Abstract
Programmed cell death (PCD) is a precise, genetically controlled cellular process with important roles in plant growth, development, and response to biotic and abiotic stress. However, the genetic mechanisms that control PCD in plants are unclear. Two Arabidopsis genes, DAL1 and DAL2 (for Drosophila DIAP1 like 1 and 2), encoding RING finger proteins with homology to DIAP1 were identified, and a series of experiments were performed to elucidate their roles in the regulation of PCD and disease resistance. Expression of DAL1 and DAL2 genes was induced in Arabidopsis plants after inoculation with virulent and avirulent strains of Pseudomonas syrinage pv. tomato (Pst) DC3000 or after infiltration with fumonisin B1 (FB1). Plants with mutations in the DAL1 and DAL2 genes displayed more severe disease after inoculation with an avirulent strain of Pst DC3000, but they showed similar disease severity as the wild-type plant after inoculation with a virulent strain of Pst DC3000. Significant accumulations of reactive oxygen species (ROS) and increased cell death were observed in the dal1 and dal2 mutant plants after inoculation with the avirulent strain of Pst DC3000. The dal mutant plants underwent extensive PCD upon infiltration of FB1 and displayed higher levels of ROS accumulation, callose deposition, and autofluorescence than the wild-type plants. Our data suggest that DAL1 and DAL2 may act as negative regulators of PCD in Arabidopsis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within all living organisms, some cells commit suicide to achieve and maintain homeostasis during normal development, environmental stress, or pathogen attack. This functionally conserved and gene-directed cell suicide is known as programmed cell death (PCD). Programmed cell death is a ubiquitous feature of all living cells, from prokaryotes to eukaryotes (Beers and McDowell 2001; Golstein et al. 2003; Lawen 2003; Lam 2004; Madeo et al. 2004). In plants, one of the most familiar forms of PCD is associated with pathogen attack. Normal plant–pathogen interactions depend on the classical gene-for-gene resistance model that requires an avirulence gene in the pathogen and a corresponding resistance gene in the plant (Jones and Dangl 2006). A rapid, localized cell death known as hypersensitive response (HR), which results in the formation of necrotic lesions around the infection sites, is the usual characteristic of an incompatible interaction between host plants and pathogens (Goodman and Novacky 1994; Lam et al. 2001). Morphologically and biochemically, the HR that occurs in response to infection from avirulent pathogens shares most features of PCD (Mur et al. 2008; Greenberg and Yao 2004; Lam 2004).
Extensive studies on the physiological, biochemical, and molecular basis of HR-PCD during plant–pathogen interactions suggest that it is controlled by endogenous signaling cascades (Torres and Dangl 2005; Delledonne 2005; Mur et al. 2008; Jones and Dangl 2006) initiated by reactive oxygen species (ROS), nitric oxide, salicylic acid (SA), ethylene, and oxylipins (Jabs et al. 1996; Rate et al. 1999; Epple et al. 2003; Torres and Dangl 2005; Torres et al. 2005; Delledonne 2005; Brodersen et al. 2005; Mur et al. 2006; Bouchez et al. 2007). On the other hand, PCD is a precise, genetically controlled cellular process that can be regulated by a number of genes. Much of our understanding on the genetic and molecular basis for the control and execution of plant PCD comes from the analysis of lesion mimic mutants that exhibit spontaneous HR-like PCD in the absence of a pathogen. Dozens of such mutants have been isolated, including accelerated cell death (acd) and lesion simulating disease (lsd) in Arabidopsis, which are unable to control the rate and extent of lesions causing chlorosis in large areas (Lorrain et al. 2003). Some of the individual genes that have been identified in these mutants provide new insights into the mechanism of PCD control in plants.
The Arabidopsis protein LSD1 is a negative regulator of PCD that modulates cellular ROS status and requires PAD4 and EDS1, two components of the SA-dependent defense signaling pathway (Dietrich et al. 1997; Jabs et al. 1996; Rusterucci et al. 2001; Aviv et al. 2002). In addition, LSD1 antagonistically interacts with bZIP10, a positive regulator of PCD (Kaminaka et al. 2006). The characterization of ACD5 and ACD11 as a ceramide kinase and a sphingosine transfer protein, respectively, suggests important roles for sphingolipids in regulating PCD (Greenberg et al. 2000; Brodersen et al. 2002; Vailleau et al. 2002; Liang et al. 2003; Raffaele et al. 2006, 2008; Shi et al. 2007; Yang et al. 2007; Wang et al. 2008; Reina-Pinto et al. 2009). Other factors have also been demonstrated to modulate PCD in plants. Such PCD-regulating factors include tomato MAPKKKα (del Pozo et al. 2004), phospholipid hydroperoxide glutathione peroxidase (Chen et al. 2004), Arabidopsis PLP2 (La Camera et al. 2009), BAP1 and BAP2 (Yang et al. 2007), FMO1 and NUDT7 (Bartsch et al. 2006), ACD2 (Mach et al. 2001; Yao and Greenberg 2006), and copine proteins (Yang et al. 2006).
Many of the signaling mechanisms of PCD are conserved among eukaryotes, including animals and plants. Some of the genes that control PCD are functionally conserved across wide evolutionary distances (Higashi et al. 2005; Williams and Dickman 2008). For example, the mammal Bax-induced cell death inhibitor BI-1 has homologues in plants, including Arabidopsis, rice, and barley (Lacomme and Santa Cruz 1999; Sanchez et al. 2000; Kawai-Yamada et al. 2001, 2004; Yu et al. 2002; Chae et al. 2003; Matsumura et al. 2003; Huckelhoven et al. 2003; Watanabe and Lam 2006; Eichmann et al. 2004). Similarly, animal apoptotic regulators such as human Bcl-2 and Bcl-xl, as well as nematode CED-9, can either induce or suppress cell death in transgenic plants (Dickman et al. 2001; Lincoln et al. 2002; Xu et al. 2004). In Drosophila melanogaster, expression of DIAP1 can suppress apoptosis, and the loss of DIAP1 function results in early embryonic death resulting from apoptosis (Wang et al. 1999; Goyal et al. 2000; Lisi et al. 2000). In this study, we identified two Arabidopsis genes, DAL1 and DAL2 (for the Drosophila inhibitor of apoptosis like), encoding RING finger proteins with homology to DIAP1. Mutations in DAL1 and DAL2 results in a significant accumulation of superoxide anions, causing PCD after inoculation with an avirulent strain of Pseudomonas syringae pv. tomato (Pst) DC3000. Similarly, both dal1 and dal2 mutants exhibit accelerated PCD progression upon treatment with fumonisin B1, a toxin produced by the necrotrophic fungal plant pathogen, Fusarium moniliforme. Our data suggest that DAL1 and DAL2 may act as negative regulators of PCD in Arabidopsis.
Materials and methods
Plant materials and growth
Seeds of Arabidopsis thaliana wild-type [ecotype Columbia-0 (Col-0)] and mutant lines were soaked in water at 4°C for 2 days to break dormancy. Afterward, the seeds were sown in pots with a mixture of perlite:vermiculite:plant ash (1:6:2). All plants were grown under fluorescent light at 150 μE m−2 s−1 at 22 ± 3°C with 60% relative humidity and long-day conditions (16-h light/8-h dark cycle).
All T-DNA insertion lines on the Col-0 background were obtained from the Arabidopsis Biological Resource Center at Ohio State University. The dal1 mutants (SALK_002099 for dal1-1 allele and SALK_063571 for dal1-2 allele) were obtained from the SALK population, whereas the dal2 mutants (SALK_024744 for dal2-1 allele and WiscDsLox383C6 for dal2-2 allele) were obtained from the SALK and Wisconsin populations, respectively. PCR genotyping was performed to identify homozygous plants using gene-specific primers and T-DNA primers (Lba1, TGG TTC ACG TAG TGG GCC ATC G for SALK lines and JL202, CAT TTT ATA ATA ACG CTG CGG ACA TCT AC-3 for Wisconsin line). The gene-specific primers used for identification of homozygous lines were as follows: SALK_002099-LP, GAA TCG AGC AAA CAC CTC ATG and SALK_002099-RP, GCT GCC ATG TATA ACA GGC TG for SALK_002099 line; CS853707-LP, AAT ATT AGG ATT CAT CCC GCG, and CS853707-RP, GCC AAC ATC TCC TGA TAC TGC for WiscDsLox383C6 line; SALK_024744-LP, GCG TTG GGT TAT CTT AAA GGC, and SALK_024744-RP, CAG CAG CAC ATA TGA CCA CAC for SALK_024774 line; SALK_063571-LP, CCC CTT TCG AAA TTC TCC TC, and SALK_063571-RP, ATT ACA TGG ACA CGG CTT GTC for SALK_063571 line. The expression levels of DAL1 and DAL2 genes in homozygous plants were analyzed by reverse transcription polymerase chain reaction (RT-PCR) using gene-specific primers DAL1-1F (ATG ATT CCT TGG GGT GGA GTT AC)/DAL2-1R (TCA GTG ACG ATA TGT CTT AAC C) and DAL2-1F (CAA GTA AGA GTG ACT TCA GAG TC)/DAL2-1R (GAA TGT TTC TGA GGC CTG AGT TG), respectively.
Assays for disease phenotype and treatment with fumonisin B1
Pst strains DC3000 and DC3000-AvrRPM1 were grown in liquid King B medium (10 g L−1 peptone, 1.5 g L−1 K2HPO4, 15 g L−1 glycerol, 5 mM MgSO4, pH 7.0) with rifampicin (25 μg mL−1) at 28°C for 8–12 h in a refrigerated shaker. Bacteria were harvested by centrifugation at 2,500×g for 10 min and resuspended in sterile 10 mM MgCl2 solution. Bacterial inocula were then adjusted to the desired concentrations. Inoculations were performed on the leaves of 4-week-old plants by infiltration with suspensions of Pst in 10 mM MgCl2 using a 1 mL needleless syringe. Mock inoculation was done by infiltrating similar amounts of 10 mM MgCl2 solution. All inocula were injected on both sides of the abaxial surface of the leaves. To determine bacterial growth, ten leaves were collected at different times after inoculation, and leaf discs of the same size were made using a hole puncher. The bacterial titer per leaf area was determined as described previously (Mengiste et al. 2003).
The mature leaves of 4-week-old wild-type and mutant plants grown in soil were infiltrated with approximately 20 μL of 10 μM fumonisin B1 (FB1, Sigma) in 10 mM MgSO4 solution, or 10 mM MgSO4 solution alone for mock treatment, using a needleless syringe. The infiltrated plants were kept in a controlled growth chamber for up to 4 days, and lesion formation was observed.
Cell death assays
Cell death was detected using Evans blue staining as described by Wright et al. (2000) with minor modifications. Detached leaves were completely submerged in a 0.1% w/v Evans blue solution and subjected to three 5 min cycles of vacuum followed by 20 min incubation under vacuum. The leaves were then washed with water and cleared by boiling for 2 min in alcoholic lactophenol (95% ethanol:lactophenol, 2:1). They were rinsed in 50% ethanol and then in distilled water. Cell death in infiltrated leaves was also assayed by measuring electrolyte leakage from leaf discs obtained at different time points following FB1 treatment. Up to 3–4 leaf discs (6 mm diameter) were floated on 4 mL of distilled water for 3 h at room temperature. After incubation, the conductivity of the bathing solution was measured using a DDS-IIAT-type conductivity meter. The leaf samples in the bathing solutions were then boiled for 5 min, and volumes of the bathing solution were brought up to the initial volumes, followed by measurement of total conductivity. The percentage of electrolyte leakage attributable to the FB1 treatment was calculated as 100% × (conductivity of the test samples)/(conductivity after boiling). Measurements for each time point were performed at least in triplicate.
RT-PCR analysis of gene expression
Leaf samples were collected at different time points after inoculation with P. syringae pv. tomato DC3000 or treatment with FB1. Total RNA was extracted using TRIZOL reagent (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Gene expression was analyzed by RT-PCR using gene-specific primers. Actin1 was used as an internal control. One microgram of total RNA was reverse transcribed using Superscript III reverse transcriptase (Invitrogen, Shanghai, China), and the synthesized first strand cDNAs were used as templates in the PCR. PCR was carried out using Taq DNA polymerase (Takara, Dalian, China) and the following cycle settings: 94°C for 15 s, 55–62°C for 30 s, and then 72°C for 30–50 s. The gene-specific primers used were as follows: DAL1-rt-1F, GTC AAG GTT GTA CAA ATA TGC CT; DAL1-rt-2R, TTC GTC TCC GAC AAA GTG GAC A; DAL2-rt-2F, GAG TAA GAC ACG TTG AGC GTG T; DAL2-rt-2R, AGC ATG GTG TGC AGC AGC ACA; PR1-1F, TCG TCT TTG TAG CTC TTG TAG GTG; PR1-1R, TAG ATT CTC GTA ATC TCA GCT CT; Actin1-1F, GGC GAT GAA GCT CAA TCC AAA CG; and Actin1-1R, GGT CAC GAC CAG CAA GAT CAA GAC G. Each experiment was done at least twice using independent samples.
Detection of callose deposition, autofluorescence, and reactive oxygen species
Callose deposition and autofluorescence were detected according to Dietrich et al. (1994) with slight modifications. The detached leaves were fixed in an autofluorescence-fixing solution (10% formaldehyde, 5% acetic acid, and 45% ethanol). The samples were then cleared in alcoholic lactophenol, rinsed with distilled water to remove the lactophenol, and stained overnight with aniline blue (0.01% aniline blue powder in 150 mM K2HPO4, pH 9.5). The samples were equilibrated in 50% glycerol before mounting onto slides. Aniline blue staining for callose deposition was visualized under a Leica TCS SPS stereo-fluorescence microscope using a blue fluorescent protein filter set (excitation 405 nm, emission 420/90 nm, and dichroic 420 nm), whereas autofluorescence was visualized using the enhanced green fluorescent protein filter set (excitation 488 nm, emission 500/60 nm, and dichroic 495 nm). Hydrogen peroxide was detected by an in situ histochemical staining procedure using 3,3′-diaminobenzidine (DAB) (Thordal-Christensen et al. 1997). The detached leaves were placed in a solution containing 1 mg mL−1 DAB (pH 5.5) for 2 h at room temperature, boiled in 95% ethanol for 2 min, and stored in distilled water. Hydrogen peroxide produces a reddish-brown coloration in the DAB staining method. Staining for the presence of superoxide (O2 −) employed the nitroblue tetrazolium (NBT) uptake method, which was performed as described (Doke 1983). The detached leaves were immersed for 30 min in 5 mL NBT staining solution (1 mg mL−1 NBT in 10 mM NaN3 and 10 mM phosphate buffer). After staining, the leaves were cleared in 96% boiling ethanol and then analyzed.
Statistical analysis
All experiments were repeated independently twice, and the data obtained were statistically analyzed using Student’s t tests.
Results
Characterization of DAL1 and DAL2 in Arabidopsis
DIAP1 is a functional anti-apoptotic protein in D. melanogaster (Vaux and Silke 2005). To determine whether higher plants also have DIAP1-like proteins, homologous searches against the Arabidopsis genome sequence database were performed using DIAP1 as the query. These searches identified two genes, At1g63900 and At1g59560, encoding putative proteins that show similarities to DIAP1 with P values at e−8. These two proteins were designated as DAL1 (At1g63900) and DAL2 (At1g59560) for Arabidopsis thaliana DIAP1-like proteins. DAL1 is annotated as a 343 amino acid protein, supported by three full-length cDNAs (AK176213, AK176755 and AY128352); DAL2 is predicted as a 338 amino acid protein, supported by two full-length cDNAs (AK228076 and AY089055) (Fig. 1a). Similar searches also identified two putative DAL proteins (Os03g24500 and Os07g45350) in rice genomes. Both DAL1 and DAL2 contain a RING domain at the C-terminus with six conserved cysteine residues (Fig. 1a). Aside from the conserved RING domain, no other conserved domains were present in the DAL proteins. Phylogenetic tree analysis revealed that DAL1 and DAL2 shared approximately 17% amino acid identity with DIAP1 (Fig. 1b). These indicate that DAL1 and DAL2 represent a group of RING finger proteins with structural and possibly functional homology to DIAP1 in Drosophila.
Expression of DAL1 and DAL2 in response to Pst infection
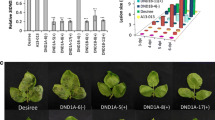
The expression patterns of DAL1 and DAL2 genes in wild-type plants were first analyzed after inoculation with virulent and avirulent strains of Pst DC3000. In the mock-inoculated plants, no significant expression of DAL1 and DAL2 genes was observed, indicating that the basal expression of these two genes is very low in healthy plants grown under normal conditions (Fig. 2). Expression of DAL1 was significantly upregulated at 18 hours post-inoculation (hpi), whereas expression of DAL2 was also upregulated at 36 hpi (Fig. 2a). Similarly, expression of DAL1 was dramatically induced after inoculation with the avirulent strain of Pst DC3000, whereas expression of DAL2 was upregulated only slightly (Fig. 2a). These indicate that the expression of DAL genes was induced through different patterns in response to virulent and avirulent strains of Pst DC3000.
Expression of DAL1 and DAL2 genes in response to Pst infection and characterization of dal mutant lines. a Expression of DAL1 and DAL2 in response to infection by virulent and avirulent strains of Pst DC3000. Four-week-old plants were inoculated by infiltration with Pst DC3000 wild-type or AvrRPM1 strains (OD600 = 0.002). Leaf samples were collected at the indicated times after inoculation for RNA isolation. Expression of DAL1 and DAL2 genes was analyzed by RT-PCR using gene-specific primers. The actin gene was used as internal control. b Exon/intron organization of the DAL1 and DAL2 genes and the T-DNA insertion lines for dal1 and dal2 mutants. Closed boxes represent exons, and bold lines represent introns. The positions of the T-DNA insertions, the initiating ATG, and stop codon (TAG) are indicated. c RT-PCR analysis of the expression of the DAL1 and DAL2 genes in the mutant plants. Leaf samples were collected from 4-week-old plants, and total RNA was used for RT-PCR. The experiments were repeated independently twice with similar results
Identification of dal mutant lines
To determine the functions of DAL1 and DAL2, T-DNA knockout mutants were identified and characterized for these two genes. The mutants SALK_002099 and SALK_063571, containing T-DNA insertions in the eighth intron and in the third exon of the DAL1 gene, respectively, were designated as dal1-1 and dal1-2 (Fig. 2b). The mutants SALK_024744 and WiscDsLox383C6, containing T-DNA insertions in the eighth intron and in the first exon of the DAL2 gene, respectively, were designated as dal2-1 and dal2-2 (Fig. 2b). PCR-based genotyping was performed to screen for the homozygous plants of these T-DNA insertion lines using gene-specific primers and T-DNA primers. Analysis using RT-PCR revealed no significant expression of DAL1 or DAL2 genes in the homozygous dal1 and dal2 plants using primers for the full-length cDNAs of the DAL1 and DAL2 genes (Fig. 2c). These results indicate that the isolated dal1 and dal2 lines are null mutants of the DAL1 and DAL2 genes.
Disease phenotypes of dal1 and dal2 mutants against Pst
To explore the possible involvement of DAL1 and DAL2 genes in defense response, the disease phenotypes of the dal1 and dal2 plants were analyzed and compared with the wild type after inoculation with a virulent strain of Pst DC3000. All tested genotypes (dal1, dal2, and wild type) showed chlorosis visible 4 days after inoculation which developed progressively on the infected leaves (Fig. 3a). The severity of chlorosis in the dal1 and dal2 plants was similar to that in the wild-type plants. The measurement of bacterial titers in the inoculated plants further demonstrated significant differences in bacterial growth among the leaves from dal1, dal2, and wild-type plants (Fig. 3a). These results indicate that mutations in the DAL1 and DAL2 genes do not alter the defense response against a virulent strain of Pst DC3000.
The disease phenotypes of the dal1 and dal2 plants after infection by virulent and avirulent strains of Pst DC3000. a Representative leaves showing disease symptoms (top) and bacterial growth (bottom) 4 dpi after infiltration with a bacterial suspension (OD600 = 0.004) of Pst DC3000. b Representative leaves showing disease symptoms (top) and bacterial growth (bottom) 2 dpi after infiltration with a bacterial suspension (OD600 = 0.002) of PstDC3000-AvrRpm1. The experiments were repeated independently twice, and the data presented are the means and standard errors from three independent experiments. Different letters above the columns indicate significant differences at p < 0.05 level by t test
Next, the disease phenotypes of the dal1 and dal2 plants were analyzed against an avirulent Pst DC3000 strain carrying the avirulence gene AvrRpm1. When a bacterial suspension of OD600 = 0.002 was infiltrated into the wild-type plants, only very slight chlorosis was observed (Fig. 3b). In contrast, very severe symptoms, resulting in extensive chlorotic lesions, were observed on the inoculated leaves of the dal1 and dal2 plants (Fig. 3b). Similarly, the dal1 and dal2 plants supported much more bacterial growth in the inoculated leaves, resulting in an average 10- to 50-fold increase in the bacterial population compared with the wild-type plants (Fig. 3b). The bacterial population in the inoculated leaves of wild-type plants was 8.1 × 103 cfu cm−2 at 2 dpi and 3.3 × 104 cfu cm−2 at 4 dpi. In contrast, bacterial populations in the inoculated leaves were 9.8 × 104 cfu cm−2 in dal1-1 and 12.1 × 104 cfu cm−2 in dal1-2 at 2 dpi. These counts rose to 1.5 × 106 cfu cm−2 in dal1-1 and 1.8 × 106 cfu cm−2 in dal1-2 plants by 4 dpi. The bacterial populations in the inoculated leaves of dal2-1 and dal2-2 plants were also higher than in the wild types, measuring 7.1 × 104 and 8.5 × 104 cfu cm−2 at 2 dpi and 1.1 × 106 and 1.3 × 106 cfu cm−2 at 4 dpi, respectively (Fig. 3b). These results indicate that mutations in the DAL1 and DAL2 genes result in susceptibility to avirulent strains of Pst DC3000.
PCD and ROS accumulation in dal1 and dal2 plants after infection with Pst
The wild-type Arabidopsis plant normally responds to infection by an avirulent strain of Pst DC3000 with HR and generation of ROS. The PCD levels and the accumulation of ROS were therefore analyzed in wild-type and dal1/dal2 plants in response to Pst DC3000-AvrRpm1. The results of the Evans blue staining indicate that cell death levels were very low and that only a few cells died in the wild-type plants (Fig. 4a). In contrast, the dal1 and dal2 plants showed higher levels of cell death compared with the wild-type plants (Fig. 4a). However, the cell death levels varied to some extent among the dal1 and dal2 mutant plants; the dal2-1 plants had severe cell death, whereas the dal1-1 and dal2-2 plants responded with moderate cell death. This was probably due to the different T-DNA locations among these mutants. The accumulation of superoxide anions (O2 −) was tested by staining with NBT (Doke 1983). In wild-type plants, no significant accumulation of O2 − was observed at 2 dpi (Fig. 4b). In contrast, significant accumulations of O2 − were detected in the leaves of the dal1 and dal2 plants at 2 dpi (Fig. 4b).
Cell death and ROS accumulation in dal1 and dal2 plants after infection with virulent and avirulent strains of Pst DC3000. a and b Four-week-old plants were inoculated by infiltration with Pst DC3000-AvrRpm1 (OD600 = 0.002), and leaf samples were taken at 3 dpi. Representative leaves showing cell death (a) and accumulation of superoxide anion (b) detected by Evans Blue and NBT staining, respectively. The experiments were repeated twice with similar results
Extensive PCD induced by FB1 in dal1 and dal2 plants
We further studied whether the dal1 and dal2 plants showed altered responses to FB1, a fungal toxin that triggers PCD in plants (Asai et al. 2000; Stone et al. 2000). Reportedly, infiltration with FB1 can trigger both necrotic lesion formation and characteristic PCD responses in Arabidopsis leaves (Stone et al. 2000). When a 10 μM FB1 solution was infiltrated into the mature leaves of 4-week-old soil-grown Arabidopsis wild-type plants under controlled environmental conditions, lesions appeared on the leaves within 2–3 dpi (Fig. 5a). At 4 dpi, infiltration with FB1 resulted in a greater number of necrotic lesions in all the dal1 and dal2 plants compared with the wild-type (Fig. 5a). Evans blue staining confirmed that cell death progression was accelerated in the dal1 and dal2 plants (Fig. 5b). Relatively high levels of cell death were observed in the dal1-1, dal1-2, and dal2-1 plants compared with the wild-type plants 48 h after infiltration (Fig. 5b). The progression of cell death was further examined by quantifying ion leakage in the wild-type, hemizygous, and homozygous dal1 and dal2 plants. The hemizygous and wild-type dal1 and dal2 plants exhibited similar cell death progression in response to FB1 infiltration (Fig. 5c). However, the cell death levels in the homozygous dal1 and dal2 plants were much higher in response to FB1 (Fig. 5c).
Responses of the dal1 and dal2 plants to fumonisin B1. The leaves of the 4-week-old wild-type and dal1 and dal2 mutant plants were infiltrated with 10 μM FB1 in 10 mM MgSO4 or with 10 mM MgSO4 as mock treatment. a Representative leaves showing necrotic symptoms 4 days after FB1 infiltration. b Representative leaves showing cell death, as revealed by Evans blue staining, 2 days after infiltration. c Cell death progression measured by ion leakage. The experiments were repeated independently twice, and the data presented are the means and standard errors from three independent experiments. Different letters above the columns indicate significant differences at p < 0.05 level by t test
We then analyzed whether the expression of DAL genes is induced in wild-type plants in response to FBI. Analysis using RT-PCR revealed that 18 h after FB1 infiltration, the expression of DAL1 and DAL2 genes was dramatically upregulated, whereas no significant expression was observed in the mock-treated mutant plants (Fig. 6a). In addition, the defense-related PR-1 gene exhibited similar upregulation in mutant plants after FB1 treatment (Fig. 6a).
Expression of DAL and PR-1 genes and accumulation of ROS in response to FB1. Four-week-old wild-type plants were infiltrated with 10 μM FB1 in 10 mM MgSO4 or with 10 mM MgSO4 as mock treatment. Leaf samples were collected at the indicated times for analysis. Expression of the DAL and PR-1 genes was analyzed by RT-PCR using gene-specific primers with actin as the internal control. Accumulation of H2O2 (b) and superoxide anion (c) in leaves was detected by DAB and NBT staining, respectively. The experiments were repeated independently twice with similar results
The ROS production in the wild-type and dal plants after infiltration of FB1 was then analyzed and compared. In mock-inoculated plants, no significant accumulation of H2O2 or O2 − was detected in either the wild-type or dal plants (data not shown). At 2 days following FBI infiltration, however, significantly greater accumulations of O2 − and H2O2 were detected in the leaves of the dal1 and dal2 plants compared with those of the wild-type plants (Fig. 6b, c). Microscopic observations revealed increased callose deposition and autofluorescence in the FB1-infiltrated leaves of the dal1 and dal2 plants compared with that of the wild types (Fig. 7). These results indicate that mutations in the DAL1 and DAL2 genes result in increased ROS accumulation and enhanced cell death in response to FB1.
Callose deposition and autofluorescence in the dal1 and dal2 plants in response to FB1. The leaves of 4-week-old plants were infiltrated with 10 μM FB1 in 10 mM MgSO4 or with 10 mM MgSO4 as mock treatment. They were detached at 72 hpi and analyzed for callose deposition and autofluorescence. The experiments were repeated independently twice with similar results
Discussion
Programmed cell death is a genetically controlled cellular process induced in response to pathogen infection, and a number of genes have been identified that are negative or positive regulators of PCD. Compared with animal studies, however, our understanding of the genetic basis and molecular biology of PCD in plants is rudimentary. In this study, two Arabidopsis RING finger proteins, DAL1 and DAL2, were identified, and these proteins were proven as negative regulators (response inhibitors) of PCD in Arabidopsis. Mutations in DAL1 and DAL2 genes result in extensive PCD in response to both avirulent bacteria and the chemical toxin FB1.
Based on their amino acid sequences and conserved domain organizations, the DAL1 and DAL2 proteins belong to the RING family whose members have diverse biological functions in plant growth and development, hormone signaling, and stress responses (Stone et al. 2005). The DAL1 and DAL2 proteins contain a conserved RING domain at their C-terminus and show moderate sequence homology with DIAP1 in fruit flies (Wang et al. 1999; Goyal et al. 2000; Lisi et al. 2000). In addition to the typical RING domain, a BIR domain is present in DIAP1 (Vaux and Silke 2005). However, no further conserved domains were identified in the DAL1 and DAL2 proteins. Our observation that mutations in the DAL1 and DAL2 genes resulted in enhanced cell death in response to an avirulent pathogen and low doses of FB1 mirrors results in Drosophila, where the loss of DIAP1 function results in early embryonic death from massive apoptosis (Wang et al. 1999; Goyal et al. 2000; Lisi et al. 2000). Most RING finger proteins have enzymatic activities that catalyze reactions within the ubiquitination/26S proteasome protein degradation system (Stone et al. 2005). DIAP1 has E3 ubiquitin ligase activity, and the RING domain is critical for its biological activity and regulation of PCD (Goyal et al. 2000; Lisi et al. 2000). Indeed, the Arabidopsis RING1, which possesses E3 ubiquitin ligase activity in vitro, is also involved in PCD (Lin et al. 2008). However, the biochemical activity and putative function of the RING domain in DAL proteins remain undetermined.
Programmed cell death and disease resistance are often intricately linked in higher plants. In Arabidopsis, most of the lesions mimic mutants like acd and lsd show spontaneous, uncontrolled PCD manifesting as large areas of chlorosis in the leaves (Lorrain et al. 2003). In this study, spontaneous lesions were not observed on the leaves of the dal plants grown under normal conditions. This phenotype is similar to Arabidopsis bap1/bap2 mutants and RING1-silenced plants in that the loss of BAP1/BAP2 and RING1 function is associated with accelerated HR-mediated cell death induced by exogenous stimuli, but this does not cause spontaneous cell death (Yang et al. 2007; Lin et al. 2008). The dal plants also exhibited elevated stress responses to an avirulent strain of Pst DC3000 that resulted in extensive PCD. Compared with the wild-type plants, the dal1 and dal2 plants supported higher levels of bacterial growth after inoculation with Pst DC3000, indicating that both DAL1 and DAL2 genes may also function in Rpm1-mediated disease resistance. In this sense, DAL1 and DAL2 genes are similar to BAP and BON genes that act both as direct repressors of PCD and as regulators of the activity of specific R genes, resulting in enhanced disease resistance against a variety of virulent pathogens (Yang and Hua 2004; Yang et al. 2007). Further research to clarify the function of DAL genes in R gene-mediated disease resistance is clearly warranted.
During incompatible interactions between plants and pathogens, cell death in HR is often associated with the development of plant disease resistance, resulting in the halting of pathogen growth in plant tissues. However, cell death can also be uncoupled from the resistance response. In defense no death 1 (dnd1) Arabidopsis mutants, for example, resistance to Pst occurs without HR-mediated cell death (Yu et al. 1998). In this study, the high levels of PCD and bacterial growth observed among the dal1 and dal2 mutant plants after inoculation with an avirulent strain of Pst DC3000 indicate that DAL1 and DAL2 have distinct functions in regulating PCD and disease resistance, a negative role in PCD, and a positive role in Rpm1-mediated resistance. Thus, DAL1 and DAL2 seemingly act as modulators to maintain the balance between the extents of PCD and disease resistance against avirulent pathogens.
Interestingly, mutations in the DAL1 and DAL2 genes did not affect the response of the corresponding mutant plants against a virulent strain of Pst DC3000 (Fig. 3). A similar phenotype was also observed in the Arabidopsis bap2 mutant plants, which showed the same disease phenotype against a virulent strain of Pst DC3000 as wild-type plants (Yang et al. 2007). Therefore, extensive PCD in dal plants may likely be unrelated to the defense response against virulent pathogens. This phenomenon was further confirmed by studies on Arabidopsis dnd1/dnd2 and bap2 mutants. The dnd1 and dnd2 plants are unable to undergo HR-mediated cell death but retain their disease resistance against avirulent pathogens in a gene-for-gene manner (Yu et al. 1998; Clough et al. 2000; Jurkowski et al. 2004). Meanwhile, the PCD in bap2 plants is not associated with an altered defense response against virulent pathogens, including Pst DC3000 and virulent strains of Hyalopernonspora parasitica (Yang et al. 2007). Therefore, in some cases, HR-mediated cell death is not strictly required for disease resistance against virulent pathogens. Furthermore, overexpression of plant negative regulators (e.g., BI-1 and BAP1/2) inhibits PCD induced by various biotic and abiotic stimuli, as well as confers enhanced disease resistance against different pathogens (Yang et al. 2007; Kawai-Yamada et al. 2001 Matsumura et al. 2003; Huckelhoven et al. 2003). Determining whether the overexpression of DAL1 and DAL2 increases disease resistance is interesting. If this is the case, DAL1 and DAL2 can be promising targets for the generation of disease-resistant transgenic plants.
In Arabidopsis, FB1-induced HR-like PCD response shared many of the features of PCD elicited by an avirulent bacterial pathogen, including increased callose deposition and increased autofluorescence (Stone et al. 2000). This FB1-induced PCD depended on multiple signaling pathways, including jasmonate, ethylene, SA, and ROS accumulation (Asai et al. 2000; Stone et al. 2000; Watanabe and Lam 2006; Shi et al. 2007). In this study, FB1 infiltration induced the expression of DAL1 and DAL2 genes in the leaves of the wild-type plants and triggered extensive PCD in the leaves of the dal plants (Figs. 5, 6). This extensive PCD was revealed by significant necrotic lesions, increased numbers of dead cells, and greater ion leakage from the leaves of the dal plants (Figs. 5, 6). In addition, increased callose deposition and autofluorescence were observed in the dal plants upon FB1 treatment (Figs. 6, 7). This is consistent with observations in atbi1 mutants, where mutant plants exhibited an accelerated PCD phenotype associated with enhanced levels of callose deposition and autofluorescence after treatment with FB1 (Watanabe and Lam 2006). In this study, relatively higher levels of H2O2 and O2 − were detected in the FB1-infiltrated leaves of the dal plants compared with wild-type plants (Fig. 6), suggesting that DAL1 and DAL2 may function upstream of ROS generation in the PCD pathways.
In summary, the results presented in this study demonstrate that the Arabidopsis proteins DAL1 and DAL2, two RING domain-containing proteins with homology to DIAP1 in fruit flies, regulate PCD. Mutations in DAL1 and DAL2 genes resulted in extensive PCD in response to an avirulent strain of Pst DC3000 and following exposure to the toxin FB1, indicating that both DAL1 and DAL2 proteins may act as negative regulators of PCD in Arabidopsis. As RING finger proteins, the DAL1 and DAL2 proteins may be E3 ubiquitin ligases within the ubiquitin/26S proteasome system. They may possibly target other positive regulators of PCD for degradation, thereby reducing the threshold for PCD. Further studies on the biochemical activities of DAL1 and DAL2 can provide new insights into our understanding of the functions of DAL1 and DAL2 in PCD.
References
Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM (2000) Fumonisin B1-induced cell death in arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 12:1823–1836
Aviv DH, Rustérucci C, Holt BF 3rd, Dietrich RA, Parker JE, Dangl JL (2002) Runaway cell death, but not basal disease resistance, in lsd1 is SA- and NIM1/NPR1-dependent. Plant J 29:381–391
Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE (2006) Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18:1038–1051
Beers EP, McDowell JM (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr Opin Plant Biol 4:561–567
Bouchez O, Huard C, Lorrain S, Roby D, Balague C (2007) Ethylene is one of the key elements for cell death and defense response control in the Arabidopsis Lesion Mimic Mutant vad1. Plant Physiol 145:465–477
Brodersen P, Petersen M, Pike HM, Olszak B, Skov S, Odum N, Jorgensen LB, Brown RE, Mundy J (2002) Knockout of Arabidopsis ACCELERATED-CELL-DEATH11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev 16:490–502
Brodersen P, Malinovsky FG, Hematy K, Newman M-A, Mundy J (2005) The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol 138:1037–1045
Chae HJ, Ke N, Kim HR, Chen S, Godzik A, Dickman M, Reed JC (2003) Evolutionarily conserved cytoprotection provided by Bax inhibitor-1 homologs from animals, plants, and yeast. Gene 323:101–113
Chen S, Vaghchhipawala Z, Li W, Asard H, Dickman MB (2004) Tomato phospholipid hydroperoxide glutathione peroxidase inhibits cell death induced by Bax and oxidative stresses in yeast and plants. Plant Physiol 135:1630–1641
Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK Jr, Bent AF (2000) The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97:9323–9328
del Pozo O, Pedley KF, Martin GB (2004) MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J 23:3072–3082
Delledonne M (2005) NO news is good news for plants. Curr Opin Plant Biol 8:390–396
Dickman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acad Sci USA 98:6957–6962
Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL (1994) Arabidopsis mutants simulating disease resistance response. Cell 77:565–577
Dietrich RA, Richberg MH, Schmidt R, Dean C, Dangl JL (1997) A novel zinc finger protein is encoded by the Arabidopsis LSD1 gene and functions as a negative regulator of plant cell death. Cell 88:685–694
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissue to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23:345–357
Eichmann R, Schultheiss H, Kogel KH, Hückelhoven R (2004) The barley apoptosis suppressor homologue BAX inhibitor-1 compromises nonhost penetration resistance of barley to the inappropriate pathogen Blumeria graminis f. sp. tritici. Mol Plant-Microbe Interact 17:484–490
Epple P, Mack AA, Morris VR, Dangl JL (2003) Antagonistic control of oxidative stress-induced cell death in Arabidopsis by two related, plant-specific zinc finger proteins. Proc Natl Acad Sci USA 100:6831–6836
Golstein P, Aubry L, Levraud JP (2003) Cell-death alternative model organisms: why and which? Nat Rev Mol Cell Biol 4:798–807
Goodman RN, Novacky AJ (1994) The hypersensitive reaction in plants to pathogens: a resistance phenomenon. St. Paul MN APS Press, USA
Goyal L, McCall K, Agapite J, Hartwieg E, Steller H (2000) Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J 19:589–597
Greenberg JT, Yao N (2004) The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 6:201–211
Greenberg JT, Silverman FP, Liang H (2000) Uncoupling salicylic acid-dependent cell death and defense-related responses from disease resistance in the Arabidopsis mutant acd5. Genetics 156:341–350
Higashi K, Takasawa R, Yoshimori A, Goh T, Tanuma S, Kuchitsu K (2005) Identification of a novel gene family, paralogs of inhibitor of apoptosis proteins present in plants, fungi, and animals. Apoptosis 10:471–480
Huckelhoven R, Dechert C, Kogel KH (2003) Overexpression of barley BAX inhibitor 1 induces breakdown of mlo-mediated penetration resistance to Blumeria graminis. Proc Natl Acad Sci USA 100:5555–5560
Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273:1853–1856
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Jurkowski GI, Smith RK Jr, Yu IC, Ham JH, Sharma SB, Klessig DF, Fengler KA, Bent AF (2004) Arabidopsis DND2, a second cyclic nucleotide-gated ion channel gene for which mutation causes the “defense, no death” phenotype. Mol Plant-Microbe Interact 17:511–520
Kaminaka H, Nake C, Epple P, Dittgen J, Schutze K, Chaban C, Holt BF 3rd, Merkle T, Schafer E, Harter K, Dangl JL (2006) bZIP10-LSD1 antagonism modulates basal defense and cell death in Arabidopsis following infection. EMBO J 25:4400–4411
Kawai-Yamada M, Jin L, Yoshinaga K, Hirata A, Uchimiya H (2001) Mammalian Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1 (AtBI-1). Proc Natl Acad Sci USA 98:12295–12300
Kawai-Yamada M, Ohori Y, Uchimiya H (2004) Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell 16:21–32
La Camera S, Balague C, Gobel C, Geoffroy P, Legrand M, Feussner I, Roby D, Heitz T (2009) The Arabidopsis patatin-like protein 2 (PLP2) plays an essential role in cell death execution and differentially affects biosynthesis of oxylipins and resistance to pathogens. Mol Plant-Microbe Interact 22:469–481
Lacomme C, Santa Cruz S (1999) Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acad Sci USA 96:7956–7961
Lam E (2004) Controlled cell death, plant survival and development. Nat Rev Mol Cell Biol 5:305–315
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848–853
Lawen A (2003) Apoptosis—an introduction. Bioessays 25:888–896
Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT (2003) Ceramides modulate programmed cell death in plants. Genes Dev 17:2636–2641
Lin SS, Martin R, Mongrand S, Vandenabeele S, Chen KC, Jang IC, Chua NH (2008) RING1 E3 ligase localizes to plasma membrane lipid rafts to trigger FB1-induced programmed cell death in Arabidopsis. Plant J 56:550–561
Lincoln JE, Richael C, Overduin B, Smith K, Bostock R, Gilchrist DG (2002) Expression of the antiapoptotic baculovirus p35 gene in tomato blocks programmed cell death and provides broad-spectrum resistance to disease. Proc Natl Acad Sci USA 99:15217–15221
Lisi S, Mazzon I, White K (2000) Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics 154:669–678
Lorrain S, Vailleau F, Balague C, Roby D (2003) Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci 8:263–271
Mach JM, Castillo AR, Hoogstraten R, Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98:771–776
Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Frohlich KU (2004) Apoptosis in yeast. Curr Opin Microbiol 7:655–660
Matsumura H, Nirasawa S, Kiba A, Urasaki N, Saitoh H, Ito M, Kawai-Yamada M, Uchimiya H, Terauchi R (2003) Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L) cells. Plant J 33:425–434
Mengiste T, Chen X, Salmeron J, Dietrich R (2003) The BOTRYTIS SUSCEPTIBLE1 gene encodes an R2R3MYB transcription factor protein that is required for biotic and abiotic stress responses in Arabidopsis. Plant Cell 15:2551–2565
Mur LA, Carver TL, Prats E (2006) NO way to live: the various roles of nitric oxide in plant–pathogen interactions. J Exp Bot 57:489–505
Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E (2008) The hypersensitive response: the centenary is upon us but how much do we know? J Exp Bot 59:501–520
Raffaele S, Rivas S, Roby D (2006) An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett 580:3498–3504
Raffaele S, Vailleau F, Leger A, Joubes J, Miersch O, Huard C, Blee E, Mongrand S, Domergue F, Roby D (2008) A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis. Plant Cell 20:752–767
Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11:1695–1708
Reina-Pinto JJ, Voisin D, Kurdyukov S, Faust A, Haslam RP, Michaelson LV, Efremova N, Franke B, Schreiber L, Napier JA, Yephremov A (2009) Misexpression of FATTY ACID ELONGATION1 in the Arabidopsis epidermis induces cell death and suggests a critical role for phospholipase A2 in this process. Plant Cell 21:1252–1272
Rusterucci C, Aviv DH, Holt BF 3rd, Dangl JL, Parker JE (2001) The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13:2211–2224
Sanchez P, de Torres Zabala M, Grant M (2000) AtBI-1, a plant homologue of Bax inhibitor-1, suppresses Bax-induced cell death in yeast and is rapidly upregulated during wounding and pathogen challenge. Plant J 21:393–399
Shi L, Bielawski J, Mu J, Dong H, Teng C, Zhang J, Yang X, Tomishige N, Hanada K, Hannun YA, Zuo J (2007) Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Res 17:1030–1040
Stone JM, Heard JE, Asai T, Ausubel FM (2000) Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis mutants. Plant Cell 12:1811–1822
Stone SL, Hauksdottir H, Troy A, Herschleb J, Kraft E, Callis J (2005) Functional analysis of the RING-type ubiquitin ligase family of Arabidopsis. Plant Physiol 137:13–30
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194
Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8:397–403
Torres MA, Jones JD, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37:1130–1134
Vailleau F, Daniel X, Tronchet M, Montillet J-L, Triantaphylides C, Roby D (2002) A R2R3-MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proc Natl Acad Sci USA 99:10179–10184
Vaux DL, Silke J (2005) IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol 6:287–297
Wang S, Hawkins C, Yoo S, Muller H-A, Hay B (1999) The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98:453–463
Wang W, Yang X, Tangchaiburana S, Ndeh R, Markham JE, Tsegaye Y, Dunn TM, Wang G-L, Bellizzi M, Parsons JF, Morrissey D, Bravo JE, Lynch DV, Xiao S (2008) An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20:3163–3179
Watanabe N, Lam E (2006) Arabidopsis Bax inhibitor-1 functions as an attenuator of biotic and abiotic types of cell death. Plant J 45:884–894
Williams B, Dickman M (2008) Plant programmed cell death: can’t live with it; can’t live without it. Mol Plant Pathol 9:531–544
Wright KM, Duncan GH, Pradel KS, Carr F, Wood S, Oparka KJ, Cruz SS (2000) Analysis of the N gene hypersensitive response induced by a fluorescently tagged tobacco mosaic virus. Plant Physiol 123:1375–1386
Xu P, Rogers SJ, Roossinck MJ (2004) Expression of antiapoptotic genes bcl-xL and ced-9 in tomato enhances tolerance to viral-induced necrosis and abiotic stress. Proc Natl Acad Sci USA 101:15805–15810
Yang S, Hua J (2004) A haplotype-specific resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 16:1060–1071
Yang S, Yang H, Grisafi P, Sanchatjate S, Fink GR, Sun Q, Hua J (2006) The BON/CPN gene family represses cell death and promotes cell growth in Arabidopsis. Plant J 45:166–179
Yang H, Yang S, Li Y, Hua J (2007) The Arabidopsis BAP1 and BAP2 genes are general inhibitors of programmed cell death. Plant Physiol 145:135–146
Yao N, Greenberg JT (2006) Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell 18:397–411
Yu IC, Parker J, Bent AF (1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA 95:7819–7824
Yu LH, Kawai-Yamada M, Naito M, Watanabe K, Reed JC, Uchimiya H (2002) Induction of mammalian cell death by a plant Bax inhibitor. FEBS Lett 512:308–312
Acknowledgments
We would like to thank the Arabidopsis Resource Center at the Ohio State University for the T-DNA insertion mutants. This work was supported in part by the Natural Science Foundation of China (Grant No. 30771399), the National Basic Research Program of China (2009CB119005), and the National Key Project for Research on Transgenic Plants (2009ZX08001-017B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Q. Zhao.
Rights and permissions
About this article
Cite this article
Basnayake, B.M.V.S., Li, D., Zhang, H. et al. Arabidopsis DAL1 and DAL2, two RING finger proteins homologous to Drosophila DIAP1, are involved in regulation of programmed cell death. Plant Cell Rep 30, 37–48 (2011). https://doi.org/10.1007/s00299-010-0941-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0941-6