Abstract
Key message
Three TaLTPs were found to enhance chilling tolerance of transgenic Arabidopsis, which were characterized by analyzes of promoter-GUS activity, subcellular localization, chromosomal location and transcriptional profile.
Abstract
Non-specific lipid transfer proteins (nsLTP) are abundantly expressed in plants, however, their functions are still unclear. In this study, we primarily characterized the functions of 3 type I TaLTP genes that were localized on chromosomes 3A, 3B, and 5D, respectively. The transcripts of TaLTPIb.1 and TaLTPIb.5 were induced under chilling, wound, and drought conditions, while TaLTPId.1 was only up-regulated by dark treatment. All the 3 TaLTP genes could be stimulated by the in vitro treatment of salicylic acid, while TaLTPId.1 was also positively regulated by methyljasmonic acid. Furthermore, the promoter-reporter assay of TaLTPIb.1 in the transgenic brachypodium showed a typical epidermis-specific expression pattern of this gene cluster. When fused with EGFP, all the 3 proteins were shown to localize on the plasma membrane in transgenic tobacco, although a signal in chloroplasts was also observed for TaLTPId.1. Heterogeneous overexpression of each of the TaLTP genes in Arabidopsis resulted in longer root length compared with wild type plants under chilling condition. These results suggest that type I TaLTPs may have a conserved functionality in chilling tolerance by lipid permeation in the plasma membrane of epidermal cells. On the other hand, the type I TaLTPs may exert functional divergence mainly through regulatory subfunctionalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant non-specific lipid transfer proteins (nsLTPs) are encoded by a multigene family, and are known for their ability to reversibly bind and transport hydrophobic molecules in vitro (Vergnolle et al. 1992). These cationic peptides contain a conserved eight-cysteine motif backbone (C-Xn-C-Xn-CC-Xn-CXC-Xn-C-Xn-C), in which the cysteine residues are engaged in four disulfide bonds to stabilize a tertiary structure of hydrophobic cavity (Lee et al. 1998; Samuel et al. 2002; Shin et al. 1995). Computational and biochemical analyzes have demonstrated that this hydrophobic cavity of nsLTPs is plastic or flexible to allow loading of a great variety of lipid compounds such as phospholipids (Vergnolle et al. 1992; Zachowski et al. 1998), palmitic acid (C16:0), and acyl chains of 1,2-dimyristoylphosphatidylglycerol (Han et al. 2001; Shin et al. 1995). According to protein molecular masses, the nsLTP genes had been divided into two families of 7 and 9 kDa which was thought to transport different kinds of lipids in plant cells. Recently, plant nsLTPs were extended over nine types using genomic information from Arabidopsis, Oryza sativa and Triticum aestivum, in which type I nsLTPs comprise the maximum number of members belonging to the classical 9 kDa nsLTPs (Boutrot et al. 2008). In cereal, a certain number of type I nsLTPs are preserved in genome via the concerted evolution between homeologous chromosomes, however, their functional redundancy or diversification is an open question (Paterson et al. 2009; Wang et al. 2012).
NsLTPs were reported to be present in abundant quantities in plants, up to as much as 4 % of the total soluble proteins of wild cabbage leaves (Pyee et al. 1994). The expression pattern of nsLTPs from different plants has shown a large divergence covering various tissues and against environmental stimulates (George and Parida 2010; Kader 1996; Pitzschke et al. 2013; Pyee et al. 1994). Consistent with their complex expression profiles, the pleiotropic functions of nsLTPs have been previously suggested to be involved in cuticle synthesis (Lee et al. 2009; Pyee et al. 1994), catabolism in lipid storage (Tsuboi et al. 1992), somatic embryogenesis (Kader 1996), stigma-pollen interaction (Huang et al. 2013; Mollet et al. 2000; Tian et al. 2013), defense signaling (Blein et al. 2002; Maldonado et al. 2002), nodule organogenesis (Lei et al. 2014) development, and antimicrobial activities (Caaveiro et al. 1997; Kristensen et al. 2000). However, the exact function of nsLTPs remains to be clearly established. Several lines of evidence support the function of nsLTPs in cuticle synthesis such as their extracellular localization, the cutin monomer binding capability (Pyee et al. 1994; Thoma et al. 1994), and the epidermis-specific expression in young aerial growing tissues, where the cuticlar wax usually localizes (Thoma et al. 1994; Wang et al. 2012). Recent research data demonstrated that the disruption of a nsLTP-like protein (LTPG1) simultaneously altered the cuticular lipid composition and enhanced the susceptibility to the fungal pathogen, Alternaria brasssicola, supporting the overlapping involvement of the nsLTP and cuticle in defense mechanism (Lee et al. 2009). However, it is unclear whether the susceptibility to the pathogens of LTP-knockout plants is attributed to cuticular lipid changes or its antimicrobial activities (Kristensen et al. 2000). An alternative explanation of nsLTPs involved in the defense against fungal pathogens is that the cutin monomer-nsLTP complex is released by the fungal cutinase at the time of infection, which can act as signaling molecules and bind to the plasmalemma receptor triggering plant defense responses (Blein et al. 2002). The role of nsLTP in defense signaling was subsequently confirmed by the study of two important nsLTP genes. DIR1, a 7 kDa nsLTP, can release a systemic signal responsible for systemic acquired resistance (SAR) in the vascular system, while an AZI1 mutation resulted in the specific loss of systemic immunity in priming defenses (Maldonado et al. 2002; Pitzschke et al. 2013). It was presumed that nsLTPs may bind to signal molecules and translocate SAR signals across long distances. Furthermore, nsLTPs were reported to be involved in pollen development. One LTP (SCA) from the Lily, sharing 56.4 % identity with AtLTP1, was proved to function in pollen tube adhesion both in vivo and in vitro (Mollet et al. 2000). Some nsLTP genes, especially in type III, were found specifically expressed in the pollen and anther tapetum, and seemed to play important roles in pollen development by the transportation of lipids between the pollen and the tapetum (Carvalho Ade and Gomes 2007; Huang et al. 2013). Although a bulk of data demonstrate that the expression of some nsLTP genes show broad responses against abiotic stresses, such as drought, chilling, and high salt, the evidence that indicates their direct association with the tolerance to these stresses is rare (Carvalho Ade and Gomes 2007; George and Parida 2010; Guo et al. 2013; Jung et al. 2005; Pitzschke et al. 2013). In a previous study, the transgenic A. thaliana, overexpressing a CALTP1 from Capsicum annuum, resulted in enhanced resistance to Pseudomonas syringae pv. tomato DC3000, Botrytis cinerea, and exhibited tolerance to NaCl and drought stresses (Jung et al. 2005). Similarly, the overexpression of AtLPT3 increased the drought tolerance of the transgenic Arabidopsis that was regulated by MYB96 (Guo et al. 2013).
In wheat, a total of 156 putative wheat nsLtp genes had been identified using the EST sequences of T. aestivum. None of these members had been functionally identified before. Previous studies of wheat nsLTPs mainly focused on the transcript level, in which a complex expression pattern was shown during the entire life cycle of the plant (Boutrot et al. 2008; Wang et al. 2010). In this study, we attempted to examine the possible functions and functional redundancies of 3 type I TaLTPs involved in abiotic stresses. The phylogeny, transcriptional response, and subcellular localization were characterized. The functionality of these genes was then identified by observing the phenotypic changes of the transgenic Arabidopsis under stress conditions.
Materials and methods
Phylogenetic analysis and chromosomal location identification
The putative sequences of nsLTPs were downloaded from National Center for Biotechnology Information. The deduced amino acid sequences of all the nsLTPs were aligned by using the ClustalW program with default parameters. The phylogenetic tree was created with MEGA using the maximum like algorithm, with 1,000 boot strap replicates (Kumar et al. 2008). The chromosomal distribution of TaLTPIb.1, TaLTPIb.5 and TaLTPId.1 was verified by a set of Chinese Spring (CS) nulli-tetrasomic lines. The specific primer pair for each gene was employed for PCR amplification using the genomic DNA of CS nulli-terasomic lines as templates.
Quantitative RT-PCR for abiotic stress and hormone treatments
Seeds of T. aestivum were allowed to germinate on filter papers and then moved to containers as previously described by Wang et al. (2012). After 36 h, the water was replaced with the Murashige and Skoog (MS) medium (Duchefa Biochemie B.V., Haarlem, Netherlands) in order to grow the plants for 10 days with the photoperiod of 14/10 h and temperature of 28/25 °C (day/night) before stress treatments. For drought stress, the healthy young seedlings were transferred to containers with 25 % (w/v) of polyethylene glycol (PEG, MW 10,000). The chilling stress was applied by subjecting plants to 4 °C, and the wounding stress was applied by piercing the leaves with 0.3 mm-diameter needles. For hormone treatments, the seedlings were transferred to containers with 100 μM of methyljasmonic acid (MeJA), 2 mM of salicylic acid (SA), 100 μM of abscisic acid (ABA), and 100 μM of indole-3-acetic acid (IAA). Leaves of 10 or more seedlings from the above treatments were harvested at different time points after treatments, frozen immediately in liquid nitrogen, and stored at −80 °C. RNA isolation and quantitative RT-PCR (qRT-PCR) were performed as described previously (Wang et al. 2010, 2012). The sequence specificity of each amplified band was cloned and confirmed by sequencing.
Construction of TaLTPIb.1 promoter::uidA fusion and transformation in brachypodium
The sequences of the TaLTP promoters were previously identified elsewhere (Jang et al. 2008). The promoter of TaLTPIb.1 (−723 to +3) was amplified from the genomic DNA of the wheat cultivar, Sumai 3, and cloned into the pMD-19 vector (Takara, Japan) with the HindIII restriction enzyme site at the 5′ end and the BamHI restriction enzyme site at the 3′ end. The plasmid was digested with the above restriction enzymes and then sub-cloned into a binary vector of pCambia1391Z. The Agrobacterium strain, AGL1, was transformed with the TaLTPIb.1 promoter::uidA construct following a freeze–thaw method (Lai et al. 2011). The brachypodium of Bd21-3 was then used for Agrobacterium-mediated transformations using the procedure described by Bragg et al. (2012). The pCambia1391Z vector was also transformed into brachypodium plants as negative controls. Histochemical analyzes were performed with the T2 plants according to the procedures previously described (Wang et al. 2012). For fluorimetrical assays of plant tissues, the concentration of protein extracted in the β-glucuronidase (GUS) assay buffer was determined by the Bradford (1976) method using a Multiskan (Labsystems, Thermo Fisher Scientific Inc., MA, USA) spectrophotometer. GUS specific activity (hydrolysis of 4-methylumbelliferone glucuronide per mg protein per hour) was determined at 460 nm with a Fluoroscan (Labsystems, Thermo Fisher Scientific Inc., MA, USA) apparatus (Hull and Devic 1995). For the sections, the GUS-stained tissue samples were fixed in the FAA buffer containing EtOH (50 %, v/v), formaldehyde (3.7 %, v/w), and acetic acid (5 %, v/v). Dehydration was performed with the graded ethanol series (80–95 %, v/v), followed by additional tert-butanol batches (25, 50, and 100 %, v/v). The samples were initially incubated in 50 % paraplast (dissolved in tert-butanol) and finally embedded in 100 % paraplast. Subsequently, the samples were sectioned by a microtome, each with a thickness of 10 μm. Deparaffinization was performed by the immersion of the slides in xylene for 5 min. The fluorescence inverted microscope system (Carl Zeiss, Oberkochen, Germany) was used to generate the images.
Subcellular localization
The coding regions of TaLTPIb.1, TaLTPIb.5 and TaLTPId.1 were amplified and cloned into the pBIN35S:EGFP vector that was then transformed into the Agrobacterium, EAH105. For transient expression of the fusion proteins in Nicotiana benthamiana, the resultant Agrobacterium culture was resuspended in an infiltration medium [10 mM 4-morpholineethanesulfonic acid hydrate (MES), pH 5.6, 10 mM MgCl2, and 200 mM acetosyringone], then injected into 3-week-old N. benthamiana leaves with an optical density of 0.6 OD at 600 nm. The transformed 35S:EGFP was used as a negative control. For colocalization, the Agrobacterium harboring TaLTP:EGFP and the Agrobacterium harboring pm-rb CD3-1008 were mixed in a 1:1 ratio and co-injected in tobacco leaves as described above (Nelson et al. 2007). Transient expression of TaLTPs for the subcellular localization in onion (Allium cepa) epidermal cells was conducted according to Das et al. with a Bio-Rad PDS-1000/He biolistic particle delivery system (Das et al. 2009). Confocal microscopy was used to assess the results, 3 days following the infection. Fluorescent images were obtained using an LSM 510 META NLO system (Carl Zeiss, Oberkochen, Germany).
Analysis of phenotypic changes in transgenic Arabidopsis
Agrobacteria GV3101 containing either pBIN35S:TaLTPIb.1, pBIN35S:TaLTPIb.5, or pBIN35S:TaLTPId.1 were used to transform Arabidopsis plants according to the floral-dip method (Clough and Bent 1998). Transgenic lines were selected on MS agar plates containing 30 mg/ml kanamycin and the T3 lines were used for further phenotypic analysis. Two independent overexpressing Arabidopsis lines were tested to observe the effects of chilling, freezing, and drought. For the chilling treatment, the transgenic seeds were germinated on 1/2 MS medium for 3 days and moved to 4 °C for 10 days. For freezing treatment, the 10 days old seedlings were subjected to −10 °C for 1–5 h, followed by 2 days of recovery under normal growth conditions (Chinnusamy et al. 2003). For drought treatment, the wild type and transgenic plants were grown in the soil for 3 weeks, and then watering was stopped until the observation of a phenotype.
Results
Phylogeny and genomic localization
The nsLTP genes in wheat, rice, and Arabidopsis were previously classified (Boutrot et al. 2008). To clarify the phylogeny of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1, putative 9 KDa nsLTP genes from wheat and rice were downloaded from NCBI (Boutrot et al. 2008). All the 3 TaLTP genes were found to belong to the type I category. TaLTPIb.1 and TaLTPIb.5 fell in a wheat-specific gene cluster with extremely high homology, which contains 23 LTP members due to independent duplications in wheat (Figure S1). TaLTPId.1, TaLTPId.1 and TaLTPId.3 localized on a sub-branch with two pairs of rice homeologs (ex. Os11g02400.1 and Os12g02330.1), which had undergone concerted evolution (Wang et al. 2012).
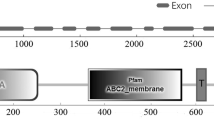
To determine the genomic distribution, wheat nulli-tetrasomic stocks and gene-specific primers were employed to examine the chromosomal location of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1, and all the amplified bands were confirmed by sequencing. The results showed that TaLTPIb.1 and TaLTPIb.5 were located on chromosomes, 3B and 3D (Fig. 1), while TaLTPId.1 was observed to be located on chromosome 5D (Fig. 1).
Expression pattern of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1
Previous studies indicated that the transcripts of TaLTPs were extensively induced by abiotic stresses, implying their possible functional roles. To study the biological function of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1, we first examined their expression pattern in chilling, drought, dark, and wounding-treated wheat seedlings by qRT-PCR. Under control conditions, no apparent changes of these transcripts were observed from 0 to 72 h (Figure S2). It was observed that TaLTPIb.1 and TaLTPIb.5 showed very similar expression patterns that were distinct from that of TaLTPId.1. Under dehydration conditions, the transcripts of TaLTPIb.1 and TaLTPIb.5 were both up-regulated from 9 h, while TaLTPId.1 showed a down-regulated response to this stress (Fig. 2a). TaLTPIb.1 was strongly up-regulated by chilling stress, 9 h after treatment, reaching the peak of 12.5-fold at 24 h, while TaLTPIb.5 was induced at a lower expression level of 3.6-fold. The expression of TaLTPId.1 was only slightly induced in the first 24 h (2.8-fold) and recovered subsequently (Fig. 2a1–3). Similarly, TaLTPIb.1 and TaLTPIb.5 were slightly down-regulated by dark treatment for 24 h, while, TaLTPId.1 was strongly up-regulated (Fig. 2a1–3). For wounding, the expression of TaLTPIb.1 and TaLTPIb.5 were strongly induced in the first hour, while no response was observed for TaLTPId.1 (Fig. 2a1–3).
Transcription profiles of TaLTPIb.1, TaLTPIb.5 and TaLTPId.1 against abiotic stresses and hormone stimuli. a 1 –a 3 The stress treatments were applied by subjecting 10-day-old seedlings to dark (3, 9, 24, 48 and 72 h), wounding (20, 40 min, 1, 3 and 9 h), drought (PEG, 25 %; 3, 9, 24, 48 and 72 h), chilling (4 °C; 3, 9, 24, 48 and 72 h) conditions; b 1 –b 3 For hormone treatments, the material samples were harvested from the plants treated with MeJA (100 μM), SA (2 mM), ABA (100 μM), and IAA (100 μM) at 1, 3, 6, 12 and 24 h after the treatments. All of the expression values were presented as fold changes compared with control. The wheat 18 s rRNA was used internal control (Supplementary Table 1). The gene expression was shown as mean ± SEM for three to four independent experiments
In order to investigate the possible involvement of a regulatory pathway, the transcriptional response of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1 against various hormones was carried out by qRT-PCR. The expression of TaLTPIb.1 and TaLTPIb.5 were both sharply induced by SA in the first 3 h. The expression of TaLTPIb.1 was depressed by MeJA and IAA, while the expression of TaLTPIb.5 was depressed by ABA (Fig. 2b1, b2). TaLTPId.1 seemed to be gradually stimulated by SA and MeJA, but depressed by IAA (Fig. 2b3).
Localization of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1
Type I TaLTP genes were found to be expressed in various organs but the expression feature was found to be highly conserved in epidermal cells (Wang et al. 2012). Here, we tested the promoter activity of TaLTPIb.1 in a transgenic brachypodium. The vector, pCambia1391Z, was transformed in plant as a negative control that showed no GUS activity (Fig. 3a, b). The promoter activity of TaLTPIb.1 was observed mainly in young leaves (Fig. 3c), shoots, and spikes (Fig. 3d), but was not observed in roots. A section of leaf revealed that the expression of TaLTPIb.1 was located in epidermal cells (Fig. 3f), including trichomes (Fig. 3e). Consistent with the results of qRT-PCR, the promoter activity could be induced by wound stress, where over fivefold of GUS activity on the leaf was stimulated 1 hour after application of the wounding treatment (Fig. 3g, i). Moreover, the major GUS activity was restricted to only young emerging leaves when applying dark treatment for 3 days. The GUS activity was depressed as much as 88 % compared with the control (Fig. 3h, i).
Tissue specific expression of TaLTPIb.1 promoter::uidA fuisions in brachypodium. The promoter region of 723 bp upstream start codon was employed for promoter-reporter construction and transformed in brachypodium. T2 seedlings were used for GUS activity observation during the plant whole life cycle. a, b The GUS staining pattern of transgenic brachypodium harboring vector of pCambia1391Z; c the expression of TaLTPIb.1p:uidA at seedling stage; d the expression of TaLTPIb.1p::uidA in spike; e the expression of TaLTPIb.1p::uidA on trichome; f the leaf section; g the wound shoot; h the expression of TaLTPIb.1p:uidA at seedling stage under dark condition; i the GUS quantities under stress conditions was evaluated using leaf tissues. wd wouding, ep epidermis, bar = 50 μm
To examine the subcellular localization of these TaLTPs, we constructed the EGFP fusion proteins under the control of the 35S promoter and transitionally expressed the proteins in N. benthamiana leaves. For the negative control, the transient expression of 35S:EGFP was observed in the cytosol and nuclei with a weak signal (data not shown). When fused with the EGFP, all of the 3 TaLTPs were exclusively expressed in the plasma membrane (Fig. 4a, b), which was confirmed by the well-merged signal with the marker line of pm-rb CD3-1008 (Nelson et al. 2007). Furthermore, small vehicle like structures were observed for TaLTPId.1 in the cytosol, which overlapped with chloroplast autofluorescence (Fig. 4c). To further confirm the localization of these TaLTPs, we transformed the three TaLTP:EGFP constructs into the onion epidermis and all of them directed the same signal pattern. For example, the TaLTP1:EGFP fusion was originally found around the cell wall, while it was subsequently confirmed in plasma-membrane via plasmolysis of onion epidermis (Figure S3).
Subcellular localization of TaLTP:EGFP fusion proteins in tobacco leaves. Agrobacterium strains GV3101 harboring each construct of TaLTP:EGFP were transiently expressed in Nicotiana leaves. a 1 –a 4 Subcellular localization of TaLTIb.1:EGFP in tobacco leaf cells; b 1 –b 4 subcellular localization of TaLTIb.5:EGFP in tobacco leaf cells; c 1 –c 4 subcellular localization of TaLTId.1:EGFP in tobacco leaf cells. Images were captured and merged by z series optical sections after 3 days of agro-infiltration. pm plasma membrane, cp chloroplast, bar = 20 μm
Overexpression of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1 in Arabidopsis
To further examine their functions, we generated transgenic Arabidopsis, overexpressing each gene, for stress tolerance test. Two independent transgenic lines (T3) were selected depending on the expression levels under normal conditions (Fig. 5a2, b2, c2). When grown in normal conditions in soil or plates, no significant difference in phenotypes was observed between the wild type and transgenic plants (data not shown). For drought stress, irrigation of 2-week-old transgenic plants was stopped for 10 days and no significant phenotypes were observed compared with the control plants (data not shown). For the chilling tolerance test, the root length test showed that the transgenic seedlings of all the 3 genes grew faster and their roots were significantly longer (t test, P < 0.01) than the wild type plants (Fig. 5a1, a3, b1, b3, c1, c3). This result implied that the heterogeneous overexpression of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1 in Arabidopsis can promote seedling growth under conditions of chilling. Interestingly, compared with wild type plants, we observed no significant changes of these transgenic lines in their phenotype when subjecting plants to freezing condition (data not shown).
TaLTPIb.1, TaLTPIb.5 and TaLTPId.1 contribute to the chilling tolerance in Arabidopsis. a 1 –c 1 Phenotype of the transgenic plants under chilling stress (4 °C); a 2 –c 2 the expression level of transgenic lines by semi-quantitative RT-PCR; a 3 –c 3 root length of the transgenic plants under chilling stress (4 °C). Each data point is average of three experiments and bars indicate SD
Discussion
Many type I nsLTPs were found to be abundantly expressed on the surface of plant aerial tissues and showed a broad transcriptional response to abiotic stresses. However, direct evidence of these small peptides in abiotic stress tolerance was rarely known. In this study, overexpression of three TaLTPs in Arabidopsis resulted in enhanced chilling tolerance of the transgenic plants, supporting their functional involvement.
The mechanism by which plants perceive changes in temperature, transfer signals and switch on the chilling tolerance pathway is relatively unknown. Numerous physiological and biochemical changes in plants were changed under chilling conditions, including the ABA level, the membrane lipid composition, and the accumulation of compatible osmolytes (Sanghera et al. 2011). The cell membranes are thought to be the primary damage sites under chilling stresses and may play important roles in signal transduction and in the chilling resistance machinery, in which the increase in unsaturated fatty acids is thought to be an effective strategy for living cells to adapt to chilling temperatures (Sanghera et al. 2011). Computational and biochemical analysis has demonstrated that some type I nsLTPs were able to accommodate various kinds of lipids such as phospholipids (Shin et al. 1995; Vergnolle et al. 1992), palmitic acid (C16:0), and acyl chains of the 1,2-dimyristoylphosphatidylglycerol (Shin et al. 1995). In this study, we found that all the 3 TaLTP genes were mainly located on the plasma membrane although small differences were found for TaLTPId.1 that was also localized in the chloroplast (Fig. 4). Thus, it is possible that these TaLTPs may function by transferring corresponding lipids and modifying the permeation of plasma membrane in order to reduce the damage caused by chilling stress. Previously, an epidermis-specific expression of type I nsLTP genes had been revealed, supporting their functional involvement in cuticle synthesis. This expression pattern was further verified in this study when transforming the construction of TaLTPIb.1::uidA in brachypodium (Fig. 3). It is not known whether these TaLTPs contribute to the chilling tolerance as well as cuticle synthesis. However, the cuticle composition in plants was also found to associate with cold and drought tolerance, indicating the common underlying mechanisms (Amid et al. 2012).
Indeed, a bulk of previous data and the current study demonstrate a strong transcriptional response of nsLTP genes against drought stress, while we observed no phenotypic changes when testing the TaLTP overexpressing Arabidopsis. One possible explanation may be the functional redundancy in Arabidopsis itself that contains over 30 members of nsLTP genes. Moreover, none of the transgenic lines in this study exhibited significant improved tolerance for freezing, which should be similar to chilling tolerance. Recently, overexpression of AtLTP3, in a native plant, was found to enhance the drought and freezing tolerance, which was observed to be located in the whole cell cytoplasm (Guo et al. 2013). Considering the different subcellular localization of these nsLTP genes, it may be presumed that the lipids transferring to different membranes may result in a functional difference in these gene copies.
The type I nsLTPs were extensively duplicated in cereal, many of which were preserved in genome via concerted evolution resulting in functional redundancy. For instance, six pairs of type I OsLTPs located on a duplication block of chromosomes 11 and 12 (Paterson et al. 2009; Wang et al. 2012), which fell together with the three TaLTPs in this study. TaLTPId.1 located in the same branch with two pairs of OsLTPs undergoing concerted evolution (Os11g02400 and Os12g02330, Os11g02424 and Os1202340). However, only three wheat members were found in the sub-branch where TaLTPId.1 located and no concerted evolution signature was observed for these TaLTPs. Considering the hexploid nature of wheat, it was supposed that some wheat members in this sub-clade were lost. In contrast, TaLTPIb.1 and TaLTPIb.5 were in a wheat-specific gene cluster containing 23 copies (Figure S1), which should be extensively duplicated recently but not only due to the polyploidy process during evolution of wheat (Brenchley et al. 2012). It is rather difficult to clarify the duplication story of these gene copies. At the transcript level, clear redundancy was found for TaLTPIb.1 and TaLTPIb.5. Both the genes were up-regulated by drought, chilling and wounding, but depressed by darkness, indicating high conservation of these 2 genes in both the coding and non-coding regions during evolution (Fig. 2a). On the other hand, TaLTPId.1 showed a relatively distinct expression profile compared with TaLTPIb.1 and TaLTPIb.5, which was up-regulated by MeJA, darkness, and showed a signal in the chloroplast, implying different biological roles. However, the transgenic Arabidopsis lines overexpressing these 3 gene copies all constitutively enhanced the tolerance to chilling stress, demonstrating the functional redundancy of TaLTPIb.1, TaLTPIb.5, and TaLTPId.1 at the protein level. Perhaps, the functional divergence between TaLTPId.1 and the other two genes may be partial through regulatory changes in the non-coding and coding regions.
References
Amid A, Lytovchenko A, Fernie AR, Warren G, Thorlby GJ (2012) The sensitive to freezing3 mutation of Arabidopsis thaliana is a cold-sensitive allele of homomeric acetyl-CoA carboxylase that results in cold-induced cuticle deficiencies. J Exp Bot 63:5289–5299
Blein JP, Coutos-Thevenot P, Marion D, Ponchet M (2002) From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci 7:293–296
Boutrot F, Chantret N, Gautier MF (2008) Genome-wide analysis of the rice and Arabidopsis non-specific lipid transfer protein (nsLtp) gene families and identification of wheat nsLtp genes by EST data mining. BMC Genom 9:86
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bragg JN, Wu J, Gordon SP, Guttman ME, Thilmony R, Lazo GR, Gu YQ, Vogel JP (2012) Generation and characterization of the Western Regional Research Center Brachypodium T-DNA insertional mutant collection. PLoS One 7:e41916
Brenchley R, Spannagl M, Pfeifer M, Barker GL, D’Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, Kay S, Waite D, Trick M, Bancroft I, Gu Y, Huo N, Luo MC, Sehgal S, Gill B, Kianian S, Anderson O, Kersey P, Dvorak J, McCombie WR, Hall A, Mayer KF, Edwards KJ, Bevan MW, Hall N (2012) Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature 491:705–710
Caaveiro JM, Molina A, Gonzalez-Manas JM, Rodriguez-Palenzuela P, Garcia-Olmedo F, Goni FM (1997) Differential effects of five types of antipathogenic plant peptides on model membranes. FEBS Lett 410:338–342
Carvalho Ade O, Gomes VM (2007) Role of plant lipid transfer proteins in plant cell physiology—a concise review. Peptides 28:1144–1153
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17:1043–1054
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Das P, Ito T, Wellmer F, Vernoux T, Dedieu A, Traas J, Meyerowitz EM (2009) Floral stem cell termination involves the direct regulation of AGAMOUS by PERIANTHIA. Development 136:1605–1611
George S, Parida A (2010) Characterization of an oxidative stress inducible nonspecific lipid transfer protein coding cDNA and its promoter from drought tolerant plant prosopis juliflora. Plant Mol Biol Rep 28:32–40
Guo L, Yang H, Zhang X, Yang S (2013) Lipid transfer protein 3 as a target of MYB96 mediates freezing and drought stress in Arabidopsis. J Exp Bot 64:1755–1767
Han GW, Lee JY, Song HK, Chang C, Min K, Moon J, Shin DH, Kopka ML, Sawaya MR, Yuan HS, Kim TD, Choe J, Lim D, Moon HJ, Suh SW (2001) Structural basis of non-specific lipid binding in maize lipid-transfer protein complexes revealed by high-resolution X-ray crystallography. J Mol Biol 308:263–278
Huang MD, Chen TL, Huang AH (2013) Abundant type III lipid transfer proteins in Arabidopsis tapetum are secreted to the locule and become a constituent of the pollen exine. Plant Physiol 163:1218–1229
Hull GA, Devic M (1995) The beta-glucuronidase (gus) reporter gene system. Gene fusions; spectrophotometric, fluorometric, and histochemical detection. Methods Mol Biol 49:125–141
Jang CS, Yim WC, Moon JC, Hung JH, Lee TG, Lim SD, Cho SH, Lee KK, Kim W, Seo YW, Lee BM (2008) Evolution of non-specific lipid transfer protein (nsLTP) genes in the Poaceae family: their duplication and diversity. Mol Genet Genomics 279:481–497
Jung HW, Kim KD, Hwang BK (2005) Identification of pathogen-responsive regions in the promoter of a pepper lipid transfer protein gene (CALTPI) and the enhanced resistance of the CALTPI transgenic Arabidopsis against pathogen and environmental stresses. Planta 221:361–373
Kader JC (1996) Lipid-transfer proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 47:627–654
Kristensen AK, Brunstedt J, Nielsen KK, Roepstorff P, Mikkelsen JD (2000) Characterization of a new antifungal non-specific lipid transfer protein (nsLTP) from sugar beet leaves. Plant Sci 155:31–40
Kumar S, Nei M, Dudley J, Tamura K (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9:299–306
Lai WC, Wang HC, Chen GY, Yang JC, Korinek M, Hsieh CJ, Nozaki H, Hayashi K, Wu CC, Wu YC, Chang FR (2011) Using the pER8:GUS reporter system to screen for phytoestrogens from Caesalpinia sappan. J Nat Prod 74:1698–1706
Lee JY, Min K, Cha H, Shin DH, Hwang KY, Suh SW (1998) Rice non-specific lipid transfer protein: the 1.6 A crystal structure in the unliganded state reveals a small hydrophobic cavity. J Mol Biol 276:437–448
Lee SB, Go YS, Bae HJ, Park JH, Cho SH, Cho HJ, Lee DS, Park OK, Hwang I, Suh MC (2009) Disruption of glycosylphosphatidylinositol-anchored lipid transfer protein gene altered cuticular lipid composition, increased plastoglobules, and enhanced susceptibility to infection by the fungal pathogen Alternaria brassicicola. Plant Physiol 150:42–54
Lei L, Chen L, Shi X, Li Y, Wang J, Chen D, Xie F (2014) A nodule-specific lipid transfer protein AsE246 participates in transport of plant-synthesized lipids to symbiosome membrane and is essential for nodule organogenesis in Chinese milk vetch. Plant Physiol 164:1045–1058
Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK (2002) A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419:399–403
Mollet JC, Park SY, Nothnagel EA, Lord EM (2000) A lily stylar pectin is necessary for pollen tube adhesion to an in vitro stylar matrix. Plant Cell 12:1737–1750
Nelson BK, Cai X, Nebenfuhr A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136
Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, Haberer G, Hellsten U, Mitros T, Poliakov A, Schmutz J, Spannagl M, Tang H, Wang X, Wicker T, Bharti AK, Chapman J, Feltus FA, Gowik U, Grigoriev IV, Lyons E, Maher CA, Martis M, Narechania A, Otillar RP, Penning BW, Salamov AA, Wang Y, Zhang L, Carpita NC, Freeling M, Gingle AR, Hash CT, Keller B, Klein P, Kresovich S, McCann MC, Ming R, Peterson DG, Mehboobur R, Ware D, Westhoff P, Mayer KF, Messing J, Rokhsar DS (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457:551–556
Pitzschke A, Datta S, Persak H (2013) Salt stress in Arabidopsis: lipid transfer protein AZI1 and its control by Mitogen-activated protein kinase MPK3. Mol Plant
Pyee J, Yu H, Kolattukudy PE (1994) Identification of a lipid transfer protein as the major protein in the surface wax of broccoli (Brassica oleracea) leaves. Arch Biochem Biophys 311:460–468
Samuel D, Liu YJ, Cheng CS, Lyu PC (2002) Solution structure of plant nonspecific lipid transfer protein-2 from rice (Oryza sativa). J Biol Chem 277:35267–35273
Sanghera GS, Wani SH, Hussain W, Singh NB (2011) Engineering cold stress tolerance in crop plants. Curr Genomics 12:30–43
Shin DH, Lee JY, Hwang KY, Kim KK, Suh SW (1995) High-resolution crystal structure of the non-specific lipid-transfer protein from maize seedlings. Structure 3:189–199
Thoma S, Hecht U, Kippers A, Botella J, De Vries S, Somerville C (1994) Tissue-specific expression of a gene encoding a cell wall-localized lipid transfer protein from Arabidopsis. Plant Physiol 105:35–45
Tian A, Jiang J, Cao J (2013) Functional analysis of a novel male fertility lipid transfer protein gene in Brassica campestris ssp. chinensis. Plant Mol Biol Rep 31:775–782
Tsuboi S, Osafune T, Tsugeki R, Nishimura M, Yamada M (1992) Nonspecific lipid transfer protein in castor bean cotyledon cells: subcellular localization and a possible role in lipid metabolism. J Biochem 111:500–508
Vergnolle C, Arondel V, Jolliot A, Kader JC (1992) Phospholipid transfer proteins from higher plants. Methods Enzymol 209:522–530
Wang HW, Kwon HJ, Yim WC, Lim SD, Moon JC, Lee BM, Seo YW, Kim W, Jang CS (2010) Expressional diversity of wheat nsLTP genes: evidence of subfunctionalization via cis-regulatory divergence. Genetica 138:843–852
Wang HW, Hwang SG, Karuppanapandian T, Liu A, Kim W, Jang CS (2012) Insight into the molecular evolution of non-specific lipid transfer proteins via comparative analysis between rice and sorghum. DNA Res 19:179–194
Zachowski A, Guerbette F, Grosbois M, Jolliot-Croquin A, Kader JC (1998) Characterisation of acyl binding by a plant lipid-transfer protein. Eur J Biochem 257:443–448
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 31200982), 973 Developmental Program (2014CB138100), and Shandong province programs (20133702120002 and BS2013NY006).
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Baochun Li.
G. Yu and W. Hou contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, G., Hou, W., Du, X. et al. Identification of wheat non-specific lipid transfer proteins involved in chilling tolerance. Plant Cell Rep 33, 1757–1766 (2014). https://doi.org/10.1007/s00299-014-1655-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1655-y