazan and Manners 2013), suggesting such function of ZmMYC2.
Expression profiles of ZmMYC2
To explore the potential involvement of ZmMYC2 in JA signaling, we investigated the expression profile with MeJA treatment. The qRT-PCR analysis revealed an inducible expression pattern of ZmMYC2 which began at 1 h and peaked at 3 h for about 25-fold higher than the control (0 h) (Fig. 3a). JA signaling plays pivotal parts in many defensive processes, such as wounding and pathogen infection (Wasternack and Hause 2013). As expected, ZmMYC2 gene expression was also induced with wounding and F. graminearum infection (Fig. S2). The inducible gene expression of ZmMYC2 in response to these treatments suggested involvement in maize JA signaling.
Expression profiles of ZmMYC2 under multiple treatments. Three-leaf stage maize seedlings were treated with different plant hormones separately including 100 μM MeJA (a), combined application of 100 μM MeJA and 50 μM EP (b), 100 μM GA3 (c) or 100 μM SA (d). The aboveground tissues were collected for qRT-PCR analysis. The hydroponic cultured seedlings were treated with 100 μM ABA (e), 250 mM NaCl (f) or 20% PEG6000 (g), respectively. The roots were collected for qRT-PCR analysis. Ef1a was used as the endogenous control and the expression level was normalized to 0 h. Asterisks indicate significant difference (Student’s t test, *P < 0.05, **P < 0.01). Error bars indicate SE (n = 3)
Considering the functions of AtMYC2 in crosstalk between JA and other plant hormones (Schmiesing et al. 2016; Verma et al. 2016; Berens et al. 2017), we examined the expression of ZmMYC2 under other phytohormone treatments (Fig. 3). The combined treatment of MeJA and EP also elevated ZmMYC2 gene expression drastically (Fig. 3b). On the contrary, GA and SA treatments significantly suppressed ZmMYC2 expression (Fig. 3c, d). These findings indicated the antagonistic effects between these two hormones and MYC2-mediated JA signaling, which has been reported in other plant species (Robert-Seilaniantz et al. 2011; Kazan and Manners 2013; Yang et al. 2015). Additionally, ABA has been proved to exert some synergistic effects with JA in roots under abiotic stress. ZmMYC2 was slightly induced by ABA in roots, and as expected, also by simulated drought stress with PEG6000 treatment and salinity stress (Fig. 3e–g). All these results suggested extensive roles of ZmMYC2 in both biotic and abiotic stress responses.
ZmMYC2 interacted with JAZ proteins
JASMONATE ZIM-domain proteins act as repressors of JA signaling by directly interacting with JA-responsive TFs like MYC2 to inhibit their transactivation on downstream genes (Pauwels and Goossens 2011). We investigated the direct binding between ZmMYC2 and JAZ proteins through yeast two-hybrid assays (Y2H). Preliminary experiments indicated the strong self-transactivation of ZmMYC2 (Fig. S3), which needs to be diminished in Y2H assays. We removed the transactivation domains and kept the JID domain of ZmMYC2 for protein interaction. Further analysis showed that ZmMYC2 directly interacted with ZmJAZ14 and ZmJAZ17, as well as AtJAZ1 and AtJAZ9 (Fig. 4). Among maize JAZ proteins, ZmJAZ14 has been reported to be associated with JA, ABA and GA signaling pathways (Zhou et al. 2015). These data indicated potential involvement of ZmMYC2 in JA signaling through interacting with JAZ proteins.
ZmMYC2 interacted with maize and Arabidopsis JAZ proteins. Yeast two-hybrid assays were used to detect the interaction between the truncated ZmMYC2 (1–139 aa) containing JID domain and JAZ proteins. Positive clones were screened on the selective medium SD/Trp-Leu-His-Ade with X-α-Gal (4 mg mL−1). The yeast concentration were adjusted to OD600 = 1 and diluted for ten times (10−1) and 100 times (10−2) to spot on the plate and grown at 30 °C for 2–4 days
ZmMYC2 restored root growth and anthocyanin accumulation in Arabidopsis myc234
Two conserved functions of MYC2 are to mediate inhibition of root growth and induction of anthocyanin accumulation by JA (Dombrecht et al. 2007). To further characterize the physiological function of ZmMYC2, transgenic Arabidopsis with ZmMYC2 overexpression in wild-type and triple-mutant myc234 were generated and the homozygous T3 plants were used for phenotype analysis. Two independent transgenic lines were used for further analysis either with overexpression in WT or in myc234, respectively. The transgenic plants grew normally with regular root length as WT (Fig. 5a, c, d). However, with treatment of 25 µM MeJA, the root length of OE plants was significantly shorter than WT lines, indicating elevated JA sensitivity by ZmMYC2 overexpression (Fig. 5b–d). The myc234 mutant plants lost the JA sensitivity partially and exhibit slight root growth inhibition by JA treatment. Overexpression of ZmMYC2 in myc234 significantly restored the sensitive to JA signaling and resulted in retarded root growth similar to WT (Fig. 5). These results indicated that ZmMYC2 exhibited the capability to compensate MYC gene mutation in Arabidopsis and played a role in the conserved JA-mediated root growth inhibition.
Overexpression of ZmMYC2 in Arabidopsis enhanced root growth inhibition by MeJA treatment. Arabidopsis WT and myc234 seeds were germinated on the ½ MS medium (a) and ½ MS medium plus 25 μM MeJA (b) and grown for 7 days for phenotype observation. c, d Root length of different lines with or without MeJA treatment. Asterisks indicate significant difference (Student’s t test, *P < 0.05, **P < 0.01). Error bars indicate SE (n = 3)
JA induces anthocyanin accumulation through MYC2 transactivation on MYB TF of anthocyanin biosynthesis (Shan et al. 2009; Al-Dhabi et al. 2015). Without JA treatment, all transgenic plants did not accumulate anthocyanin, as well as WT and myc234 (Fig. 6a). Once these Arabidopsis plants were treated with 25 µM MeJA, WT plants accumulated anthocyanin, in contrast, myc234 did not show anthocyanin induction (Fig. 6b). Overexpression of ZmMYC2 in myc234 resulted in inducible anthocyanin accumulation upon MeJA treatment (Fig. 6b), indicating restored JA regulation on anthocyanin biosynthesis via ZmMYC2.
Overexpression of ZmMYC2 in myc234 restored induction of anthocyanin accumulation by MeJA treatment. a ZmMYC2 overexpression lines were grown on the ½ MS medium for 7 days. b ZmMYC2 overexpression lines were grown on the ½ MS medium plus 25 μM MeJA for 7 days. Anthocyanin accumulation was induced in all lines except myc234
Overexpression of ZmMYC2 in Arabidopsis enhanced the resistance to B. cinerea
Another important function of JA is to regulate plant-defensive responses (Robert-Seilaniantz et al. 2011). As the master regulator of JA signaling, MYC2 plays pivotal roles in JA-mediated resistance to pathogens, especially for necrotrophic fungi. To explore the function of ZmMYC2 in defense against necrotrophic fungi, the detached leaves of transgenic Arabidopsis with ZmMYC2 overexpression were infected with B. cinerea. Compared with WT, the ZmMYC2 overexpression lines with Col-0 background exhibited smaller lesions and slighter disease symptom (Fig. 7), indicating enhanced resistance by ZmMYC2 overexpression in Col-0. However, both myc234 and its ZmMYC2 overexpression lines developed grievous disease symptom. ZmMYC2 overexpression in myc234 did not elevate disease resistance, suggesting that ZmMYC2 is not sufficient to compensate MYC function loss in this triple mutant and AtMYC2, AtMYC3 and AtMYC4 might have differential functions in pathogen defense.
Overexpression of ZmMYC2 enhanced the resistance to Botrytis cinerea. a Detached Arabidopsis leaves were infected with B. cinerea hypha for 7 days. b The foliar incidences of different Arabidopsis lines were calculated based on the percentage of infected leaf area to the whole leaf area, which was counted using the Image J software. Different lowercase letters indicate significant difference (LSD test, P < 0.05). Error bars indicate SE (n = 6)
Identification of ZmMYC2 target genes in maize
MYC2 regulates gene expression as the transcription factor by binding gene promoters. To explore regulatory function of ZmMYC2 in maize, we analyzed its target genes through in vitro ChIP-Seq. 47156 fragments were detected to be bound by ZmMYC2 and most of them (58.64%) were located in intergenic regions (Fig. 8a). Other fragments were mostly identified as the promoter sequences (12.06%) at the 2 kb upstream of the TSS, and the potential regulatory sequences (14.61%) at the 2 kb downstream of the stop codon. The promoter region is the main target site of transcription factors. ZmMYC2 target sequences in promoter regions were mapped to 4437 genes and the binding sites were enriched at ~ 500 bp upstream of the TSS (Fig. 8b). Further analysis by Gene Ontology (GO) indicated that the 4437 genes were mainly involved biological processes including terpene synthase activity, defense response, response to abiotic and biotic stimuli, response to oxygen levels, response to hormone and regulation of gene expression (Fig. 8c, d). Among these genes, many of them were annotated as transcription factors fallen into a number of categories (Fig. 8e), suggesting diverse function of ZmMYC2 through targeting these transcription factors to regulate different biological processes.
Genome-wide identification of ZmMYC2 binding sites and Gene Ontology (GO) analysis of ZmMYC2 target genes. a Binding-peak distribution of ZmMYC2 across maize genomic regions. b Distance of identified ZmMYC2 binding sites relative to the TSS. c GO enrichment analysis of ZmMYC2 target genes and classification of biological process. Part of ZmMYC2 target genes are shown including hormone-related genes (d) and transcription factors (e). Gene numbers for each category are labeled in pie charts
ZmMYC2 bound to defense gene promoters to regulate gene expression
MYC2 plays key roles as the master to regulate various biological processes, particularly defense response. In the ChIP-Seq analysis, ZmMYC2 bound to a lot of defense gene promoters (Supplemental Dataset 1). To validate its potential regulation on these defense genes, ZmMYC2 was transiently overexpressed in maize protoplasts and qRT-PCR was conducted to analyze gene expression for some selected marker genes. As shown in Fig. 9a, JA biosynthetic gene LOX1 and JA-Ile biosynthetic gens JAR1 were both targeted and up-regulated by ZmMYC2. Meanwhile, pathogenesis-related gene PR4 exhibited higher expression through ZmMYC2 transient overexpression. Furthermore, ROS scavenging is an important mechanism in plant defense. POD1 and SOD3 were targeted by ZmMYC2 as indicated by ChIP-Seq analysis (Supplemental Dataset 1). ZmMYC2 promoted gene expression of POD1 and SOD3 in maize protoplasts, which might contribute to suppressed ROS level and corresponding defense response (Fig. 9a).
Transient overexpression of ZmMYC2 regulated gene expression in maize protoplasts. qRT-PCR analysis of defense-related genes (a), ARF genes (b) and AUX/IAA genes (c, d) in maize protoplasts with transient overexpression of ZmMYC2. Ef1a was used as the endogenous control. Gene expression was normalized to that in the control. Black and gray bars indicate gene expression in maize protoplasts of the control (empty vector transformation) or with ZmMYC2 transient overexpression. Asterisks indicate significant difference (Student’s t test, *P < 0.05, **P < 0.01). Error bars indicate SE (n = 3)
ZmMYC2 played roles in plant hormone signal crosstalk
Signaling crosstalk among plant hormones has been investigated in numerous literatures. MYC2 was also involved in the signaling crosstalk between JA and other plant hormones such as ethylene and GA. We identified a lot of plant hormone biosynthetic and signal genes as ZmMYC2 targets by ChIP-Seq analysis (Fig. 8d; Supplemental Dataset 2). Ethylene-related genes were the main targets of ZmMYC2, consistent with JA/ethylene signaling crosstalk as reported by many investigations (Kazan 2015). We also observed many ABA-related genes bound by ZmMYC2, implying JA/ABA signaling crosstalk that has also been explored intensively (Abe et al. 2003; Vishwakarma et al. 2017) (Supplemental Dataset 2). Although some downstream genes in auxin signaling were reported to be regulated by MYC2 (Sun et al. 2009; Hentrich et al. 2013), JA and auxin signaling crosstalk has not been explored thoroughly. Unexpectedly, a number of auxin-related genes were also detected in ZmMYC2 target profile (Supplemental Dataset 3), suggesting involvement of ZmMYC2 in potential JA/auxin signaling crosstalk. The major auxin-related genes were identified as Aux/IAA and ARF family genes (Supplemental Dataset 3). Further analysis indicated that ZmMYC2 transient overexpression up-regulated most ARF gene expression, while ARF28 and ARF30 were down-regulated (Fig. 9b). For Aux/IAA genes, opposite regulation was observed. Some IAA genes accumulated higher transcripts and others exhibited compromised gene expression (Fig. 9c, d), indicating differential regulation by ZmMYC2 and potential diverse function of Aux/IAA genes. ZmMYC2 bound to various genes related to plant hormones suggests involvement in signal crosstalk as the master regulator, also implicating the complicated regulatory network among different biological processes including growth and defense in plants.
Discussion
MYC2 is considered to be the most important and extensively studied transcription factor in JA signaling (Dombrecht et al. 2007; Kazan and Manners 2013). AtMYC2 can be rapidly induced by exogenous JA treatment and further activate a large number of JA early responsive genes (Dombrecht et al. 2007). In our study, ZmMYC2 also exhibited inducible expression pattern in response to JA treatment at very early stage, indicating its potential role in JA signaling. Previous transcriptome analysis revealed that the F. graminearum infection stimulated a number of JA-related genes in maize, implying the participation of JA signaling in maize disease defense (Liu et al. 2016). As shown in Fig. S2, ZmMYC2 was highly induced by F. graminearum infection. Hence, we speculated that ZmMYC2 might be involved in this defensive process and play a part in controlling such transcriptional rearrangement. Moreover, ZmMYC2 was also induced by insect elicitors with increased JA level, indicating a potential role of ZmMYC2 in JA-mediated herbivores resistance (Engelberth et al. 2012). However, those regulatory mechanisms still need to be elucidated.
In Arabidopsis, AtMYC2 regulates multiple pathways and interactions between JA and other plant hormones (Robert-Seilaniantz et al. 2011; Schmiesing et al. 2016). AtMYC2 has been found as a negative regulator of ethylene response transcription factor ORA59 and ERF1 (Dombrecht et al. 2007; Zander et al. 2010; Verhage et al. 2011). These findings suggest putative antagonism effects of ZmMYC2 on JA and ETH. In addition, the T-DNA insert mutant of AtMYC2 exhibited less sensitive to both JA and ABA, highlighting the synergistic effect of AtMYC2-mediated JA and ABA (Abe et al. 2003; Lorenzo et al. 2004; Nakata et al. 2013). Interestingly, ZmMYC2 was induced by the combined treatment of MeJA and EP in maize leaves, as well as ABA treatment in maize roots (Fig. 3b, e). These results suggest coordinated effects of ZmMYC2 on JA-ETH and JA-ABA interactions, which is different from that in Arabidopsis. This implies specific regulating aspects of ZmMYC2 in maize JA signaling, which deserves further study. Furthermore, maize terpenoid phytoalexins were accumulated in response to both MeJA/EP treatment and ABA treatment (Huffaker et al. 2011; Schmelz et al. 2011; Vaughan et al. 2015). Our recent study characterized a transcription factor ZmWRKY79 to regulate maize phytoalexin biosynthesis (Fu et al. 2018). The promoter of ZmWRKY79 contains a lot of G-boxes, which are the target cis-elements of MYC transcription factors. Hence, ZmMYC2 might act with ZmWRKY79 to be involved in the JA-mediated phytoalexins regulation.
In many plant species, MYC2 has been well established to function in positive feedback regulation of JA biosynthesis and signaling. In this study, we also demonstrated that ZmMYC2 directly bound to the promoters of many JA biosynthetic genes. And overexpression of ZmMYC2 in maize protoplasts enhanced the expression of these genes, such as LOX1 and JAR1, suggesting an elevated JA level and activated JA signaling in ZmMYC2-OE protoplasts (Fig. 9a). However, the expression of pathogen resistance gene PR4 was also increased in ZmMYC2-OE protoplasts (Fig. 9a). This is opposite with previous studies about AtMYC2, which was reported to be a negative regulator on PR4 in Arabidopsis by suppressing the positive regulators such as ethylene-related ORA59 and ERF1. These studies indicate an antagonistic role of AtMYC2 in modulation of JA/ET related defense responses (Pre et al. 2008). However, in our study, the enhanced expression of PR4 echoes the results that the expression of ZmMYC2 can be induced by joint treatment of JA and ET, which together proposing a novel synergistically role of ZmMYC2 in these two pathways. In consistent with the hypothesis, we observed an enhanced resistance in ZmMYC2-OE Arabidopsis against the necrotrophic pathogen B. cinerea, while atmyc2 mutant exhibited increased resistance against this pathogen (Dombrecht et al. 2007). Furthermore, the suppressed ROS level during infection caused by activated JA signaling has been considered as defense response to necrotrophic pathogens (Govrin and Levine 2000). Therefore, the enhanced expression of ROS scavenging genes might also contribute to ZmMYC2-mediated resistance against B. cinerea (Fig. 9a).
As the repressor of JA signaling, JAZ proteins suppress transactivation of MYC2 through direct interactions, thereby regulating JA signaling. There are 13 JAZ proteins in Arabidopsis genome and most of them can interact with AtMYC2 except AtJAZ4 and AtJAZ7 (Fernandez-Calvo et al. 2011). In rice, OsMYC2 have been found to interact with 14 rice JAZ proteins except OsJAZ14 (Uji et al. 2016). In maize genome, 48 members of JAZs proteins have been identified, suggesting the interactions between MYCs and JAZs in maize should be more complicated (Zhou et al. 2015). Here, we detected protein interactions between ZmMYC2 with ZmJAZ14 and ZmJAZ17 (Fig. 4). All these three genes are all highly expressed in the anther and staminal filament based on RNA-Seq data of MaizeGDB (Sekhon et al. 2011). In Arabidopsis, AtMYC2, AtMYC3, AtMYC4 and AtMYC5 were together involved in JA-mediated stamen development and seed production (Qi et al. 2015). Hence, ZmMYC2 might also play a role in JA-mediated stamen development by interacting with ZmJAZ14 and ZmJAZ17. Additionally, ZmJAZ14 exhibited increased expression under JA, ABA and PEG6000 treatment (Zhou et al. 2015), which is similar to ZmMYC2, implicating their interaction in these stress responses.
JA has been widely studied to suppress root growth (Dombrecht et al. 2007). In our study, the Arabidopsis myc234 mutant did not exhibit much root growth inhibition by MeJA treatment, indicating partial loss of JA sensitivity (Fig. 5b, d). By complementation expression of ZmMYC2 in this myc234 mutant, the root growth inhibition by JA was restored (Fig. 5b, d). In addition, compared to WT, the ZmMYC2-OE lines exhibited even shorter root growth under MeJA treatment, suggesting a hypersensitivity effect of these lines to JA (Fig. 5b, d). This is consistent with the previous findings that MYC2 played the major role in promoting hypersensitivity of primary root towards exogenous JA (Dombrecht et al. 2007). Additionally, anthocyanin accumulation was induced with MeJA treatment in WT, OE plants and complementation plants except for the myc234 line (Fig. 6), indicating involvement of ZmMYC2 in this process. However, overexpression of ZmMYC2 in WT did not cause more anthocyanin accumulation with MeJA treatment, suggesting that the endogenous MYC genes in Arabidopsis WT plants are sufficient to regulate this phenotype.
ChIP-Seq analysis indicated that ZmMYC2 bound to a large number of hormone-related gene promoters, suggesting its roles in plant hormone signaling crosstalk (Fig. 8c, d; Supplemental dataset 2). Surprisingly, among those plant hormone-related genes, a high proportion of genes are involved in auxin signaling including many AUX/IAA genes and ARFs (Fig. 8d, e; Supplemental dataset 3). Some JAZ proteins and JA-responsive genes were under control of ARFs (Grunewald et al. 2009), but regulation of ARFs by JA-related regulators remains unknown. qRT-PCR analysis uncovered a positive role of ZmMYC2 on most ARF genes in maize protoplasts (Fig. 9b). Although the effects of ZmMYC2 on AUX/IAA genes were conflicted (Fig. 9c, d), it is undoubted that ZmMYC2 is involved in modulation of those auxin-related regulators, and the underlying mechanism should be illuminated in further studies.
Taken together, we identified a bHLH transcription factor ZmMYC2, which is a functional ortholog of AtMYC2, and not only involved in many JA-mediated conserved pathways but also shows some putative-specific functions. Further studies of ZmMYC2 might help to understand the JA signaling in maize and explore new aspects of JA signaling in plant kingdom. Given the importance of auxin in plant growth and development, the regulation of ZmMYC2 on auxin-related genes is the valuable clue to explore the balance between growth and defense.
Author contribution statement
QW conceived the research. JF, LL, QL, CW, QS and PY, CZ conducted the experiments and collected all the data. JF, LL and QW analyzed all the data and wrote the paper.
Abbreviations
- ABA:
-
Abscisic acid
- bHLH:
-
Basic helix–loop–helix
- EP:
-
Ethephon
- GA:
-
Gibberellin
- JA:
-
Jasmonate
- JA-Ile:
-
Jasmonoyl-l-isoleucine
- JAZ:
-
JASMONATE ZIM-domain protein
- MeJA:
-
Methyl jasmonate
- SA:
-
Salicylic acid
- TF:
-
Transcription factors
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Al-Dhabi NA, Arasu MV, Kim SJ, Romij Uddin M, Park WT, Lee SY, Park SU (2015) Methyl jasmonate- and light-induced glucosinolate and anthocyanin biosynthesis in radish seedlings. Nat Prod Commun 10:1211–1214
Amoutzias GD, Robertson DL, Van de Peer Y, Oliver SG (2008) Choose your partners: dimerization in eukaryotic transcription factors. Trends Biochem Sci 33:220–229
Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16:3460–3479
Berens ML, Berry HM, Mine A, Argueso CT, Tsuda K (2017) Evolution of hormone signaling networks in plant defense. Annu Rev Phytopathol 55:401–425
Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60:183–205
Campos ML, Kang JH, Howe GA (2014) Jasmonate-triggered plant immunity. J Chem Ecol 40:657–675
Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martinez-Garcia JF, Bilbao-Castro JR, Robertson DL (2010) Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol 153:1398–1412
Cevik V, Kidd BN, Zhang P, Hill C, Kiddle S, Denby KJ, Holub EB, Cahill DM, Manners JM, Schenk PM, Beynon J, Kazan K (2012) MEDIATOR25 acts as an integrative hub for the regulation of jasmonate-responsive gene expression in Arabidopsis. Plant Physiol 160:541–555
Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448:666–671
Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG (2002) COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J 32:457–466
Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, Reid JB, Fitt GP, Sewelam N, Schenk PM, Manners JM, Kazan K (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19:2225–2245
Du M, Zhao J, Tzeng DTW, Liu Y, Deng L, Yang T, Zhai Q, Wu F, Huang Z, Zhou M, Wang Q, Chen Q, Zhong S, Li CB, Li C (2017) MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato. Plant Cell 29:1883–1906
Engelberth J, Contreras CF, Viswanathan S (2012) Transcriptional analysis of distant signaling induced by insect elicitors and mechanical wounding in Zea mays. PLoS One 7:e34855
Fernandez-Calvo P, Chini A, Fernandez-Barbero G, Chico JM, Gimenez-Ibanez S, Geerinck J, Eeckhout D, Schweizer F, Godoy M, Franco-Zorrilla JM, Pauwels L, Witters E, Puga MI, Paz-Ares J, Goossens A, Reymond P, De Jaeger G, Solano R (2011) The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23:701–715
Fu J, Ren F, Lu X, Mao H, Xu M, Degenhardt J, Peters RJ, Wang Q (2016) A tandem array of ent-kaurene synthases in maize with roles in gibberellin and more specialized metabolism. Plant Physiol 170:742–751
Fu J, Liu Q, Wang C, Liang J, Liu L, Wang Q (2018) ZmWRKY79 positively regulates maize phytoalexin biosynthetic gene expression and is involved in stress response. J Exp Bot 69:497–510
Glazebrook J (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43:205–227
Govrin EM, Levine A (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea.pdf. Curr Biol 10:751–757
Grunewald W, Vanholme B, Pauwels L, Plovie E, Inze D, Gheysen G, Goossens A (2009) Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin. EMBO Rep 10:923–928
Gutierrez L, Mongelard G, Flokova K, Pacurar DI, Novak O, Staswick P, Kowalczyk M, Pacurar M, Demailly H, Geiss G, Bellini C (2012) Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 24:2515–2527
Hentrich M, Bottcher C, Duchting P, Cheng Y, Zhao Y, Berkowitz O, Masle J, Medina J, Pollmann S (2013) The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J 74:626–637
Hong GJ, Xue XY, Mao YB, Wang LJ, Chen XY (2012) Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. Plant Cell 24:2635–2648
Hou X, Lee LY, Xia K, Yan Y, Yu H (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19:884–894
Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D (2017) Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot 68:1361–1369
Huang H, Liu B, Liu L, Song S (2017) Jasmonate action in plant growth and development. J Exp Bot 68:1349–1359
Huffaker A, Kaplan F, Vaughan MM, Dafoe NJ, Ni X, Rocca JR, Alborn HT, Teal PE, Schmelz EA (2011) Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol 156:2082–2097
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, Katayama T, Kawashima S, Okuda S, Tokimatsu T, Yamanishi Y (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36:D480–D484
Kazan K (2015) Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci 20:219–229
Kazan K, Manners JM (2013) MYC2: the master in action. Mol Plant 6:686–703
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Li T, Xu Y, Zhang L, Ji Y, Tan D, Yuan H, Wang A (2017a) The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell 29:1316–1334
Li W, Zhu Z, Chern M, Yin J, Yang C, Ran L, Cheng M, He M, Wang K, Wang J, Zhou X, Zhu X, Chen Z, Wang J, Zhao W, Ma B, Qin P, Chen W, Wang Y, Liu J, Wang W, Wu X, Li P, Wang J, Zhu L, Li S, Chen X (2017b) A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 170(114–126):e115
Liu T (2014) Use model-based analysis of ChIP-Seq (MACS) to analyze short reads generated by sequencing protein-DNA interactions in embryonic stem cells. Methods Mol Biol 1150:81–95
Liu Y, Guo Y, Ma C, Zhang D, Wang C, Yang Q (2016) Transcriptome analysis of maize resistance to Fusarium graminearum. BMC Genomics 17:477
Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16:1938–1950
Nagpal P, Ellis CM, Weber H, Ploense SE, Barkawi LS, Guilfoyle TJ, Hagen G, Alonso JM, Cohen JD, Farmer EE, Ecker JR, Reed JW (2005) Auxin response factors ARF6 and ARF8 promote jasmonic acid production and flower maturation. Development 132:4107–4118
Nakata M, Mitsuda N, Herde M, Koo AJ, Moreno JE, Suzuki K, Howe GA, Ohme-Takagi M (2013) A bHLH-type transcription factor, ABA-INDUCIBLE BHLH-TYPE TRANSCRIPTION FACTOR/JA-ASSOCIATED MYC2-LIKE1, acts as a repressor to negatively regulate jasmonate signaling in arabidopsis. Plant Cell 25:1641–1656
Ogawa S, Miyamoto K, Nemoto K, Sawasaki T, Yamane H, Nojiri H, Okada K (2017) OsMYC2, an essential factor for JA-inductive sakuranetin production in rice, interacts with MYC2-like proteins that enhance its transactivation ability. Sci Rep 7:40175
Park PJ (2009) ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet 10:669–680
Pauwels L, Goossens A (2011) The JAZ proteins: a crucial interface in the jasmonate signaling cascade. Plant Cell 23:3089–3100
Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Perez AC, Chico JM, Bossche RV, Sewell J, Gil E, Garcia-Casado G, Witters E, Inze D, Long JA, De Jaeger G, Solano R, Goossens A (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791
Pepke S, Wold B, Mortazavi A (2009) Computation for ChIP-seq and RNA-seq studies. Nat Methods 6:S22–S32
Pre M, Atallah M, Champion A, De Vos M, Pieterse CM, Memelink J (2008) The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense. Plant Physiol 147:1347–1357
Qi T, Huang H, Song S, Xie D (2015) Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in arabidopsis. Plant Cell 27:1620–1633
Robert-Seilaniantz A, Grant M, Jones JD (2011) Hormone crosstalk in plant disease and defense: more than just jasmonate–salicylate antagonism. Annu Rev Phytopathol 49:317–343
Salmon-Divon M, Dvinge H, Tammoja K, Bertone P (2010) PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC Bioinform 11:415
Santner A, Calderon-Villalobos LI, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5:301–307
Schmelz EA, Kaplan F, Huffaker A, Dafoe NJ, Vaughan MM, Ni X, Rocca JR, Alborn HT, Teal PE (2011) Identity, regulation, and activity of inducible diterpenoid phytoalexins in maize. Proc Natl Acad Sci USA 108:5455–5460
Schmiesing A, Emonet A, Gouhier-Darimont C, Reymond P (2016) Arabidopsis MYC transcription factors are the target of hormonal salicylic acid/jasmonic acid cross talk in response to Pieris brassicae egg extract. Plant Physiol 170:2432–2443
Schweizer F, Fernandez-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P (2013) Arabidopsis basic helix–loop–helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25:3117–3132
Sekhon RS, Lin H, Childs KL, Hansey CN, Buell CR, de Leon N, Kaeppler SM (2011) Genome-wide atlas of transcription during maize development. Plant J 66:553–563
Shan X, Zhang Y, Peng W, Wang Z, Xie D (2009) Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J Exp Bot 60:3849–3860
Shoji T, Hashimoto T (2011) Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant Cell Physiol 52:1117–1130
Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, Wu X, Cohen JD, Palme K, Li C (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448:661–665
Toledo-Ortiz G, Huq E, Quail PH (2003) The Arabidopsis basic/helix–loop–helix transcription factor family. Plant Cell 15:1749–1770
Uji Y, Taniguchi S, Tamaoki D, Shishido H, Akimitsu K, Gomi K (2016) Overexpression of OsMYC2 results in the up-regulation of early JA-Rresponsive genes and bacterial blight resistance in rice. Plant Cell Physiol 57:1814–1827
Vaughan MM, Christensen S, Schmelz EA, Huffaker A, McAuslane HJ, Alborn HT, Romero M, Allen LH, Teal PE (2015) Accumulation of terpenoid phytoalexins in maize roots is associated with drought tolerance. Plant Cell Environ 38:2195–2207
Verhage A, Vlaardingerbroek I, Raaymakers C, Van Dam NM, Dicke M, Van Wees SC, Pieterse CM (2011) Rewiring of the jasmonate signaling pathway in arabidopsis during insect herbivory. Front Plant Sci 2:47
Verma V, Ravindran P, Kumar PP (2016) Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16:86
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:161
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann Bot 111:1021–1058
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Xu L, Liu F, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang D, Xie D (2002) The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14:1919–1935
Yang YX, Ahammed GJ, Wu C, Fan SY, Zhou YH (2015) Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr Protein Pept Sci 16:450–461
Yuan Z, Zhang D (2015) Roles of jasmonate signalling in plant inflorescence and flower development. Curr Opin Plant Biol 27:44–51
Zander M, La Camera S, Lamotte O, Metraux JP, Gatz C (2010) Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. Plant J 61:200–210
Zhang X, Henriques R, Lin SS, Niu QW, Chua NH (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1:641–646
Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137
Zhou X, Yan S, Sun C, Li S, Li J, Xu M, Liu X, Zhang S, Zhao Q, Li Y, Fan Y, Chen R, Wang L (2015) A maize jasmonate Zim-domain protein, ZmJAZ14, associates with the JA, ABA, and GA signaling pathways in transgenic Arabidopsis. PLoS One 10:e0121824
Zhou W, Lozano-Torres JL, Blilou I, Zhang X, Zhai Q, Smant G, Li C, Scheres B (2019) A jasmonate signaling network activates root stem cells and promotes regeneration. Cell 177(942–956):e914
Acknowledgements
This work was supported by the funds of NSFC (31671708, 31971825) and the 1000-Talent Program of Sichuan Province to QW. We appreciate the support of Prof. Roberto Solano at Universidad Autónoma de Madrid and Prof. Benke Kuai at Fudan University for providing the myc234 triple mutant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to this work.
Additional information
Communicated by Renate Schmidt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fu, J., Liu, L., Liu, Q. et al. ZmMYC2 exhibits diverse functions and enhances JA signaling in transgenic Arabidopsis. Plant Cell Rep 39, 273–288 (2020). https://doi.org/10.1007/s00299-019-02490-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-019-02490-2