Abstract
Key message
Potato and tobacco cells are differentially suited to study oxylipin pathway and elicitor-induced responses.
Abstract
Synthesis of oxylipins via the lipoxygenase (LOX) pathway provides plant cells with an important class of signaling molecules, related to plant stress responses and innate immunity. The aim of this study was to evaluate the induction of LOX pathway in tobacco and potato cells induced by a concentrated culture filtrate (CCF) from Phytophthora infestans and lipopolysaccharide (LPS) from Pectobacterium atrosepticum. Oxylipin activation was evaluated by the measurement of LOX activity and metabolite quantification. The basal levels of oxylipins and fatty acids showed that potato cells contained higher amounts of linoleic (LA), linolenic (LnA) and stearic acids than tobacco cells. The major oxylipin in potato cells, 9(S),10(S),11(R)-trihydroxy-12(Z),15(Z)-octadecadienoic acid (9,10,11-THOD), was not detected in tobacco cells. CCF induced a sharp increase of LA and LnA at 8 h in tobacco cells. In contrast they decreased in potato cells. In CCF-treated tobacco cells, colneleic acid increased up to 24 h, colnelenic acid and 9(S)-hydroxyoctadecatrienoic acid (9(S)-HOT) increased up to 16 h. In potato cells, only colneleic acid increased slightly until 16 h. A differential induction of LOX activity was measured in both cells treated by CCF. With LPS treatment, only 9,10,11-THOD accumulation was significantly induced at 16 h in potato cells. Fatty acids were constant in tobacco but decreased in potato cells over the studied time period. These results showed that the two elicitors were differently perceived by the two Solanaceae and that oxylipin pathway is strongly induced in tobacco with the CCF. They also revealed that elicitor-induced responses depended on both cell culture and elicitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant responses to pathogen attack involve early and late defense reactions. Early signal transduction pathways include changes of ionic fluxes (Ca2+, K+ and H+) (Garcia-Brugger et al. 2006), production of reactive oxygen species (ROS) and protein phosphorylation (Grant et al. 2000). They activate many genes related to plant defense responses. Some genes encode enzymes such as phenylalanine ammonia-lyase (PAL), which catalyze the synthesis of phenolic compounds, and lipoxygenases, which are key enzymes in the oxylipin pathway (Ingle et al. 2006). Oxylipin is a collective name for oxidized polyunsaturated fatty acids and their metabolites. The first step of this pathway leads to the synthesis of polyunsaturated fatty acid (PUFA) hydroperoxides (HPO) non-enzymatically (Hamberg 2011) or enzymatically via the action of lipoxygenase (linoleate oxygen oxidoreductase, LOX, E.C 1.13.11.12) or α-dioxygenase (E.C 1.13.11) (Blée 2002; Andreou and Feussner 2009). LOX are ubiquitous enzymes that can be found in both animals and plants (Feussner and Wasternack 2002). In plants, the dioxygenases catalyze oxygenation of PUFAs such as linoleic acid (LA, C18:2) and linolenic acid (LnA, C18:3) and mainly arachidonic acid (C20) in mammals. The release of PUFAs starts through an action of phospholipases (PLAs) as lipid acyl hydrolases (LAHs). In potato tubers, patatin, the major potato storage protein, shows LAH activity (Galliard 1971). The oxygenation of PUFAs by LOX can occur either at carbon 9 (9-LOX) or carbon 13 (13-LOX) (Liavonchanka and Feussner 2006). Then HPO can be metabolized by at least seven different pathways leading to various compounds: LOX, peroxigenase, divinyl ether synthase, hydroperoxide lyase; allene oxide synthase, epoxy alcohol synthase and hydroperoxide reductase (Mosblech et al. 2009).
The oxylipin pathway is implicated in physiological processes such as plant growth, development, and senescence or response to stress (Schaller 2001). In addition, Montillet et al. (2002) have shown that the oxylipin pathway was also activated in tobacco during biotic stress. Many oxylipins have been found to stimulate plant defense genes expression (Weber 2002) or modulate hypersensitive reaction (HR) characterized by cell death in tobacco leaves (Rustérucci et al. 1999; Hamberg et al. 2003).
Moreover, oxylipins have antimicrobial activity (Göbel et al. 2001; Prost et al. 2005; Kishimoto et al. 2008) and they are described as potential defense compounds. Prost et al. (2005) showed that HPO (13-HPOT, 9-HPOT, 9-HPOD and 13-HPOD) and the reduced forms (13-HOT, 9-HOT, 13-HOD and 9-HOD) have antifungal and antioomycete activity but they had no antibacterial effect against Pectobacterium carotovorum carotovorum.
Vellosillo et al. (2007) showed that 9-HOT regulated stress responses during the plant’s response to pathogen. In addition, it was demonstrated that 9-HOT enhance ROS production suggesting the participation of the 9-LOX oxylipin pathway in controlling oxidative stress and lipid peroxidation (López et al. 2011) and its implication in plant resistance. Tobacco cells treated with an elicitor from Phytophthora parasitica var. nicotianae (Ppn) showed a rapid and transient increase in jasmonic acid levels from the 13-LOX metabolism. It acted as a signal messenger in the transduction chain before the activation of defense genes expression (Rickauer et al. 1997; Kenton et al. 1999). In Solanaceae species, oxylipins from the 9-LOX pathway participate in the defense against oomycetes (Rustérucci et al. 1999; Göbel et al. 2001, 2002, 2003; Fammartino et al. 2007, 2010). 9-LOX products were preferentially stimulated in elicitor-treated potato cells (Göbel et al. 2001) and in tobacco (Fournier et al. 1993; Rustérucci et al. 1999). Thus, the accumulation of pathogen-induced transcripts was reported in potato infected by P. infestans (Kolomiets et al. 2000; Göbel et al. 2001; Stumpe et al. 2001; Göbel et al. 2002). These results suggest a role of these compounds in plant defense responses (Göbel et al. 2001; Weber et al. 1999). In tobacco, the accumulation of pathogen-induced 9-LOX transcripts was reported after infection with Ppn. Moreover, LOX gene overexpression is sufficient to reduce susceptibility to P. nicotianae (Mène-Saffrané et al. 2003). Other investigations in elicitor-treated tobacco leaves revealed that transcripts of genes encoding patatin-like proteins (NtPat) and 9-lox accumulated with similar profiles (Dhondt et al. 2002). In the same way, Cacas et al. (2005) showed that increased levels of transcripts of 9-lox and NtPat in cryptogein-elicited tobacco leaves were followed by the biosynthesis of active oxylipins.
We previously focused on the biochemical and physiological reactions induced in cell suspensions of three Solanaceae species (tobacco, tomato and potato) by purified lipopolysaccharide (LPS) from Pectobacterium atrosepticum (Pa) (pectinolytic Gram-negative bacterium, causing soft rot on potato tubers). LPS caused a significant acidification of potato, tomato and tobacco extracellular media, but it did not induce the formation of ROS in any of the cell suspensions. We also reported that LOX activity is induced in both cell suspensions, by a concentrated culture filtrate (CCF) of P. infestans (Pi) (an oomycete, causal agent of potato late blight) (Desender et al. 2006, 2007; Val et al. 2008). We therefore chose elicitor preparations derived from two pathogens inducing disease symptoms on potato (host), but not in tobacco (non host), and able to generate defense responses as pathogen-associated molecular patterns (PAMPs) without the presence of the pathogen itself. The aim of this study was to assess the induction of oxylipin pathway in tobacco BY and potato cv Bintje cell suspensions induced by CCF and LPS. To confirm the cell viability, we measured the PAL activity, a key enzyme of the phenylpropanoid pathway after induction. We studied oxylipin induction by measurement of 9-LOX activity, fatty acids and metabolite quantification. We therefore wondered (i) if LPS and CCF induced the oxylipin pathway in both cell cultures and (ii) if this potential activation is different between potato and tobacco cells.
Materials and methods
Plant cell cultures
Cell suspension cultures of Nicotiana tabacum cv. BY-2 and Solanum tuberosum cv Bintje were maintained in 80 ml of liquid, sterile MS medium at pH 5.8 and multiplied as described by Desender et al. (2006). Tobacco and potato cells were grown at 25 °C in the dark on a rotary shaker (130 rpm). Both cell cultures were subcultured before the stationary phase: tobacco cells were subcultured every week and potato cells every 2 weeks. Elicitation experiments were performed on the day before the stationary phase.
Elicitor preparation and elicitation
LPS of Pa (strain CFBP 5889, INRA Angers, France) were extracted using the hot phenol–water method of Westphal and Jann (1965) modified as described by Desender et al. (2006). CCF of Pi (strain isolated from Ploudaniel, France) was prepared as described by Desender et al. (2006). P.infestans was grown on sterile pea broth for 3 weeks. The culture broth was prepared by boiling 125 g of frozen peas in 1.2 L of distilled water, discarding the peas and autoclaving the broth for 20 min at 120 °C. The filtrate was obtained by separating the mycelium from the culture broth on 0.45 μm Whatman filter paper and lyophilized before use. For LOX activity, CCF concentrations were previously tested in potato cells from 0.1 to 100 μg ml−1. No differences were observed in LOX activity after induction by 1 or by 100 μg.ml−1. For all LOX activity experiments, we adjusted CCF concentration to 1 μg ml−1. CCF and LPS concentrations were adjusted to 200 μg.ml−1 for oxylipin analysis. Cryptogein from P. cryptogea and capsicein from P. capsici (provided by INRA Antibes, France) were used at 10 μg ml−1 as positive controls of LOX induction. Elicitors or water (control) were added to tobacco and potato cell suspensions. After 0, 8, 16, 18, 24, 42, 48 or 72 h cells were harvested by filtration and aliquots of 300 mg were frozen at −20 °C. One, two or three independent experiments with two independent replicates were realized for each analysis.
PAL activity measurement
PAL activity is estimated measuring the formation of cinnamic acid from phenylalanine as described by Zucker (1968) with some modifications. Cells (300 mg) were ground with extraction buffer (25 mM boric acid, 10 mg mL−1 polyvinylpyrrolidone and 0.3 % β-mercaptoethanol, pH 8.8). The homogenate was centrifuged at 10,000g at 4 °C for 30 min. The modified method of Bradford (1976) was applied to quantify the proteins in supernatants and adjusted to 300 μg per sample. PAL activity was measured in continuous for 50 mn in supernatants at 290 nm on a spectrophotometer, using phenylalanine as substrate. The reaction mixture contained 50 μM phenylalanine, 25 mM borate buffer (pH 8.8) and 335 μL of enzymatic supernatant. PAL activity was calculated in pmol of t-cinnamic acid produced per min and mg fresh weight (FW) with a molar absorption coefficient of ε = 10,000 L mol−1 cm−1.
LOX activity measurement
LOX activity was measured as described by Desender et al. (2006). Harvested cells (300 mg) of potato and tobacco were ground in 0.6 ml of cold Tris buffer (0.1 % Triton X-100, 3 mM EDTA, 0.04 % Na2S2O5, and 0.1 % polyvinylpyrrolidone, pH 6.8). The homogenate was centrifuged at 10,000g at 4 °C for 15 min. LOX activity in supernatants was measured at 234 nm on a spectrophotometer, using linolenic acid as substrate. The reaction mixture contained 100 μM of linolenic acid, 0.1 M Tris buffer (pH 6.8) and 200 μl enzyme. The increase in absorbance was followed for 15 min and the rate of increase was calculated from the initial slope. LOX activity was evaluated in nkat g−1 FW. Experiments were performed twice.
Analysis of oxylipin content
Oxylipin extraction
Oxylipin extraction was performed according to Gaquerel et al. (2007) with modifications: 1 ml of phosphate buffered saline (PBS) was added to 300 mg of cells filtered and ground in a hemolysis tube. Ethyl acetate (2 ml) containing internal standard (12-hydroxylauric acid at 0.05 mg ml−1) was added. The mixture was shaken for 1 h at 4 °C and put on ice for 5 min. Tubes were centrifuged at 4,000g for 10 min at 4 °C. The upper phase was removed and evaporated under nitrogen flux and then the dry extract was suspended in 200 μl of methanol.
Oxylipin derivatization
The samples were subjected to two derivatization procedures according to Christie (1993) and Pinot et al. (1992). For the methylation by diazomethane in ether, the reaction took place at room temperature for 15 min on 50 μl of extract in methanol with 300 μl of diazomethane. Then the excess of diazomethane was rapidly cold-evaporated. The dry residues were silylated with Sylon BFT [N–O-bis-silyltrifluoroacetamide (BSTFA)/trimethylchlorosilane (TMCS); 99:1] (Supelco, Bellafonte) for 1 h at 60 °C. The excess of reactive was evaporated under nitrogen flux and oxylipin derivates were suspended in hexane. For quantification, standards were established according to the same procedure using commercially available linoleic, linolenic, colneleic and colnelenic acids, 9(S),10(S),11(R)-trihydroxy-12(Z),15(Z)-octadecadienoic (9,10,11-THOD) and 9(S)-hydroxyoctadecatrienoic acid (9(S)-HOT) (Larodan Fine Chemicals, Sweden).
Oxylipin analysis by GC–MS
Derivatized oxylipins were analyzed by GC–MS (Le Quéré et al. 2004) on an Agilent GC 6890+coupled to a 5973 MS Detector (Agilent, Les Ullis, France) and with a DB-5MS column (low-bleeding, 5 % phenyl/95 % dimethylpolysiloxane phase) 30 m × 0.25 mm I.D. × 0.25 μm film thickness (J and W Scientific, Agilent) in the electron ionization mode at 70 eV. The temperature gradient was 60 °C for 5 min, 60–120 °C at 30 °C min−1, 120–290 °C at 4 °C min−1, and 290 °C for 10 min.
Statistical analysis
All statistical analyses were performed using the statistical software R GUI version 2.10.1. Analyses of variance were performed using the function ‘aov.’ Transformations were performed if necessary to approximate normality. Multiple comparisons of means were carried out using the TukeyHSD (honestly significant difference) Test (p = 0.05 **, p = 0.01 ***) with the glht () function of the package ‘multcomp’.
Results
Effect of CCF and LPS on PAL activity
In potato cells, CCF, but not LPS, induced a significant increase of PAL activity with a maximum activity 8 h after treatment (Fig. 1a). PAL activity then slowly decreased until 48 h reaching almost the control level. In tobacco cells, the challenge with CCF and LPS induced a significant and transient activation of PAL that increased from 8 h and slowly decreased until 48 h (Fig. 1b). For both elicitors, the maximum activity is reached 8 h after treatment (35 and 15 pmol min−1 g−1 FW, respectively).
Phenylalanine ammonia-lyase (PAL) activity induced in potato (a) and tobacco (b) cells after treatment with CCF (200 μg ml−1), LPS (200 μg ml−1) and water (control). PAL activity was calculated as pmol/min/g FW. Values are means of two independent experiments with two replicates each ± standard error. Bars with different stars superscript are significantly different (Tukey HSD, ***p = 0.001)
This preliminary experiment confirmed the viability of the cell culture used during all the experiments.
Effect of CCF and LPS on the concentrations of LOX substrates and 9-LOX products
At the initial time (0 h), comparison of basal levels of free PUFAs and oxidation products showed high differences between potato and tobacco cells (Fig. 2) (F value of 21.18). Large amounts of linoleic and linolenic acids were found in potato cells, 12-fold higher for linoleic acid (60 nmol g−1 FW against 5 nmol g−1 FW) and 5-fold for linolenic acid (25 nmol g−1 FW against 5 nmol g−1 FW) than in tobacco cells, whereas stearic acid was undetectable in tobacco suspensions. It can be noticed that in tobacco, both precursors of oxylipins, LA and LnA, were equally represented at a low concentration. 9-hydroxyoctadecatrienoic acid (9(S)-HOT) amounts were similar (approximately 3 nmol g−1 FW) in both cell suspensions and 9(S),10(S),11(R)-trihydroxy-12(Z),15(Z)-octadecadienoic acid (9,10,11-THOD) was highly represented in potato cells (120 nmol g−1 FW) and undetectable in tobacco suspensions. Colneleic and colnelenic acids were not found in either cell suspensions.
Basal levels of studied LOX products [9(S)-HOT, 9,10,11-THOD, colneleic (CA) and colnelenic acids (CnA)] and their substrates [linoleic (LA) and linolenic acids (LnA)] in potato (black bars) and tobacco (white bars) cells induced by water (control) at 0 h. Values are means of two independent experiments ± standard error. ND is for non-detected
In potato cells after treatment with CCF and LPS (Fig. 3a), the linoleic and linolenic acid contents significantly decreased from 8 to 72 h. No significant effect on stearic acid is observed after elicitors’ treatment (Table 1). In contrast, in tobacco, the challenge with CCF induced a rapid and transient increase of the release of free fatty acids with a maximum 8 h after treatment (Fig. 3b), and then the amounts slowly decreased until 72 h reaching almost the basal level (0 h). The linoleic acid contents reached 80 nmol g−1 FW (16-fold more than at 0 h) and 55 nmol g−1 FW for the linolenic acid (about 16-and 11-fold more than at 0 h respectively). LPS had no effect on tobacco cells.
Time course of accumulation of linoleic and linolenic acids in potato (a) and tobacco (b) cells treated with CCF (200 μg ml−1), LPS (200 μg ml−1) and water (control). Values are means of replicates of two or three independent experiments ± standard error. Bars with different stars superscript are significantly different (Tukey HSD, ***p = 0.001, **p = 0.05)
A slight increase of 9(S)-HOT was observed 8 and 16 h after treatment by CCF (5 nmol g−1 FW) in potato cells, whereas no accumulation was significantly increased after induction by LPS or in control (Fig. 4a). In tobacco cells elicited by CCF, 9(S)-HOT increased transiently with a maximum at 16 h (about 15 nmol g−1 FW) (Fig. 4b). Then the amounts slowly decreased until 72 h. No significant variation was observed after treatment by LPS or in control. In LPS-treated potato cells, 9,10,11-THOD accumulated with a maximum 16 h after treatment. No significant variation was observed after treatment by CCF (Fig. 5).
Time course of accumulation of 9(S)-HOT in potato (a) and tobacco (b) cells after treatment with CCF (200 μg ml−1), LPS (200 μg ml−1) and water (control). Values are the means of replicates of two or three independent experiments ± standard error. Bars with different stars superscript are significantly different (Tukey HSD, ***p = 0.001, **p = 0.05)
Time course of accumulation of 9,10,11-THOD in potato cells after treatment with CCF (200 μg ml−1), LPS (200 μg ml−1) and water (control). Values are the means of replicates of two or three independent experiments ± standard error. Bars with different stars superscript are significantly different (Tukey HSD, ***p = 0.001)
Colneleic acid increased in CCF-treated potato cells (Fig. 6a). The maximum is reached 16 h after treatment (1.3 nmol g−1 FW) and then CA was no more detectable. In our experiments colnelenic acid was not induced. In tobacco cells, CCF induced a rapid and transient increase of the two divinyl ether colneleic and colnelenic acids (Fig. 6b, c). The most significant increase was observed for colneleic acid, 24 h after treatment. Its amount raised to 100 nmol g−1 FW and slowly decreased until 72 h. Colnelenic amounts varied in the same way and the maximum was reached at 16 and 24 h (6 nmol g−1 FW). Both colneleic and colnelenic acids were not induced in potato and tobacco cells treated with LPS or in controls.
Time course of accumulation of colneleic acid in potato (a) and tobacco (b) cells and colnelenic acid in tobacco (c) cells after treatment with CCF (200 μg ml−1). Colneleic and colnelenic acids were not detected in water and LPS treated cells. Values are the means of replicates of two or three independent experiments ± standard error
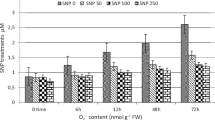
Effect of CCF and LPS on LOX activity
All challenges with elicitors were tested in comparison with water-treated cells (negative control). In potato cells challenged with CCF 1 μg ml−1, LOX activity reached a maximum rapidly, 18 h after treatment, and then decreased to the basal rate at the end of time (Fig. 7a). Capsicein (10 μg ml−1) alone induced a significant and strong activation of LOX activity with a maximum 24 h after treatment. This activity then decreased at 42 h (about 4 nkat g−1 FW to 2 nkatal g−1 FW). Cryptogein induced a slight but not significant activity of LOX. In contrast, in tobacco cells, the challenge with 1 μg ml−1 CCF induced a significant increase of LOX from 18 h to reach a maximum (2.5 nkat g−1 FW) 72 h after treatment (Fig. 7b). Cryptogein and capsicein used as positive controls induced similar patterns of LOX activation. With both elicitors, LOX activity increased to 3 nkat g−1 FW and it decreased 42 h after treatment with capsicein.
Lipoxygenase (LOX) activity induced in potato (a) and tobacco (b) cells after treatment with CCF (1 μg ml−1), elicitins (capsicein and cryptogein 10 μg ml−1) and water (control). LOX activity was calculated in nkatal/g FW. Values are means of one or two independent experiments with two replicates each ± standard error. Bars with different stars superscript are significantly different (Tukey HSD, ***p = 0.001, **p = 0.05). NM is for non measured
Discussion
In our study, we compared the induction of the oxylipin pathway in tobacco and potato cells by analysis of 9-LOX products and LOX activity. To confirm the cell viability, PAL activity was measured in the two Solanaceae cell suspensions challenged by LPS and CCF. PAL activity was strongly induced in tobacco cells by both elicitors while only CCF activated PAL in potato cells. This is in accordance with Kröner et al. (2011) who showed that in potato tubers, PAL activity increased and reached a maximum 7.5 h after CCF treatment, but not with LPS.
Basal levels of PUFAs varied between potato and tobacco cells
At 0 h, in potato cells induced with water (control), GC–MS analysis revealed a high amount of free PUFAs (stearic, linoleic and linolenic acids). The same results were reported by Griffiths et al. (2000) in potato leaves but the LA and LnA concentrations are inversed. Our results showed that linoleic acid quantities were more abundant than linolenic acid quantities that were found predominant in potato leaves (Griffiths et al. 2000; Göbel et al. 2002). In tobacco cells basal levels are very low. Basal level of PUFAs could depend on Solanaceae species.
LPS only induced 9,10,11 THOD in oxylipin pathway
LPS only induced 9,10,11-THOD at 16 h in potato cells but had no effect on the other studied oxylipins. This trihydroxyoxylipin could have an antimicrobial activity as shown by Kato et al. (1985) for 9,12,13-THOD and 9,12,13-THOE. These compounds were induced in the rice inoculated by Pyricularia grisea. LPS did not induce LOX activity in both cell suspensions. In the same way, Desender et al. (2006) showed that LPS did not induce LOX activity in both potato and tobacco cells but only in tomato cells. However, these authors showed that LPS caused a significant acidification of potato extracellular media, but did not induce the formation of ROS. In our study, PAL activity increased in tobacco cells treated with LPS, but not in potato cells. Such results were also observed for PAL induction in different potato cultivars elicited by LPS (Kröner et al. 2011). On the contrary, in potato cells, LPS induced a decrease of free PUFAs.
CCF induced the oxylipin pathway differentially in potato and tobacco cells
In both cell cultures, CCF induced LOX activities and oxylipin accumulation. We used different concentrations of CCF for the experiments. For oxylipin induction we used CCF and LPS at 200 μg ml−1 to compare the effect of two elicitors. For LOX activity, we previously tested different concentrations for CCF but no difference was observed between 1 μg ml−1 and the highest concentration. This result suggests that a minimal concentration is necessary to induce LOX activity and probably oxylipin pathway.
Our results showed in elicitor-treated potato cells an activation of LOX activity with a maximum at 18 h as described by Desender et al. (2006). Metabolite profiling using GC–MS revealed that high amounts of free PUFAs are constitutively present but these high amounts of LA and LnA are not correlated with an increase of oxylipins after elicitation. CCF and LPS induced a decrease of LA and LnA amounts suggesting that (i) this response is not elicitor-specific (ii) lipases which release free unsaturated fatty acids are not activated in potato cell suspensions. These enzymes, crucial for the production of oxylipins, are often involved in the regulation of defense reactions in higher plants (Roy et al. 1995; Kallenbach et al. 2010) and mammals (Schaloske and Dennis 2006). No subsequent increase of LOX products was observed except for 9(S)-HOT at 8 h suggesting that (i) other enzymes involved in the formation of LOX products (such as divinyl ether synthase, epoxy alcohol synthase or reductase) are not present or induced or (ii) fatty acids are metabolized by other reactions, such as conjugation with the most abundant cellular thiol, glutathione or remobilized in phospholipids and thus are not implicated in the oxylipin pathway.
In contrast, in tobacco cells, basal levels of PUFAs are very low and the release of high amounts of substrates is followed by a rise of LOX activity from 8 h and an increase in LOX products upon elicitation. Accumulation of oxylipins is often correlated with increased amounts of LnA and LA (Conconi et al. 1996; Ryu and Wang 1998; Zien et al. 2001). High release of LA and LnA probably resulted in an activation of galactolipases (EC 3.1.1.26) from chloroplastic membranes. Cacas et al. (2005) showed that fatty acids hydroperoxide accumulation in cryptogein-elicited leaves was preceded by the coordinated rise in 9-LOX and galactolipase activities. Besides, Yaeno et al. (2004) found that LnA activated in vitro NADPH oxidase responsible for ROS generation. They pointed out the major role of these PUFAs in plants responses. In addition it was demonstrated that 9-HOT enhances ROS production suggesting the participation of the 9-LOX oxylipin pathway in controlling oxidative stress and lipid peroxidation and plant defense (López et al. 2011).
LPS and CCF perception is different in potato and tobacco cells
LPS and CCF were differently perceived by the two cell suspensions. The CCF contained an α-elicitin, infestin, and a mix of oligosaccharides (data not shown). Ponchet et al. (1999) showed that elicitins induced defense reactions. They are generally structurally similar to lipid-transfer proteins of plant cells (Blein et al. 2002). A biochemical characterization of a specific binding site to cryptogein on tobacco cell membranes revealed that it is a glycoprotein (Bourque et al. 1999). For N. benthamiana, Kanzaki et al. (2008) suggested that the “lectin-like receptor kinase” (NbLRK1) contribute to the perception of infestin INF1. In the same way, Kim et al. (2010) identified in N. glutinosa, NgRLK1, a potential receptor to capsicein from P. capsici. In tobacco cells, the perception of cryptogein from P. cryptogea is followed by activation of protein kinases or inhibition of protein phosphatases. These modifications trigger early signaling events including fluxes of ions, variations in free calcium concentrations, MAPK activation, and production of nitric oxide or active oxygen species (Garcia-Brugger et al. 2006; Plešková et al. 2011). In most Nicotiana species, these events lead to HR (Attard et al. 2008). All these results suggest that cell suspensions of N. tabacum cv. BY-2 have receptors for elicitins which could be involved in the activation of the oxylipin pathway. In potato cells elicitin receptors have never been described.
After treatment with LPS, only 9,10,11-TriHOD is induced in potato cells. In tobacco cells, neither LOX activity nor oxylipin accumulation was observed. These results could reflect the ability of the cells to recognize the LPS. The mechanisms of recognition of LPS in plants are still unknown and the consequent transduction steps remain unclear (Erbs and Newman 2012). Gross et al. (2005) showed that the LPS from Xanthomonas campestris pv campestris was internalized 2 h after its addition in tobacco cells. The authors suggested that the LPS could contribute to the defense responses regulation. In mammals, Poltorak et al. (1998) identified a Toll-like receptor 4 (TLR4) as a lipid A receptor, but to date no LPS receptor has been isolated in plants. A potentially different perception between potato and tobacco could explain the differential activation of the oxylipin pathway.
In conclusion, we showed that CCF strongly activated the oxylipin pathway in tobacco cells and in a lesser extent in potato cells. LPS induced only 9,10,11-THOD in potato cells. The oxylipin pathway could be involved in the defense responses against pathogens in both cell suspensions. Our results showed that cell cultures could discriminate between LPS and CCF. Major differences in the PAMPs perception between tobacco and potato cells could induce plant-specific signal transduction cascades. Tobacco cell cultures are well suited to study CCF induction of the oxylipin pathway. To confirm the potential involvement of the oxylipin pathway in potato cells, we could test other PAMPs as P. atrosepticum culture filtrate. On the other hand, to compare the role of the oxylipin pathway in potato and tobacco defense responses, it is now necessary to use whole plants inoculated with specific pathogens.
Abbreviations
- CCF:
-
Concentrated culture filtrate
- Cv:
-
Cultivar
- HOD:
-
Hydroxy octadecadienoic acid
- HOE:
-
Hydroxy octadecenoic acid
- HOT:
-
Hydroxy octadecatrienoic acid
- HPO:
-
Hydroperoxide
- HPOD:
-
Hydroperoxy octadecadienoic acid
- HPOT:
-
Hydroperoxy octadecatrienoic acid
- HR:
-
Hypersensitive response
- LAH:
-
Lipid acyl hydrolase
- LOX:
-
Lipoxygenase
- LPS:
-
Lipopolysaccharide
- PAL:
-
Phenylalanine ammonia-lyase
- PLA:
-
Phospholipase
- Pv:
-
Pathovar
- PUFA:
-
Polyunsaturated fatty acid
- ROS:
-
Reactive oxygen species
References
Andreou A, Feussner I (2009) Lipoxygenases—structure and reaction mechanism. Phytochemistry 70(13–14):1504–1510. doi:10.1016/j.phytochem.2009.05.008
Attard A, Gourgues M, Galiana E, Panabières F, Ponchet M, Keller H (2008) Strategies of attack and defense in plant-oomycete interactions, accentuated for Phytophthora parasitica Dastur (syn. P. Nicotianae Breda de Haan). J Plant Physiol 165(1):83–94. doi:10.1016/j.jplph.2007.06.011
Blée E (2002) Impact of phyto-oxylipins in plant defense. Trends Plant Sci 7(7):315–322
Blein J-P, Coutos-Thévenot P, Marion D, Ponchet M (2002) From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci 7(7):293–296
Bourque S, Binet MN, Ponchet M, Pugin A, Lebrun-Garcia A (1999) Characterization of the cryptogein binding sites on plant plasma membranes. J Biol Chem 274(49):34699–34705. doi:10.1074/jbc.274.49.34699
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Cacas J-L, Vailleau F, Davoine C, Ennar N, Agnel J-P, Tronchet M, Ponchet M, Blein J-P, Roby D, Triantaphylides C, Montillet J-L (2005) The combined action of 9 lipoxygenase and galactolipase is sufficient to bring about programmed cell death during tobacco hypersensitive response. Plant Cell Environ 28(11):1367–1378
Christie WW (1993) Preparation of ester derivatives of fatty acids for chromatographic analysis. Adv Lipid Methodol 2:69–111
Conconi A, Miquel M, Browse JA, Ryan CA (1996) Intracellular levels of free linolenic and linoleic acids increase in tomato leaves in response to wounding. Plant Physiol 111(3):797–803. doi:10.1104/pp.111.3.797
Desender S, Klarzynski O, Potin P, Barzic M-R, Andrivon D, Val F (2006) Lipopolysaccharides of Pectobacterium atrosepticum and Pseudomonas corrugata induce different defence response patterns in tobacco, tomato, and potato. Plant Biol 8(5):636–645
Desender S, Andrivon D, Val F (2007) Activation of defence reactions in Solanaceae: where is the specificity? Cell Micro 9(1):21–30
Dhondt S, Gouzerh G, Müller A, Legrand M, Heitz T (2002) Spatio-temporal expression of patatin-like lipid acyl hydrolases and accumulation of jasmonates in elicitor-treated tobacco leaves are not affected by endogenous levels of salicylic acid. Plant J 32(5):749–762. doi:10.1046/j.1365-313X.2002.01465.x
Erbs G, Newman M-A (2012) The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe-associated molecular patterns (MAMPs), in plant innate immunity. Mol Plant Pathol 13(1):95–104. doi:10.1111/j.1364-3703.2011.00730.x
Fammartino A, Cardinale F, Gobel C, Mene-Saffrane L, Fournier J, Feussner I, Esquerre-Tugaye M-T (2007) Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol 143(1):378–388. doi:10.1104/pp.106.087304
Fammartino A, Verdaguer B, Fournier J, Tamietti G, Carbonne F, Esquerré-Tugayé M-T, Cardinale F (2010) Coordinated transcriptional regulation of the divinyl ether biosynthetic genes in tobacco by signal molecules related to defense. Plant Physiol Biochem 48(4):225–231
Feussner I, Wasternack C (2002) The lipoxygenase pathway. Annu Rev Plant Biol 53(1):275–297. doi:doi:10.1146/annurev.arplant.53.100301.135248
Fournier J, Pouénat M-L, Rickauer M, Rabinovltch-Chable H, Rigaud M, Esquerré-Tugayé M-T (1993) Purification and characterization of elicitor-induced lipoxygenase in tobacco cells. Plant J 3(1):63–70. doi:10.1111/j.1365-313X.1993.tb00011.x
Galliard T (1971) The enzymic deacylation of phospholipids and galactolipids in plants. Purification and properties of a lipolytic acyl-hydrolase from potato tubers. Biochem J 121:379–390
Gaquerel E, Hervé C, Labrière C, Boyen C, Potin P, Salaün J-P (2007) Evidence for oxylipin synthesis and induction of a new polyunsaturated fatty acid hydroxylase activity in Chondrus crispus in response to methyl jasmonate. Biochim Biophys Acta 1771(5):565–575
Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant Microbe Interact 19(7):711–724. doi:10.1094/mpmi-19-0711
Göbel C, Feussner I, Schmidt A, Scheel D, Sanchez-Serrano J, Hamberg M, Rosahl S (2001) Oxylipin profiling reveals the preferential stimulation of the 9-lipoxygenase pathway in elicitor-treated potato cells. J Biol Chem 276(9):6267–6273. doi:10.1074/jbc.M008606200
Göbel C, Feussner I, Hamberg M, Rosahl S (2002) Oxylipin profiling in pathogen-infected potato leaves. Biochim Biophys Acta 1584(1):55–64
Göbel C, Feussner I, Rosahl S (2003) Lipid peroxidation during the hypersensitive response in potato in the absence of 9-lipoxygenases. J Biol Chem 278(52):52834–52840. doi:10.1074/jbc.M310833200
Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J (2000) The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J 23(4):441–450. doi:10.1046/j.1365-313x.2000.00804.x
Griffiths G, Leverentz M, Silkowski H, Gill N, Sánchez-Serrano JJ (2000) Lipid hydroperoxide levels in plant tissues. J Exp Bot 51(349):1363–1370. doi:10.1093/jexbot/51.349.1363
Gross A, Kapp D, Nielsen T, Niehaus K (2005) Endocytosis of Xanthomonas campestris pathovar campestris lipopolysaccharides in non-host plant cells of Nicotiana tabacum. New Phytol 165(1):215–226. doi:10.1111/j.1469-8137.2004.01245.x
Hamberg M (2011) Stereochemistry of hydrogen removal during oxygenation of linoleic acid by singlet oxygen and synthesis of 11(< i > s </i >)-deuterium-labeled linoleic acid. Lipids 46(2):201–206. doi:10.1007/s11745-010-3510-4
Hamberg M, Sanz A, Rodriguez MJ, Calvo AP, Castresana C (2003) Activation of the fatty acid α-dioxygenase pathway during bacterial infection of tobacco leaves. J Biol Chem 278(51):51796–51805. doi:10.1074/jbc.M310514200
Ingle RA, Carstens M, Denby KJ (2006) PAMP recognition and the plant-pathogen arms race. BioEssays 28(9):880–889. doi:10.1002/bies.20457
Kallenbach M, Alagna F, Baldwin IT, Bonaventure G (2010) Nicotiana attenuata SIPK, WIPK, NPR1, and fatty acid-amino acid conjugates participate in the induction of jasmonic acid biosynthesis by affecting early enzymatic steps in the pathway. Plant Physiol 152(1):96–106. doi:10.1104/pp.109.149013
Kanzaki H, Saitoh H, Takahashi Y, Berberich T, Ito A, Kamoun S, Terauchi R (2008) NbLRK1, a lectin-like receptor kinase protein of Nicotiana benthamiana, interacts with Phytophthora infestans INF1 elicitin and mediates INF1-induced cell death. Planta 228(6):977–987. doi:10.1007/s00425-008-0797-y
Kato T, Yamaguchi Y, Abe N, Uyehara T, Namai T, Kodama M, Shiobara Y (1985) Structure and synthesis of unsaturated trihydroxy C-18 fatty-acids in rice plant suffering from rice blast disease. Tetrahedron Let. 26:2357–2360
Kenton P, Mur LAJ, Atzorn R, Wasternack C, Draper J (1999) (−)-Jasmonic acid accumulation in tobacco hypersensitive response lesions. Mol Plant Microbe Interact 12(1):74–78. doi:10.1094/mpmi.1999.12.1.74
Kim Y-T, Oh J, Kim K-H, Uhm J-Y, Lee B-M (2010) Isolation and characterization of NgRLK1, a receptor-like kinase of Nicotiana glutinosa that interacts with the elicitin of Phytophthora capsici. Mol Biol Rep 37(2):717–727. doi:10.1007/s11033-009-9570-y
Kishimoto K, Matsui K, Ozawa R, Takabayashi J (2008) Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 69(11):2127–2132
Kolomiets MV, Chen H, Gladon RJ, Braun EJ, Hannapel DJ (2000) A leaf lipoxygenase of potato induced specifically by pathogen Infection. Plant Physiol 124(3):1121–1130. doi:10.1104/pp.124.3.1121
Kröner A, Hamelin G, Andrivon D, Val F (2011) Quantitative resistance of potato to Pectobacterium atrosepticum and Phytophthora infestans: integrating PAMP-triggered response and pathogen growth. PLoS ONE 6(8):e23331
Le Quéré V, Plée-Gautier E, Potin P, Madec S, Salaün J-P (2004) Human CYP4F3s are the main catalysts in the oxidation of fatty acid epoxides. J Lipid Res 45(8):1446–1458. doi:10.1194/jlr.M300463-JLR200
Liavonchanka A, Feussner I (2006) Lipoxygenases: occurrence, functions and catalysis. J Plant Physiol 163(3):348–357. doi:DOI:10.1016/j.jplph.2005.11.006
López MA, Vicente J, Kulasekaran S, Vellosillo T, Martínez M, Irigoyen ML, Cascón T, Bannenberg G, Hamberg M, Castresana C (2011) Antagonistic role of 9-lipoxygenase-derived oxylipins and ethylene in the control of oxidative stress, lipid peroxidation and plant defence. Plant J 67(3):447–458. doi:10.1111/j.1365-313X.2011.04608.x
Mène-Saffrané L, Esquerre-Tugaye M-T, Fournier J (2003) Constitutive expression of an inducible lipoxygenase in transgenic tobacco decreases susceptibility to Phytophthora parasitica var. nicotianae. Mol Breed 12:271–282. doi:10.1023/B:MOLB.0000006754.19398.d4
Montillet J-L, Agnel J-P, Ponchet M, Vailleau F, Roby D, Triantaphylidès C (2002) Lipoxygenase-mediated production of fatty acid hydroperoxides is a specific signature of the hypersensitive reaction in plants. Plant Physiol Biochem 40(6–8):633–639
Mosblech A, Feussner I, Heilmann I (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem 47(6):511–517. doi:DOI:10.1016/j.plaphy.2008.12.011
Pinot F, Salaün J-P, Bosch H, Lesot A, Mioskowski C, Durst F (1992) ω-Hydroxylation of Z9-octadecenoic, Z9,10-epoxystearic and 9,10-dihydroxystearic acids by microsomal cytochrome P450 systems from Vicia sativa. Biochem Biophys Res Commun 184(1):183–193
Plešková V, Kašparovský T, Obořil M, Ptáčková N, Chaloupková R, Ladislav D, Damborský J, Lochman J (2011) Elicitin-membrane interaction is driven by a positive charge on the protein surface: role of Lys13 residue in lipids loading and resistance induction. Plant Physiol Biochem 49(3):321–328
Poltorak A, He XL, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282(5396):2085–2088. doi:10.1126/science.282.5396.2085
Ponchet M, Panabières F, Milat ML, Mikes V, Montillet JL, Suty L, Triantaphylides C, Tirilly Y, Blein JP (1999) Are elicitins cryptograms in plant-oomycete communications? Cell Mol Life Sci 56(11):1020–1047. doi:10.1007/s000180050491
Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ, Kift N, Carbonne F, Griffiths G, Esquerre-Tugaye M-T, Rosahl S, Castresana C, Hamberg M, Fournier J (2005) Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol 139(4):1902–1913. doi:10.1104/pp.105.066274
Rickauer M, Brodschelm W, Bottin A, Véronési C, Grimal H, Esquerré-Tugayé MT (1997) The jasmonate pathway is involved differentially in the regulation of different defence responses in tobacco cells. Planta 202(2):155–162. doi:10.1007/s004250050114
Roy S, Pouénat M-L, Caumont C, Cariven C, Prévost M-C, Esquerré-Tugayé M-T (1995) Phospholipase activity and phospholipid patterns in tobacco cells treated with fungal elicitor. Plant Sci 107(1):17–25
Rustérucci C, Montillet J-L, Agnel J-P, Battesti C, Alonso B, Knoll A, Bessoule J–J, Etienne P, Suty L, Blein J-P, Triantaphylidès C (1999) Involvement of lipoxygenase-dependent production of fatty acid hydroperoxides in the development of the hypersensitive cell death induced by cryptogein on tobacco leaves. J Biol Chem 274(51):36446–36455. doi:10.1074/jbc.274.51.36446
Ryu SB, Wang X (1998) Increase in free linolenic and linoleic acids associated with phospholipase D-mediated hydrolysis of phospholipids in wounded castor bean leaves. Biochim Biophys Acta 1393(1):193–202
Schaller F (2001) Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J Exp Bot 52(354):11–23. doi:10.1093/jexbot/52.354.11
Schaloske RH, Dennis EA (2006) The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761(11):1246–1259
Stumpe M, Kandzia R, Göbel C, Rosahl S, Feussner I (2001) A pathogen-inducible divinyl ether synthase (CYP74D) from elicitor-treated potato suspension cells. FEBS Lett 507(3):371–376. doi:Doi:10.1016/s0014-5793(01)03019-8
Val F, Desender S, Bernard K, Potin P, Hamelin G, Andrivon D (2008) A culture filtrate of Phytophthora infestans primes defense reaction in potato cell suspensions. Phytopathology 98(6):653–658. doi:10.1094/PHYTO-98-6-0653
Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, Hamberg M, Castresana C (2007) Oxylipins produced by the 9-lipoxygenase pathway in arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell Online 19(3):831–846. doi:10.1105/tpc.106.046052
Weber H (2002) Fatty acid-derived signals in plants. Trends Plant Sci 7(5):217–224
Weber H, Chetelat A, Caldelari D, Farmer EE (1999) Divinyl ether fatty acid synthesis in late blight—diseased potato leaves. Plant Cell 11(3):485–494. doi:10.1105/tpc.11.3.485
Westphal O, Jann K (1965) Bacterial lipopolysaccharides: extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem 5:83–91
Yaeno T, Matsuda O, Iba K (2004) Role of chloroplast trienoic fatty acids in plant disease defense responses. Plant J 40(6):931–941. doi:10.1111/j.1365-313X.2004.02260.x
Zien CA, Wang C, Wang X, Welti R (2001) In vivo substrates and the contribution of the common phospholipase D, PLDα, to wound-induced metabolism of lipids in Arabidopsis. Biochim Biophys Acta 1530(2–3):236–248
Zucker M (1968) Sequential induction of phenylalanine ammonia-lyase and a lyase-inactivating system in potato tuber disks. Plant Physiol 43(3):365–374. doi:10.1104/pp.43.3.365
Acknowledgments
This work was funded through funds from the “Région Bretagne” PRIR Program, France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Petersen.
Rights and permissions
About this article
Cite this article
Saubeau, G., Goulitquer, S., Barloy, D. et al. Differential induction of oxylipin pathway in potato and tobacco cells by bacterial and oomycete elicitors. Plant Cell Rep 32, 579–589 (2013). https://doi.org/10.1007/s00299-012-1377-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1377-y