Abstract
Five intergeneric hybrids between the chrysanthemum cultivar ‘Zhongshanjingui’ (as female) and Ajania przewalskii (as male) were obtained with the help of embryo culture. While ‘Zhongshanjingui’ bears a standard anemone type flower and A. przewalskii a non-anemone type one, the inflorescence type of the hybrids varied. The diameter of the hybrids’ flowers was intermediate between those of the parents. The chromosome number of the hybrids was 2n = 45, of which GISH analysis was able to establish that 27 were inherited from ‘Zhongshanjingui’ and the other 18 from A. przewalskii. A combination of various assays was used to show that the cold tolerance of the hybrids was equivalent to that of the highly tolerant A. przewalskii parent. Enhanced cold tolerance was correlated with an increase in free proline and a decrease in malondialdehyde content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As cold stress is a major contributory factor in crop loss, breeding for low temperature tolerance has become a priority in a number of plant breeding programmes, especially in herbaceous perennial ornamentals (Still et al. 1988; Griesbach and Berberich 1995; Wang et al. 2003). The chrysanthemum (Chrysanthemum grandiflorum (Ramat.) Kitam., syn. Dendranthema morifolium (Ramat.) Tzvelv.) is a commercially important ornamental species, but most of the cultivars are not cold hardy (Anderson and Gesick 2004; Kim and Anderson 2006). The field-grown chrysanthemum roots and rhizomes become irreversibly damaged if the temperature falls below about −12ºC (Holley 1945), while controlled freezing studies have shown that acclimated rhizomes are injured at temperatures in the range −10 to −15ºC (Widmer 1958). Previously, efforts have been made to enhance the cold tolerance of chrysanthemum using intervarietal or interspecific crossing (Anderson 2007; Cheng et al. 2010). However, above efforts has enjoyed only limited success, because of the paucity of relevant genetic variation within the Chrysanthemum spp. gene pool (Zhao et al. 2009; Cheng et al. 2010). Intergeneric hybridization succeeded in improving aphid resistance of chrysanthemum (Deng et al. 2010a). Herein, we attempted to enhance the cold tolerance of chrysanthemum using intergeneric sexual cross.
Ajania represents a secondary genepool for chrysanthemum genetic improvement (Zhao et al. 2009). Some of the cross compatibility does exist between Ajania and Chrysanthemum (Fukai 2003; Zhao et al. 2009), and a number of Chrysanthemum × Ajania hybrids have been successfully obtained (Abd El-Twab et al. 1999; Zhao et al. 2008; Tang et al. 2010). However, these have for the most part been made to establish taxonomical relationships rather than being seen as a prelude to trait introgression. A. przewalskii Poljak. is native to China, and since its major range lies in the northwestern alpine and arid regions above an altitude of 2,800 m (Shih and Fu 1983), the species is expected to have a high level tolerance to low temperatures (Zhao et al. 2009). Unfortunately, strong post-fertilization barriers inhibit the formation of hybrids between Chrysanthemum spp. and A. przewalskii, such that soon after the formation of a hybrid embryo, the structure rapidly collapses (Deng et al. 2010b). Here, however, we show that it is possible to obtain this hybrid by rescuing the immature hybrid embryo, an approach which has also proven successful in a number of intergeneric cross combinations involving Chrysanthemum spp. (Tang et al. 2009, 2010; Deng et al. 2010a). We describe the morphology and cytology of the hybrid progeny, and compare their cold tolerance with that of their two parents.
Materials and methods
Plant materials, crossing and embryo rescue
The C. grandiflorum variety ‘Zhongshanjingui’ was used as the female parent and an accession of A. przewalskii as the male. ‘Zhongshanjingui’ is an important commercial cultivar with excellent ornamental quality owed to its standard anemone (SA) type inflorescence—but low cold tolerance, whereas A. przewalskii is extremely cold tolerant. Our previous study showed that the selected accession of A. przewalskii was the most compatible one with chrysanthemum as compared to others. All the plants were grown in a greenhouse (day/night temperature 25/18ºC, photoperiod 16 h, light intensity 50 μmol m−2 s−1, relative humidity 70%) at Nanjing Agricultural University, China. Crossing was performed following the method detailed by Deng et al. (2010b), using 15 female flowers (containing ~300 ray florets). Plump ovaries were removed from the female flower 10–12 days after pollination, and were surface-sterilized and washed, following Deng et al. (2010a). The ovary coat and integument were aseptically removed using a pair of dissecting needles under anatomic microscopy (SZ-T2, Olympus Optical Co. Ltd., Tokyo, Japan), and the rescued embryos were immediately transferred to Medium I (MS medium supplemented with 1.0 mg L−1 6-BA, 0.1 mg L−1 α-NAA). Shoots regenerated from embryos were transferred to Medium II (MS supplemented with 0.1 mg L−1 6-BA, 0.05 mg L−1 α-NAA) and cultured for 2–3 weeks; thereafter, vigorous shoots were moved to Medium III (MS supplemented with 2.0 mg L−1 6-BA, 0.5 mg L−1 α-NAA) for a further 3–4 weeks to allow proliferation. Then the proliferated shoots (~20 shoots per embryo) were transferred to Medium IV (half strength MS medium supplemented with 0.1 mg L−1 α-NAA) to promote rooting.
Hybridity testing
Hybridity testing initially considered the morphological traits, including flower type, plant height, crown width, leaf shape and flower shape. Flower type was classified as the SA, the mid-anemone (MA) type or the non-anemone (NA) type (Chen et al. 2009). Flower shape, derived from a measurement of ten inflorescences, was quantified in the form of a flower core diameter index, an inflorescence diameter index, ray floret quantity and tubular floret quantity (Li 1993). Leaf shape comprised a combination of length and width, measured on the fifth leaf below the apex, and sampled from ten leaves (Li 1993). The density and morphology of trichomes were assessed by inspection under stereo light microscopy (Zeiss Stemi 2000-C, Carl Zeiss Ltd., Jena, Germany), following Deng et al. (2010a).
Hybridity was then confirmed by counting mitotic chromosomes sampled from root tips, as described previously (Deng et al. 2010a). Young ~1 cm long root tips were pre-treated in ice water for 20–22 h, fixed in Carnoy’s fluid (3:1 ethanol : acetic acid (v/v)), stored at 4ºC for 24 h and squashed in a drop of 45% (v/v) acetic acid. The resulting mitotic chromosome spreads were observed by phase contrast microscopy (BX41, Olympus Optical Co. Ltd., Tokyo, Japan). Some of the chromosome preparations were also subjected to genomic in situ hybridization (GISH), with the genomic DNA extracted from young leaves using the CTAB method (Doyle and Doyle 1987). A. przewalskii DNA was labelled with fluorescein-12-dUTP (Roche Ltd., Berlin, Germany) by nick translation following the manufacturer’s protocol, and unlabelled maternal chrysanthemum DNA was used for blocking. The GISH procedure followed that described by Heslop-Harrison et al. (1991) and Tang et al. (2009). Cover slips were removed by the liquid nitrogen freezing method and the preparations were dried at room temperature, and then denatured in 75% (v/v) deionized formamide at 78ºC for 70 s, dehydrated by passing through an ethanol series (each 5 min in 75, 95 and 100% v/v) and air-dried once more at room temperature. The hybridization mixture was denatured by boiling for 7 min, and then quenched for 15 min. A 15 μl aliquot of the hybridization solution was applied to each slide, which was covered with a 20 × 20 mm cover slip and incubated in a humid chamber at 37ºC for 12 h. The slides were, thereafter, washed four times in 2× SSC at 42ºC and in 1× PBS for 5 min at room temperature. After air drying at room temperature, the preparations were finally stained with 100 μg ml−1 propidium iodide (Sigma Co. Ltd.) for 5 min and washed with 1× PBS. The resulting preparations were inspected by fluorescence microscopy (Zeiss Axioskop 40, Carl Zeiss Ltd., Jena, Germany), in which the labelled DNA fluoresced greenish and the unlabelled DNA appeared red.
Evaluation of cold tolerance
The number of emerged rhizomes (NER) of ten field-grown plants of each hybrid line was counted (Anderson and Gesick 2004). Following this, a semi-lethal low temperature (LT50) was determined using 10 g young leaves emerging from uniformly growing rhizomes of each parental and putative hybrid plant, according to methods described by Cheng et al. (2010) with minor revision. The temperature treatments were 0, −5, −10, −15, −20 and −25ºC. Then, the electrical conductivity (EC) both before and after boiling was measured for each temperature treatment using a dedicated meter (Model: DDS-320, KangYi Ltd., Shanghai, China). The relative EC (REC %) = (EC before boiling/EC after boiling) × 100. REC% and temperature gradient fitted Logistic Equation, y = K/(1 + ae −bx), where ‘x’ represents the treatment temperature, ‘K’ is saturation capacity of cell damage rate, and ‘a, b’ is equation parameters, was calculated using Rcpsys software (Gai 2000). The LT50 was given by the expression ln(1/a)/b. Each measure represented the mean of four replicates.
The survival rate (SR, %) of detached rhizomes subjected to a period of low temperature was assessed following the method suggested by Kim and Anderson (2006). A sample of 30 rhizome segments per hybrid, each of ~5 cm in length was held at 0ºC for 2 h, then chilled over 2 h to reach −5ºC, at which temperature it was then held for 1 h; the temperature was thereafter reduced over a further 2 h to −10ºC, at which temperature the material was held for 1 h; finally the rhizome segments were thawed at 4ºC for 24 h. The material was then planted in an artificial climate chamber (day/night temperature 24/18ºC, photoperiod 14 h, light intensity 50 μmol m−2 s−1, relative humidity 85%) and the number of surviving rhizomes was counted after a further 15 days. All low temperature treatments were conducted in a Programmable and Digital Temperature Controller (Model: 9610; PolyScience Inc., Niles, Illinois, USA).
Proline and malondialdehyde content
Three replicate samples of ~0.5 g fresh leaves were detached from the young plants of each line held at 0, −5, −10, −15, −20 and −25ºC, for the analysis of leaf free proline and malondialdehyde (MDA) content. The analysis relied, respectively, on the acid ninhydrin (Bates et al. 1973) and the thiobarbituric acid methods (Li 2000), with minor modification. For the former, samples were homogenized in 3% (w/v) aqueous sulfosalicylic acid and the homogenate was boiled at 100ºC for 10 min. Then the homogenate was cooled down to room temperature and centrifuged at 4,000g for 10 min. The reaction mixture consisted of 2 ml supernatant, 3 ml 2.5% (w/v) acid ninhydrin and 2 ml of glacial acetic acid, and was boiled at 100ºC for 40 min. Subsequently, the reaction was quenched on ice, the mixture was extracted with 4 ml of toluene and the absorbance was read at 520 nm. For the later, each sample was first ground in a mortar along with 10% (w/v) trichloroacetic acid, and the homogenate was then centrifuged at 4,000g for 30 min. A 2 ml supernatant was mixed with 2 ml 10% (w/v) trichloroacetic acid containing 0.5% (w/v) thiobarbituric acid. Then the mixture was boiled at 100ºC for 30 min, and the absorbance was read at 532 nm. The values were corrected by subtracting the absorbance at 600 nm, and the MDA content was calculated according to its extinction coefficient.
Statistical analysis
All data were analysed by a one-way analysis of variance using the software package SYSTAT v7.0 (SYSTAT 1997). Tukey’s Honestly Significant Difference test was applied to identify significantly different trait values.
Results
Embryo rescue and in vitro culture
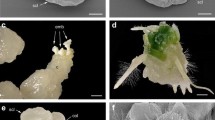
At 10–12 days after pollination, the ~0.5 mm long immature embryos in plump ovaries appeared white and almost transparent, and had reached either the torpedo or the cotyledon embryonic stage (Fig. 1a). Of 120 embryos cultured, 46 germinated to some extent during their first 2 weeks in vitro, and grew to a height of ~5 mm with both cotyledons visible (Fig. 1b). However, after a further week they started to become bleached, especially the stem tip and the emerging true leaves (Fig. 1c). When transferred to Medium II for 2 weeks, some of the buds became green again (Fig. 1d). Further culture on Media III and IV promoted bud proliferation (Fig. 1e) and rooting (Fig. 1f). Finally, five putative hybrid lines with well developed roots were selected and potted into autoclaved vermiculite in the greenhouse.
Shoot growth from rescued embryos of the intergeneric cross C. grandiflorum ‘Zhongshanjingui’ × A. przewalskii. a Embryos freshly exacted from ovaries. b Shoots with two cotyledons developing on Medium I. c Shoots became bleached before d their transfer to Medium II. e Proliferating and f rooting shoots. Bars 5 mm
Morphology of the putative hybrids
The plants flowered in the autumn, but varied with respect to their actual flowering date (Table 1). While the ‘Zhongshanjingui’ inflorescence consisted of an outer ring of ray florets and a central mass of elongated and pigmented SA type disc florets (Fig. 2a), and the A. przewalskii one developed short ray florets and NA type disc florets (Fig. 2b), the five hybrids exhibited a mixture of SA, MA and NA types disc florets (Fig. 2c–h; Table 1). The mean plant height of ‘Zhongshanjingui’ was 59.4 cm and its corolla width was 94.8 cm, while those of A. przewalskii were, respectively, 40.6 and 70.1 cm; these two traits were also highly variable among the hybrids (Table 1). Both the leaf length and width of ‘Zhongshanjingui’ were greater than those of A. przewalskii, and the hybrids were intermediate in terms of these traits (Table 1). The hybrids’ leaf shape was rather similar to that of male parent A. przewalskii (Fig. 3a, b). ‘Zhongshanjingui’ leaves were only sparsely covered by trichomes, while the hybrid leaves were quite densely covered, giving them a silvery appearance, similar to that of A. przewalskii leaf (Fig. 3c–e).
Inflorescences of a C. grandiflorum ‘Zhongshanjingui’ (a standard anemone type (SA) inflorescence), b A. przewalskii (a non-anemone type (NA)), and (c–g) the five ‘Zhongshanjingui’ × A. przewalskii intergeneric hybrids. The hybrid inflorescence types varied, including SA, mid-anemone (MA) and NA types. h Various disc florets formed. Bars 10 mm
Chromosome number and GISH analysis
The somatic chromosome number of ‘Zhongshanjingui’ is 54 (Deng et al. 2010a), while that of A. przewalskii is 36 (Fig. 4a). As expected, all of the hybrids showed 2n = 45 (Fig. 4b). The GISH technique was unable to differentiate between the parental genomes without blocking, but worked successfully with the addition of blocking DNA up to the ratio of 50:1 of unlabelled chrysanthemum genomic DNA to labelled A. przewalskii genomic DNA. The application of the GISH technique confirmed that 18 of the hybrids’ chromosomes were inherited from A. przewalskii and 27 from ‘Zhongshanjingui’ (Fig. 4c, d).
GISH analysis of mitotic chromosomes. (a) A. przewalskii (2n = 36). b All 45 chromosomes present in the ‘Zhongshanjingui’ × A. przewalskii intergeneric hybrid nucleus counter-stained with propidium iodide. (c) An intergeneric hybrid cell containing 18 labeled chromosomes inherited from A. przewalskii. (d) Merged image of (b) and (c), showing the 18 A. przewalskii chromosomes indicated by (arrow heads) and the 27 non-hybridized ‘Zhongshanjingui’ chromosomes. Bars 5 μm
Cold tolerance of the hybrid plants
The mean NER of ‘Zhongshanjingui’ was 7.7, while that of A. przewalskii was 34.5 (Fig. 5a, b; Table 2). The five hybrids produced an NER of between 40.8 and 46.3 (Fig. 5c–g; Table 2), demonstrating a level of cold tolerance even higher than that of the cold tolerant A. przewalskii. The LT50 of ‘Zhongshanjingui’ and A. przewalskii was, respectively, −9.2 and −21.1ºC, while those of the five hybrids were from −17.7 to −21.4ºC, none of which differed significantly from that of A. przewalskii, but all of which were much greater than that of ‘Zhongshanjingui’ (Table 3). Finally, the recovery test revealed that the hybrids’ SR (88.3-93.3%) was similarly to that of A. przewalskii (95.0%), but substantially >that of ‘Zhongshanjingui’ (16.7%) (Table 3).
Leaf proline and MDA content under conditions of low temperature
The leaf proline content of the parents and the five hybrids was affected by cold stress, but the dynamics of these changes differed between the lines. The leaves of ‘Zhongshanjingui’ consistently contained the least proline, which accumulated steadily as the temperature fell, but stabilized once a temperature of −15ºC had been reached. In contrast, the proline content in A. przewalskii leaves increased rapidly at temperatures below −10ºC. In the five hybrids, the leaf proline content also increased as the temperature was lowered, but the extent of the change was much less drastic than in A. przewalskii (Fig. 6a). The content of MDA increased in the leaves of all plants as the temperature was reduced. In A. przewalskii, the content of MDA and its rate of increase were least over the temperature range 0 to −15ºC. The hybrids’ performance was intermediate between that of the two parents; and their leaf MDA content was consistently significantly lower than that in ‘Zhongshanjingui’ leaves. Their performance resembled that of A. przewalskii over the temperature range 0 to −15ºC, and that of ‘Zhongshanjingui’ over the range −15 to −25ºC (Fig. 6b).
Discussion
Embryo rescue as a technique for chrysanthemum improvement
As one of the most effective methods to overcome hybrid breakdown, embryo rescue has been widely applied in plant improvement programmes, including in chrysanthemum. The embryo rescue technique includes ovary culture, ovule culture and embryo culture (Sharma et al. 1996). Where embryo abortion occurs as early as the globular or heart-shaped embryo stage, it has proven difficult to culture them directly (Raghavan 2007). In such cases, the embryo can sometimes be rescued by either culturing the ovary (Cheng et al. 2010) or ovule (Deng et al. 2010a). However, embryos which remain normal until the torpedo, cotyledon or mature embryo stage are generally amenable to direct embryo culture (Sharma et al. 1996; Raghavan 2007). In the case of the present ‘Zhongshanjingui’ × A. przewalskii hybrids, systematical anatomical studies showed that most of the embryos were able to develop up to the torpedo or even later stages by 10–12 days post pollination, making feasible the use of embryo culture. Embryo germination directly avoiding a period of callus formation and bud differentiation, the hybrid embryo culture method can save breeding time and effectively guarantee the progeny’s hybridity. However, the success of embryo culture depends largely on optimizing the in vitro culture technology, including the timing of intervention, aseptic dissecting operation and medium components, etc. A particular problem encountered with the present crossing combination was that the shoots started to bleach at early stage post germination (Fig. 1), which greatly compromised the success rate achieved.
Intergeneric hybridization as a means of improving abiotic tolerance in chrysanthemum
Wide hybridization (including interspecific and intergeneric hybridization) has been exploited to improve the level of abiotic stress tolerance in a number of crop species (Roy et al. 2004; Nakamura et al. 2005). Regarding to chrysanthemum, recent studies have reported enhancement of tolerance to cold (Cheng et al. 2010), drought (Sun et al. 2010) and heat stresses (Cheng et al. 2011) via different interspecific combinations. In the present study, the LT50 of chrysanthemum ‘Zhongshanjingui’ (around −9ºC) was higher than that of either A. przewalskii or the five intergeneric hybrids (around −20ºC) (Table 3). Meanwhile, the NER and SR of the hybrids were superior to that of their chrysanthemum parent (Tables 2, 3). The evidence, therefore, was conclusive that much of the cold tolerance of A. przewalskii was transferred to these intergeneric hybrids. Their level of cold tolerance was higher than that of an interspecific hybrid described by Cheng et al. (2010). Therefore, this study suggested that intergeneric sexual crossing with wild species is an effective way for developing chrysanthemum germplasm with improved abiotic tolerance.
According to a previous report, chrysanthemum cold tolerance has been identified as a quantitative trait under polygenic control with different regulating characteristic in interspecific hybrid progenies (Cheng et al. 2010). In this study, the five intergeneric hybrids varied somewhat among one another with respect to their LT50 (Table 3), as did their scores for both NER and SR. This variation implies that one or other (or both) of the hybrids’ parents were heterozygous for some or all of the genes underlying cold tolerance. The implication of this finding is that selection for greater cold tolerance should be possible among a population of intergeneric hybrid progeny, provided that a sufficient number of hybrids can be made; this represents one of our major future research goals.
Proline is frequently used by abiotically stressed plants as an osmotic regulator, and it serves as an osmotic protectant for a number of cellular structures during episodes of abiotic stress (Yue et al. 2001; Ueda et al. 2008). As a product of membrane lipid peroxidation, MDA content can be viewed as an indicator of the degree of cellular membrane lipid peroxidation occurring as a response to stress (Li 2000). The high proline content of cold stressed A. przewalskii leaves indicated that proline was synthesized as a means of regulating cell osmosis and metabolism, and thereby conferring enhanced cold tolerance; the effect of these adaptations is that cellular damage could be kept to a minimum under conditions of very low temperature. In contrast, the stressed leaves of ‘Zhongshanjingui’ contained much less proline and more MDA, consistent with its lesser level of winter hardiness (Fig. 6). Although the intergeneric hybrids showed similar levels of cold tolerance as that of A. przewalskii, their leaves responded variably to cold stress with respect to both proline and MDA content (Fig. 6). This suggests that the intergeneric hybrid may develop a physiological response to cold stress which is rather different from that of either of their parents.
Chrysanthemum cultivars are generally described as being either large- or small-flowered types, with the latter class comprising single, double, honeycomb and anemone type flowers (Chen et al. 2009). According to this classification, ‘Zhongshanjingui’ is a typical small-flowered SA type potted chrysanthemum, while A. przewalskii is an NA type (Fig. 2). The inflorescence type of the five intergeneric hybrids varied from line to line, with one forming SA, one MA and three NA flowers. The ornamental value of the hybrids, in terms of their inflorescence diameter, flower core diameter and the volume of ray and disc florets, was lower than that of ‘Zhongshanjingui’ (Table 1). In certain wide hybrids involving chrysanthemum, Cheng et al. (2011) were able to show that the first backcross hybrid was highly similar to the recurrent chrysanthemum in terms of inflorescence diameter and the number of ray florets, while its level of heat tolerance was significantly superior. Our intended strategy, therefore, is to perform a backcrossing programme with ‘Zhongshanjingui’, imposing selection for the cold tolerance of the donor and ornamental trait of the recurrent parent.
In summary, we have shown that the C. grandiflorum × A. przewalskii hybrid can be obtained using embryo rescue. The hybrids out-performed their chrysanthemum parent with respect to cold tolerance, a trait which is most conveniently measured by a LT50 test. At the same time, leaf proline and MDA content appeared to be reasonable indicators of cold tolerance. The intergeneric hybrids represent the basis of a breeding approach towards improving the level of low temperature tolerance in chrysanthemum, and also contribute to a more general expansion of the genetic base of this important ornamental species.
Abbreviations
- MA:
-
Mid-anemone type flower
- SA:
-
Standard anemone type flower
- NA:
-
Non-anemone type flower
- NER:
-
The number of emerged rhizomes
- GISH:
-
Genomic in situ hybridization
- EC:
-
Electrical conductivity
- REC:
-
The relative EC
- LT50 :
-
Semi-lethal low temperature
- Pro:
-
Proline
- MDA:
-
Malondialdehyde
- SR:
-
Survival rate
References
Abd El-Twab MH, Kondo K, Hong DY (1999) Isolation of a particular chromosome of Ajania remotipinna in a chromosome complement of an artificial F1 hybrid of Dendranthema lavandulifolia × Ajania remotipinna by use of genomic in situ hybridization. Chromosom Sci 3:21–28
Anderson NO (2007) Chrysanthemum (Dendranthema × grandiflora Tzvelv.). In: Anderson NO (ed) Flower breeding and genetics. Springer, Netherlands, pp 389–437
Anderson N, Gesick E (2004) Phenotypic markers for selection of winter hardy garden chrysanthemum (Dendranthema × grandiflora Tzvelv.) genotypes. Sci Hortic 101:153–167
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Chen FD, Li FT, Chen SM, Guan ZY, Fang WM (2009) Meiosis and pollen germinability in small-flowered anemone type chrysanthemum cultivars. Plant Syst Evol 280:143–151
Cheng X, Chen SM, Chen FD, Fang WM, Deng YM, She LF (2010) Interspecific hybrids between Dendranthema morifolium (Ramat.) Kitamura and D. nankingense (Nakai) Tzvel. achieved using ovary rescue and their cold tolerance characteristics. Euphytica 172:101–108
Cheng X, Chen SM, Chen FD, Deng YM, Fang WM, Tang FP, Liu ZL, Shao W (2011) Creating novel chrysanthemum germplasm via interspecific hybridization and backcrossing. Euphytica 177:45–53
Deng YM, Chen SM, Lu AM, Chen FD, Tang FP, Guan ZY, Teng NJ (2010a) Production and characterisation of the intergeneric hybrids between Dendranthema morifolium and Artemisia vulgaris exhibiting enhanced resistance to chrysanthemum aphid (Macrosiphoniella sanbourni). Planta 231:693–703
Deng YM, Teng NJ, Chen SM, Chen FD, Guan ZY, Song AP, Chang QS (2010b) Reproductive barriers in the intergeneric hybridization between Chrysanthemum grandiflorum (Ramat.) Kitam. and Ajania przewalskii Poljak. (Asteraceae). Euphytica 174:41–50
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 9:11–15
Fukai S (2003) Dendranthema species as Chrysanthemum genetic resources. Acta Hort 620:223–230
Gai JY (2000) Statistics for experiments. Agricultural Press, Beijing
Griesbach RJ, Berberich SM (1995) The early history of research on ornamental plants at the US Department of Agriculture from 1862 to 1940. Hortic Sci 30:421–425
Heslop-Harrison JS, Schwarzacher T, Anamthawat-Jonsson K, Leitch AR, Shi M, Letich IJ (1991) In situ hybridization with automated chromosome denaturation. Technor 3:109–116
Holley WD (1945) Winter hardiness in chrysanthemums. Plant Gard 1:163–164
Kim DC, Anderson NO (2006) Analysis of laboratory freezing methods to establish cold tolerance of detached rhizomes and intact crowns in garden chrysanthemums (Dendranthema grandiflora Tzvelv.). Sci Hortic 109:345–352
Li HJ (1993) Chrysanthemums in China. Jiangsu Scientific and Technical Press, Nanjing
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing, pp 258–261
Nakamura T, Kuwayama S, Tanaka S, Oomiya T, Saito H, Nakano M (2005) Production of intergeneric hybrid plants between Sandersonia aurantiaca and Gloriosa rothschildiana via ovule culture (Colchicaceae). Euphytica 142:283–289
Raghavan V (2007) Double fertilization: embryo and endosperm development in flowering plants (transl:Yang HY). Science Press, Beijing
Roy AK, Malaviya DR, Kaushal P, Kumar B, Tiwari A (2004) Interspecific hybridization of Trifolium alexandrinum with T. constantinopolitanum using embryo rescue. Plant Cell Rep 22:705–710
Sharma DR, Kaur R, Kumar K (1996) Embryo rescue in plants: a review. Euphytica 89:325–337
Shih C, Fu G (1983) Angiospermae, dicotyledoneae, compositae (3) anthemideae. In: Flora Reipublicae Popularis Sinicae, vol 76 (3). Science Press, Beijing
Still S, Disabato A, Brenneman G (1988) Cold hardiness of herbaceous perennials. Proc Int Plant Prop Soc 37:386–392
Sun CQ, Chen FD, Teng NJ, Liu ZL, Fang WM, Hou XL (2010) Interspecific hybrids between Chrysanthemum grandiflorum (Ramat.) Kitamura and C. indicum (L.) Des Moul. and their drought tolerance evaluation. Euphytica 174:51–60
SYSTAT (1997) SYSTAT, Version 7.0. SPSS Inc, Chicago
Tang FP, Chen FD, Chen SM, Teng NJ, Fang WM (2009) Intergeneric hybridization and relationship of genera within the tribe Anthemideae Cass. (I. Dendranthema crassum (Kitam.) Kitam. × Crossostephium chinense (L.) Makino). Euphytica 169:133–140
Tang FP, Chen SM, Deng YM, Chen FD (2010) Intergeneric hybridization between Dendranthema crassum and Ajania myriantha. Acta Hortic 855:267–272
Ueda A, Shi W, Shimada T, Miyake H, Takabe T (2008) Altered expression of barley proline transporter causes different growth responses in Arabidopsis. Planta 227:277–286
Wang WX, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Widmer RE (1958) The determination of cold resistance in the garden chrysanthemum and its relation to winter survival. Proc Am Soc Hortic Sci 71:537–546
Yue W, Xia G, Zhi D, Chen H (2001) Transfer of salt tolerance from Aeleuropus littorulis sinensis to wheat (Triticum aestivum L.) via asymmetric somatic hybridization. Plant Sci 161:259–266
Zhao HB, Chen FD, Fang WM, Guo WM, Xie W (2008) Creating novel germplasms of chrysanthemum by employing the Ajania pacifica. Sci Agric Sin 41:2077–2084
Zhao HE, Liu ZH, Hu X, Yin JL, Li W, Rao GY, Zhang XH, Huang CL, Anderson N, Zhang QX, Chen JY (2009) Chrysanthemum genetic resources and related genera of Chrysanthemum collected in China. Genet Resour Crop Evol 56:937–946
Acknowledgments
This work was supported by the Basic Infrastructure Construction Plan of Science and Technology of Jiangsu Province (Grant No. BM2008008), the Shanghai Key Science and Technology for Agriculture Promotion Program (2009 No.3-3), the Fundamental Research Funds for the Central Universities (KYJ200907), Fund for Independent Innovation of Agricultural Sciences in Jiangsu Province (CX(10)114).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Register.
Rights and permissions
About this article
Cite this article
Deng, Y., Chen, S., Chen, F. et al. The embryo rescue derived intergeneric hybrid between chrysanthemum and Ajania przewalskii shows enhanced cold tolerance. Plant Cell Rep 30, 2177–2186 (2011). https://doi.org/10.1007/s00299-011-1123-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1123-x