Abstract
A method for the genetic transformation of several citrus cultivars is described, including cultivars observed to be recalcitrant to conventional epicotyl-mediated transformation. Embryogenic cell suspension cultures, established from unfertilized ovules were used as target tissues for Agrobacterium-mediated transformation. Several modifications were made to the culture environment to investigate factors required for efficient transfer of the T-DNA and the subsequent regeneration of transgenic citrus plants. It was determined that co-cultivation of citrus cells and Agrobacterium in EME medium supplemented with maltose (EME-M) and 100 μM acetosyringone for 5 days at 25°C was optimum for transformation of each of the citrus cultivars. Efficient selection was obtained and escapes were prevented when the antibiotic hygromycin B was used as a selection antibiotic following transformation with an Agrobacterium strain containing hptII in the T-DNA region. Transgenic embryo regeneration and development was enhanced in medium that contained a liquid overlay consisting of a 1:2 mixture of 0.6 M BH3 and 0.15 M EME-M media. PCR and Southern blot analyses confirmed the presence of the T-DNA and the stable integration into the genome of regenerated plants, while RT-PCR demonstrated variable amounts of RNA being transcribed in different transgenic lines. This protocol can create an avenue for insertion of useful traits into any polyembryonic citrus cultivar that can be established as embryogenic cell suspension cultures, including popular specialty mandarins and seedless cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a major thrust in citrus improvement as competition from international citrus markets, disease and pest pressure, and other abiotic and biotic conditions stimulate worldwide interest (Grosser et al. 2000). Several strategies exist for the genetic improvement of citrus including conventional breeding and genetic transformation (Pena et al. 2007). Currently, genetic transformation of citrus as a tool for citrus improvement is gaining in popularity. This method is especially useful in cases where it is not possible to introduce a particular trait of interest to an otherwise elite cultivar using conventional breeding (Dutt et al. 2010a).
Citrus cultivars vary in their response to in vitro organogenesis and genetic transformation. This results in the need for cultivar-specific optimization of in vitro protocols (Dutt et al. 2009). Of the several methods available for the genetic transformation of citrus, the most popular method to transform a wide range of citrus cultivars is Agrobacterium-mediated transformation using epicotyl explants as target cells for incorporation of the T-DNA (Dutt and Grosser 2009). However, this method is not suitable for the transformation of any seedless cultivar. Also, specialty cultivars in the mandarin group remain difficult to transform using this method (Khawale et al. 2006; Dutt et al. 2009).
Genetic transformation using embryogenic cell suspension cultures offers a practical alternative to the transformation of epicotyl explants obtained from germinating seedlings, since almost all polyembryonic cultivars can be introduced in vitro as embryogenic cell suspension cultures. Availability of established cultures is year round in contrast to the seasonal availability of fresh citrus fruits, which limits the seed availability to certain times of the year. Also, the single cell origin of somatic embryos eliminates non-chimeric plant production (Polito et al. 1989), which can be a major problem in citrus transformation (Domínguez et al. 2004). In this study, we investigate a method of transformation using cell suspension cultures. Amenability of cell suspension cultures to transformation using Agrobacterium would allow the transformation of any cultivar that can be introduced as embryogenic cell masses, including specialty seedless sweet oranges or Satsuma mandarins and other difficult-to-transform cultivars of the mandarin or lemon group. We also outline modifications to the culture environment for efficient transformation and regeneration of transgenic citrus plants.
Materials and methods
Initiation of embryogenic suspension cell masses
Unfertilized ovules were extracted from surface-sterilized fruits of Citrus sinensis (L.) Osbeck cvs. ‘Hamlin’, ‘Valencia’, and ‘OLL8’ (an early maturing, low seeded ‘Valencia’-like somaclone); C. reticulata Blanco. cvs. ‘Ponkan’ and ‘W Murcott’; C. amblycarpa (Hassk.) Ochse, and C. depressa Hayata cv ‘Shekwasha’ and plated onto solid callus induction medium (DOG medium consisting of MT (Murashige and Tucker 1969) salts and vitamins supplemented with 50 g L−1 sucrose, 500 mg L−1 malt extract, 8 g L−1 agar, and 5 mg L−1 kinetin, pH 5.8). Ovules were subcultured on a monthly basis until production of embryogenic callus. The callus was maintained by monthly transfer on a hormone-free EME-S callus-maintenance medium (EME medium supplemented with 50 g L−1 sucrose, 8 g L−1 agar, pH 5.8; Grosser and Gmitter 1990a). Embryogenic callus of C. unshiu Marcow. cv. ‘Okitsu wase’ was kindly provided by the National Institute of Horticultural and Herbal Science, Jeju, Korea and maintained on EME-S medium. For embryogenic cell suspension cultures, approximately 5 g of callus was incubated in 25 ml of liquid H + H cell proliferation medium and subsequently subcultured on a 2-week transfer cycle (Grosser and Gmitter 1990a). Actively dividing embryogenic suspension cells were treated with Agrobacterium 7 days after the third subculture.
Construction of transformation vectors
All vectors were constructed based on either pCAMBIA 1300 (containing hptII as a selectable marker gene) or pCAMBIA2300 (containing nptII as a selectable marker gene). Restriction digestion and DNA manipulation were similar for the construction of both vectors. The egfp gene was excised as a BamHI/NotI fragment from pEGFP (Clontech Laboratories, Inc., USA) and ligated into a BamHI/NotI cloning site between the doubly enhanced cauliflower mosaic virus (CaMV) 35S promoter and a CaMV 3′ terminator in a pUC18-derived plasmid pUCLON to form plasmid pUEGFP. A 1.8-kb HindIII/EcoRI cassette containing p35s-EGFP-3′CaMV was isolated and cloned into compatible restriction sites of the pCAMBIA-based cloning vectors, pCAMBIA1300 or pCAMBIA2300, to form plasmid pCAMBIA1300-GFP or pCAMBIA2300-GFP (Fig. 1). E. coli strain DH5α was used for the cloning of all plasmids. All cloning was verified first by restriction analysis and then by DNA sequencing. Both binary plasmids were introduced into A. tumefaciens strain EHA105 (Hood et al. 1993) by the freeze–thaw method (Burrow et al. 1990).
Agrobacterium-mediated transformation
All initial transformation experiments to establish a working protocol were carried out using ‘Hamlin’. The protocol thus developed was applied to the other cultivars evaluated in this study. Agrobacterium-mediated transformation was carried out either with pCAMBIA1300-GFP or pCAMBIA2300-GFP (Fig. 1). Two milliliter of a vigorously growing Agrobacterium culture, initiated the night before, was seeded into 48 ml YEP medium containing appropriate antibiotics as described by Dutt and Grosser (2009). The cells were cultured for 3 h before being collected by centrifugation at 5,000×g for 6 min at 25°C and resuspended to an OD600 of 0.3 using liquid EME-S medium.
Embryogenic cells were incubated in liquid EME-S medium containing the appropriate Agrobacterium strain for 10 min. Subsequently, cells were blotted dry on sterile Whatman filter paper disks, plated on solid EME-M medium (sucrose was replaced with maltose), supplemented with 100 μM acetosyringone, and incubated in the dark at 25°C for 5 days before transfer to EME-M selection medium. Selection medium was prepared by the addition of 400 mg L−1 timentin (Duchefa Biochemie B.V., Netherlands) to precooled EME-M followed by the addition of either 25 mg L−1 hygromycin B (Sigma–Aldrich Corp., USA) or 100 mg L−1 kanamycin (Fisher Scientific, USA). The embryogenic cell cultures were maintained at 28°C on a standard 16-h light/8-h dark cycle using cool white fluorescent light (75 μmol s−1 m−2) and transferred into fresh antibiotic containing selection medium on a bi-weekly basis. During the second subculture, cells were transferred either solely onto solid EME-M medium or on solid EME-M medium overlaid with 2 ml of a 1:2 (v:v) mixture of 0.6 M BH3 and 0.15 M EME-M liquid media (henceforth called 1:2 medium; Dutt et al. 2010b). The liquid overlay medium was supplemented with 25 mg L−1 hygromycin B and 400 mg L−1 timentin.
Selection and further development of transformants
Developing embryos were observed for EGFP-specific fluorescence. EGFP-specific fluorescence was evaluated using a Zeiss SV11 epi-fluorescence stereomicroscope equipped with a light source consisting of a 100-W mercury bulb and an FITC/GFP filter set with a 480 nm excitation filter and a 515 nm long-pass emission filter (Chroma Technology Corp., USA). Globular embryoids observed to be EGFP positive were cultured over 0.22 mm cellulose acetate membrane filters on solid EME-M medium to normalize and enlarge the embryoids (Niedz et al. 2002). Transgenic embryos were subsequently enlarged on EME-1500 embryo maturation medium and germinated on B+ medium. Plantlets were transferred for further root development and growth into RMAN rooting medium. After 2 months of growth in vitro, the rooted plantlets were potted into Metromix potting medium (Sun Gro Horticulture, USA) and acclimated to greenhouse conditions. Unless otherwise mentioned, all media formulations were as described by Grosser and Gmitter (1990a).
Ex vitro micrografting
A rapid ex vitro micrografting technique as described by Skaria (2000) was modified for shoots that did not root in vitro. Tender shoots from young PCR-positive plants in RMAN medium were excised, cut into pieces along with 1 cm of attached stem and prepared for micrografting either onto diploid Carrizo or tetraploid Orange16 rootstock (Grosser et al. 2003). Vigorously growing 6 months old rootstocks were chosen. The rootstock was decapitated and a cut made in the center of the stem through the pith. A tapering cut was made on the transgenic stem exposing the pith, and a wedge graft union was made between the rootstock and transgenic scion. To stabilize the graft union, a thin strip of Nescofilm® was wrapped around the graft union. A 200 μl pipette tip “cap” was placed on top of the graft to maintain a high level of humidity around the bud. The grafted plant was placed under low light and room temperature conditions for 2 weeks following grafting. The plants were subsequently transferred to 80% shade and high humidity levels (80–90%) maintained in a greenhouse. After 3 weeks, plants were transferred into another greenhouse that was shaded with a 40% shade cloth. The pipette tip was then removed and all side branches as well as rootstock leaves were removed to allow the bud to grow.
Analysis of transgene integration
Citrus genomic DNA was used to confirm the presence of transgene(s) in the citrus genome. A 0.5 cm leaf disk, obtained from regenerating transgenic plants in culture, was extracted as per manufacturer’s instructions with the Extract-N-Amp™ Plant PCR Kit (Sigma–Aldrich Corp., USA). Duplex PCR was performed with 4 μL of the leaf disk extract in Extract-N-Amp PCR Ready Mix with hptII (HP51, 5′-ATG AAA AAG CCT GAA CTC AC-3′ and HP32, 5′-CTA TTT CTT TGC CCT CGG ACG A-3′) and egfp (EG-51, 5′-ATG GTG AGC AAG GGC GAG GAG CTG T-3′ and EG-32, 5′-CTT GTA CAG CTC GTC CAT GCC GAG A-3′)-specific primers. Amplified DNA fragments were electrophoresed on a 1% agarose gel containing GelRed™ Nucleic Acid Gel Stain (Biotium Inc., USA) and visualized under UV light.
RNA was isolated from 100 mg of leaf tissue using an RNeasy Mini Kit (Qiagen Inc., USA). After treatment with DNAse I(Qiagen) to remove contaminating DNA, the RNA was quantified spectrophotometrically. RNA concentration of each sample was adjusted to 500 ng ml−1 and quality was checked by agarose gel electrophoresis. cDNA was synthesized from 500 ng total RNA using gene-specific primers as detailed earlier and a QIAGEN OneStep RT-PCR Kit as described by the manufacturer.
For Southern blot analysis, genomic DNA was isolated using the Qiagen DNeasy Plant Maxi Kit. Fifteen microgram EcoRI digested DNA was loaded on a 0.8% agarose gel in 1× TAE buffer. Following gel electrophoresis of digested genomic DNA and subsequent depurination, denaturation, and neutralization treatments, DNA was transferred onto a positively charged nylon membrane by capillary transfer. A DIG-labeled egfp probe was produced by PCR labeling. The DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Applied Science, USA) was used for subsequent hybridization steps. Following hybridization to the probe, chemiluminescence substrate CDP-Star was used for detection of hybridization signals using X-ray film autography. Genomic DNA extracted from a non-transformed control plant served as negative control.
Data analysis
Each experiment was repeated at least twice and treatments were replicated six times. A replicate consisted of one Petri dish. Data were analyzed to calculate standard error using MS Excel.
Results and discussion
Hygromycin B for efficient selection of transformed cells
We report herein an efficient and reproducible transformation system using citrus embryogenic suspension cells as target tissue for Agrobacterium-mediated transformation. Embryogenic suspension cells derived from callus of different citrus cultivars have been observed to have a similar morphology and can be cultured on the same hormone-free medium and under the same culture conditions on a 2-week cycle (Grosser and Gmitter 1990b; Jiménez and Guevara 1995). Cells in a liquid medium undergo a ‘conditioning’ phase that delays the occurrence of changes in the endogenous hormone levels in response to the carbohydrate treatment (Jiménez et al. 2001). Liquid medium also improves and facilitates somatic embryo development (Cabasson et al. 1997). These factors make suspension cell cultures a promising target for the incorporation of transgenes.
Initial experiments to establish a reproducible transformation system using pCAMBIA2300-GFP were not successful. Kanamycin selection resulted in erratic and low transgenic embryo production (Fig. 2). Inefficient kanamycin selection was either due to cells overcoming the effects of the antibiotic or to the protection of cells from kanamycin by the surrounding cells (Moore et al. 1992; Cervera et al. 1998).
A kill-curve experiment was subsequently performed using embryogenic suspension cells to evaluate the effectiveness of hygromycin B as a suitable selection antibiotic. Cells were plated in EME-M medium containing varying levels of kanamycin or hygromycin B. It was observed that kanamycin was indeed ineffective in the selection of ‘Hamlin’ cells. Even very high levels of kanamycin (200 mg L−1, Fig. 3) were not adequate to completely prevent escapes. Hygromycin B in general is more toxic than kanamycin and has been observed to rapidly kill nontransformed cells (Angenon et al. 1994). Our results demonstrated that 25 mg L−1 hygromycin B could inhibit the proliferation of non-transgenic embryogenic suspension cells (Fig. 3). Transformation of cells with pCAMBIA1300-GFP and subsequent selection demonstrated the effectiveness of using this antibiotic for the selection of transgenic cells (Fig. 2). Co-cultivation for 5 days was determined to be optimum, as longer durations of time resulted in bacterial overgrowth. Li et al. (2002), demonstrated successful callus transformation of ‘Valencia’ sweet orange using 50 mg L−1 hygromycin B, but our studies demonstrated that 25 mg L−1 of the antibiotic was adequate for efficient selection. In contrast to our results, Han et al. (2005) reported GFP expression in three callus clumps of ‘Miyagawa Wase’ mandarin on a kanamycin containing medium, while none was observed in medium containing hygromycin B. No transgenic plants were, however, recovered in that study. In our results, hygromycin B efficiently discriminated between the transgenic and non-transgenic cells by suppressing the non-transgenic cells and allowing efficient proliferation of transformed cells (Fig. 2). Necrosis was observed in the non-transformed cells, which made identification and selection of transgenic tissues easier. In subsequent experiments, hygromycin B was used for selection and discrimination between transgenic and non-transgenic cells.
Addition of acetosyringone results in vir gene activation in Agrobacterium (Stachel et al. 1985). Acetosyringone has been reported to influence the transformation rate in some citrus cultivars, while it does not benefit others (Dutt and Grosser 2009). In the current study, addition of 100 μM acetosyringone was necessary for transformation in all cultivars evaluated. Similar results have been observed by Li et al. (2002), who concluded that acetosyringone was required due to the absence of any wounding treatments on the citrus cells co-cultivated with Agrobacterium.
Use of a 1:2 medium overlay
Putative transgenic cells were transferred either onto EME-M medium or on EME-M medium overlaid with 2 ml 1:2 medium mixture during the second subculture. It was observed that there was a threefold improvement in the production of transgenic embryos over the control treatment, which did not include any 1:2 medium mixture overlay (Fig. 4). The 1:2 medium mixture has been extensively used during production of tetraploid citrus following protoplast fusion (Grosser and Gmitter 1990a; Grosser et al. 1992; Grosser and Chandler 2004). Recently, we used this medium to develop a technique that resulted in the production of a large number of autotetraploid Ponkan mandarin plants (Dutt et al. 2010b). The 1:2 medium mixture effectively mimics the conditions prevalent in developing seeds by providing higher osmotic levels around the developing somatic embryo. Higher osmotic levels have been observed to be an important factor associated with embryogenesis and storage reserve accumulation (Fujii et al. 1990; Attree et al. 1992), since it helps to promote a cascade of events that are necessary for embryogenesis to occur (Brisibe et al. 1994). Subsequent transfer to embryo maturation medium (EME-1500) brought down osmoticum levels and allowed further embryo development and germination to occur. Removal of high osmotic levels is critical for further development, as high osmotic levels can result in inhibition of embryo germination by blocking water uptake (Finkelstein and Crouch 1986).
Embryo proliferation following transformation of embryogenic cell suspension cultures of ‘Hamlin’ sweet orange with an Agrobacterium strain containing pCAMBIA1300-GFP. Putatively transformed cells on EME-M selection medium was overlaid with 2 ml of a 1:2 (v:v) mixture of 0.6 M BH3 medium and 0.15 M EME-M liquid medium. This liquid medium was supplemented with 25 mg L−1 hygromycin B and 400 mg L−1 timentin
Age of culture on transformation
Two groups of ‘Hamlin’ suspension cell lines were evaluated for their competence to transform. The first, an old cell line, had been routinely maintained for 6 years on a monthly subculture regime. The second was a young line that had been initiated from unfertilized ovules in the previous season and therefore less than a year old. Both suspension cell lines were treated similarly with the Agrobacterium strain containing pCAMBIA1300-GFP and after 5 days of co-cultivation plated in EME-M medium supplemented with antibiotics. We observed some EGFP expression in the callus phase of the old cell line. However, it was not possible to regenerate actual transgenic embryos and plants from such cells. The new cell line, however, produced 53 embryos on average that expressed EGFP (Fig. 5). Normal plants could also be regenerated from such embryos.
A change in carbohydrate source has been reported to be useful in the induction of somatic embryogenesis in citrus cells (Ben-Hayyim and Neumann 1983; Gavish et al. 1991). Carbohydrate such as glycerol are especially effective in inducing further development of citrus somatic embryos (Jiménez et al. 2001) by forming embryo initials that develop into globular embryos (Gavish et al. 1992). However, we were not able to induce embryo formation by plating the callus cells of the old ‘Hamlin’ line in medium containing glycerol as a carbohydrate source (results not shown). Similar results were observed following transformation of other citrus lines that had been maintained over time in culture such as C. amblycarpa and C. depressa cv ‘Shekwasha’. Transgenic plants of the sweet orange cultivars ‘Valencia’ and ‘OLL8’, as well as the mandarin/tangerine cultivars ‘Ponkan’, ‘Okitsu wase’, and ‘W Murcott’ could be regenerated (Table 1). The age of cells has been correlated with cell competence for transformation due to the availability of higher concentration of auxins available in the younger cells (Huang et al. 1993; Cervera et al. 1998).
It was observed that time required for proliferation of EGFP expressing cells was dependent on the initial age of cells used for transformation. Younger cell lines were quicker to proliferate into embryogenic callus than older lines (Table 2). There was no difference in the time taken for regeneration of embryogenic callus and the subsequent embryo production in sweet orange cells, while ‘Ponkan’ cells regenerated 2 weeks earlier than ‘Okitsu Wase’ or ‘W Murcott’. Small, globular stage EGFP expressing somatic embryos were visible on the proliferating callus after 2 weeks for the sweet oranges, while it took a further 4 weeks for embryo proliferation in the mandarin group. Average number of embryos that expressed EGFP ranged from 15 in ‘Okitsu Wase’ to 53 in ‘Hamlin’. It has been observed, in other explants types, that aging decreased the competence of plant cells to Agrobacterium infection (Cervera et al. 1998). Citrus cell lines can be maintained for years in an embryogenic competence state (Gavish et al. 1991). Over time, these cells do become habituated in culture. This can lead to buildup of mutations, which can result in low differentiation capability (Grosser and Gmitter 1990a; Etienne et al. 1993). Thus, our data indicate that the use of newly initiated embryogenic cultures are necessary for efficient transgenic plant recovery, which is in contrast to the efficiency of somatic hybridization experiments, where older cultures facilitate somatic hybrid plant recovery by reducing or eliminating regeneration from unfused embryogenic cell line protoplasts (Grosser et al. 2000).
In contrast to Agrobacterium-mediated transformation using epicotyl explants, chimeric transgenic plants were not observed in any of the cultivars evaluated in the study (Fig. 6d–f). Chimeric transgenic plant production is a major problem during citrus transformation (Pena et al. 1995). Due to the single cell origin of somatic embryos (Haccius 1978), we did not observe any chimeric plants in our system. Transgenic EGFP expressing cells developed into globular stage embryos within 3 months of transformation. Secondary embryogenesis (Fig. 6e) from primary somatic embryos also resulted in embryo production. Embryo maturation was achieved by cultivating individual somatic embryos in EME-1500 embryo maturation medium. In most cases, normal transgenic embryos germinated into normal plants on B+ medium. The only exception was ‘Okitsu Wase’, which did not produce normal embryos. Many embryos in this cultivar did not develop further following the globular stage. In several, there was a lack of dedifferentiation of protoderm and absence of apical meristems. Abnormal embryo formation has also been observed in citrus by Tomaz et al. (2001). Chromosomal variations occur when the calluses are subcultured for a long time and it is possible that mutations in the ‘Okitsu Wase’ embryogenic suspension line resulted in abnormal embryos.
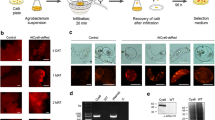
Steps in Agrobacterium-mediated genetic transformation of citrus embryogenic suspension cultures. a Citrus cell suspension cultures, b cell suspension with Agro, c transgenic callus/embryos regenerating on selection medium, d EGFP expressing transgenic citrus callus cells, e secondary embryo formation from globular stage primary embryo, f close-up of EGFP expressing transgenic citrus somatic embryos
Transgenic plant recovery
Ex vitro micrografting is a useful technique to rapidly generate a population of clones in a short period of time. Citrus, being a woody fruit tree species, is slow in growth in vitro. Over 90% survival rate was observed using this technique and has become the technique of choice for us to rapidly multiply select transgenic lines. The waterproof and self-adhering properties of Nescofilm were found suitable to provide a barrier that kept moisture inside the graft union while allowing gaseous exchange to occur. Providing a high humidity to the grafted shoot tip by the utilization of the 200 μL pipette tip ‘cap’ prevented the shoot tip from drying (Fig. 7a). The graft union healed within 2 weeks after grafting and plants could be transferred to a heavily shaded greenhouse within a month. Removal of the cap while maintaining high humidity allowed the transgenic buds to push within 5 weeks after micrografting (Fig. 7b, c).
Ex vitro micrografting of transgenic shoots and recovery of transgenic plants. a A 200 μl pipette tip “cap” placed on top of the graft union to maintain a high level of humidity around the bud. b The same graft union wrapped with Nescofilm and without the cap. c A transgenic plant 3 months after grafting. Inset (left) is a young transgenic leaf as visualized under an epi-fluorescent microscope. Non-transgenic leaf (inset, right) appears red under blue light due to auto-fluorescence of chlorophyll
Molecular analysis of transgenic plants
EGFP was visualized in embryogenic citrus cells within a month after transformation (Fig. 6). Depending on the cultivar, 70–80% of transgenic embryos germinated on B+ medium and were transferred into RMAN medium for further growth. Plants obtained from somatic embryos exhibited a normal phenotype. A total of 49 regenerated transgenic shoots in RMAN medium were evaluated by PCR to confirm the presence of both the hptII selectable marker gene and the egfp reporter gene. PCR analysis indicated that all plants that expressed the EGFP protein contained both genes in the plant’s genome. Results from seven randomly selected samples tested are shown in Fig. 8a. Thus, the observable EGFP expression ensured the early and efficient detection of transgenic plants for subsequent molecular analysis and evaluation.
Amplification products obtained from a duplex PCR of genomic DNA of transgenic citrus plants with egfp and hptII gene-specific primers, which successfully amplified the expected 720 bp egfp gene fragment and the 1,026 bp hptII gene fragment M, 1 kb marker; 1–7 are seven individual transgenic lines. b Reverse transcriptase PCR of total RNA isolated from the same seven transgenic lines evaluated using PCR with egfp and hptII gene-specific primers. Equal loading of RNA was confirmed by measuring the 28S rRNA
To confirm transcription of transgene mRNA, transgenic lines was analyzed using RT-PCR. Our results confirmed the production of full length hptII and egfp mRNA (Fig. 8b). The amount of mRNA produced depended on the transgenic line tested. Transgenic lines 4 and 5 for example produced relatively lower amounts of hptII mRNA than others, while transgenic line 3 produced relatively lower amounts of egfp mRNA. Transgene inactivation generally occurs at highest frequency when multiple copies of the gene are integrated either at a single insertion site or dispersed throughout the genome (Ottaviani et al. 1993). We did not observe gene silencing in any of the transgenic lines tested. Also, no visual difference in EGFP expression was observed in any of the transgenic plants evaluated.
Southern blot analysis determined the stable integration of transgene into the plant’s genome. Analysis was subsequently performed on five randomly selected transgenic plants and a non-transgenic control using a DIG-labeled DNA probe corresponding to the egfp gene. Genomic DNA was digested with EcoRI, which is present as a single restriction site downstream of the egfp probe sequence in the T-DNA. This ensured that any hybridization fragments corresponded to the number of integrated T-DNA sequences. Among the transgenic plants analyzed, two independent lines (lines 2, 3, and 4) had one copy of the egfp gene, while the other two lines (lines 1 and 5) had three copies (Fig. 9). Transgenic plants obtained via Agrobacterium-mediated transformation often contain single copy insertions (Hansen and Wright 1999). Levels of transgene expression in plants vary between independent transformants, and this variation is usually related to differences in transgene copy number and/or site of gene integration (Prols and Meyer 1992). Variation in the copy number between different transgenic lines could lead to variable gene expression levels in independent transformants as was observed following RT-PCR. Our results demonstrated that gene integration via embryogenic suspension cell-mediated transformation followed a similar trend to that observed using the more popular epicotyl-mediated transformation (Dutt and Grosser 2009; Dutt et al. 2010a).
In conclusion, we report an efficient Agrobacterium-mediated transformation system using embryogenic citrus cells of several mandarin and sweet orange cultivars. Efficient transformation results were obtained using young cell suspension cultures. Molecular analysis confirmed that transgenes were integrated into the plant’s genome, and individual citrus plants were from independent transformation events. The efficiency of the reported transformation system made it possible to generate a large number of transgenic citrus plants in a relatively short period, which would facilitate the testing of various transgenes for citrus improvement. Amenability of embryogenic cell suspension cultures to transformation using Agrobacterium would allow the transformation of any cultivar that can be introduced in vitro as embryogenic cell masses, including specialty seedless sweet oranges or Satsuma mandarins and other difficult-to-transform cultivars of the mandarin group. It is also expected that this technology could be easily adapted to important commercial lemon cultivars.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- EGFP:
-
Enhanced green fluorescent protein
- hptII:
-
Hygromycin phosphotransferase gene
- MES:
-
2-(N-morpholino)ethane sulfonic acid
- MS:
-
Murashige and Skoog medium
- NAA:
-
Naphthaleneacetic acid
- nptII:
-
Neomycin phosphotransferase gene
- YEP:
-
Yeast extract peptone
References
Angenon G, Dillen W, Van Montagu M (1994) Antibiotic resistance markers for plant transformation. In: Gelvin SB, Schilperoort RA (eds) Plant molecular biology manual. Kluwer, Dordrecht, pp 1–13
Attree SM, Pomeroy MK, Fowke LC (1992) Manipulation of conditions for the culture of somatic embryos of white spruce for improved triacylglycerol biosynthesis and desiccation tolerance. Planta 187:395–404
Ben-Hayyim G, Neumann H (1983) Stimulatory effect of glycerol on growth and somatic embryogenesis in citrus callus cultures. Z Pflanzenphysiol 110:331–337
Brisibe EA, Miyake H, Taniguchi T, Maeda E (1994) Abscisic acid and high osmoticum regulation of development and storage reserve accumulation in sugarcane somatic embryos. Jpn J Crop Sci 63:689–698
Burrow MD, Chlan CA, Sen P, Murai N (1990) High frequency generation of transgenic tobacco plants after modified leaf disk co-cultivation with Agrobacterium tumefaciens. Plant Mol Biol Rep 8:124–139
Cabasson C, Alvard D, Dambier D, Ollitrault P, Teisson C (1997) Improvement of Citrus somatic embryo development by temporary immersion. Plant Cell Tiss Org Cult 50:33–37
Cervera M, Pina JA, Juarez J, Navarro L, Pena L (1998) Agrobacterium mediated transformation of citrange: factors affecting transformation and regeneration. Plant Cell Rep 18:271–278
Domínguez A, Cervera M, Pérez RM, Romero J, Fagoaga C, Cubero J, López MM, Juárez JA, Navarro L, Peña L (2004) Characterization of regenerants obtained under selective conditions after Agrobacterium-mediated transformation of citrus explants reveals production of silenced and chimeric plants at unexpected high frequencies. Mol Breed 14:171–183
Dutt M, Grosser J (2009) Evaluation of parameters affecting Agrobacterium-mediated transformation of citrus. Plant Cell Tiss Org Cult 98:331–340
Dutt M, Orbovic V, Grosser JW (2009) Cultivar dependent gene transfer into citrus using Agrobacterium. Proc Fla State Hortic Soc 122:85–89
Dutt M, Madhavaraj J, Grosser JW (2010a) Agrobacterium tumefaciens-mediated genetic transformation and plant regeneration from a complex tetraploid hybrid citrus rootstock. Sci Hortic 123:454–458
Dutt M, Vasconcellos M, Song K, Gmitter F, Grosser JW (2010b) In vitro production of autotetraploid Ponkan mandarin (Citrus reticulata Blanco) using cell suspension cultures. Euphytica 173:235–242
Etienne H, Sotta B, Montoro P, Miginiac E, Carron MP (1993) Relation between exogenous growth regulators and endogenous indole-3-acetic acid and abscisic acid in the expression of somatic embryogenesis in Hevea brasiliensis (Müll. Arg). Plant Sci 88:91–96
Finkelstein RR, Crouch ML (1986) Rapeseed embryo development in culture on high osmoticum is similar to that in seeds. Plant Physiol 81:907–912
Fujii JAA, Slade D, Olsen R, Ruzin SE, Redenbaugh K (1990) Alfalfa somatic embryo maturation and conversion to plants. Plant Sci 72:93–100
Gavish H, Vardi A, Fluhr R (1991) Extracellular proteins and early embryo development in Citrus nucellar cell cultures. Physiol Plant 82:606–616
Gavish H, Vardi A, Fluhr R (1992) Suppression of somatic embryogenesis in Citrus cell cultures by extracellular proteins. Planta 186:511–517
Grosser JW, Chandler JL (2004) Production of twelve new allotetraploid somatic hybrid citrus breeding parents with emphasis on late maturity and cold-hardiness. J Amer Pomol Soc 58:21–28
Grosser JW, Gmitter FG Jr (1990a) Protoplast fusion and citrus improvement. Plant Breed Rev 8:339–374
Grosser JW, Gmitter FG Jr (1990b) Wide-hybridization of Citrus via protoplast fusion: progress, strategies, and limitations. In: Bennet AB, O’Neill SD (eds) Horticultural biotechnology, plant biology, vol 25. Wiley-Liss, New York, pp 31–41
Grosser JW, Gmitter FG, Louzada ES, Chandler JL (1992) Production of somatic hybrid and autotetraploid breeding parents for seedless citrus development. HortScience 27:1125–1127
Grosser JW, Ollitrault P, Olivares-Fuster O (2000) Somatic hybridization in Citrus: an effective tool to facilitate variety improvement. In Vitro Cell Dev Biol-Plant 36:434–449
Grosser JW, Graham JH, McCoy CW, Hoyte A, Rubio HM, Bright DB, Chandler JL (2003) Development of ‘‘tetrazyg’’ rootstocks tolerant of the diaprepes/phytophthora complex under greenhouse conditions. Proc Fla State Hortic Soc 116:262–267
Haccius B (1978) Question of unicellular origin of non-zygotic embryos in callus cultures. Phytomorphology 28(1):74–81
Han SH, Ahn HJ, Kang SK, Kim HY (2005) Expression of green fluorescent protein gene in the callus of Satsuma mandarin (Citrus unshiu cv. Miyagawa Wase) by Agrobacterium-mediated transformation. J Korean Soc Hortic Sci 46(1):39–42
Hansen G, Wright MS (1999) Recent advances in the transformation of plants. Trends Plant Sci 4:226–232
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Huang Y, Stokke DD, Diner AM, Barnes WM, Karnosky DF (1993) Virulence of Agrobacterium on Larix deciduas and their cellular interactions as depicted by scanning electron microscopy. J Exp Bot 44:1191–1201
Jiménez VM, Guevara E (1995) Regeneración in vitro mediante embriogénesis somática de variedades de cítricos. I. Obtención de callo friable y suspensiones celulares de naranja dulce (Citrus sinensis) y naranja agria (Citrus aurantium) cultivadas en Costa Rica. Agron Costarric 19:7–18
Jiménez VM, Guevara E, Herrera J, Bangerth F (2001) Endogenous hormone levels in habituated nucellar Citrus callus during the initial stages of regeneration. Plant Cell Rep 20:92–100
Khawale RN, Singh SK, Garg G, Baranwal VK, Ajirlo SA (2006) Agrobacterium-mediated genetic transformation of Nagpur mandarin (Citrus reticulata Blanco). Curr Sci 91(12):1700–1705
Li DD, Shi W, Deng XX (2002) Agrobacterium-mediated transformation of embryogenic calluses of Ponkan mandarin and the regeneration of plants containing the chimeric ribonuclease gene. Plant Cell Rep 21:153–156
Moore GA, Jacona CC, Neidigh JL, Lawrence SD, Cline K (1992) Agrobacterium-mediated transformation of citrus stem segments and regeneration of transgenic plants. Plant Cell Rep 11:238–242
Murashige T, Tucker DP (1969) Growth factor requirements of citrus tissue culture. Proc 1st Int Citrus Symp, pp 1155–1161
Niedz RP, Hyndman SE, Wynn ET, Bausher MG (2002) Normalizing sweet orange (C. sinensis (L.) Osbeck) somatic embryogenesis with semi-permeable membranes. In Vitro Cell Dev Biol 38:552–557
Ottaviani MT, Smith T, Haenisch CH (1993) Differential methylation and expression of the β-gus and npt genes in transgenic potato. Plant Sci 88:73–81
Pena L, Cervera M, Juarez J, Ortega C, Pina JA, Duran-Vila N, Navarro L (1995) High efficiency Agrobacterium-mediated transformation and regeneration of citrus. Plant Sci 104:183–191
Pena L, Cervera M, Ghorbel R, Domínguez A, Fagoaga C, Juarez J, Pina JA, Navarro L (2007) Genetic transformation. In: Khan IA (ed) Citrus genetics, breeding, and biotechnology. CABI International, Wallingford, UK, pp 329–344
Polito VS, McGranahan G, Pinney K, Leslie C (1989) Origin of somatic embryos from repetitively embryogenic cultures of walnut (Juglans regia L.): implications for Agrobacterium-mediated transformation. Plant Cell Rep 8:219–221
Prols F, Meyer P (1992) The methylation pattern of chromosomal integration region influence gene activity of transferred DNA in petunia. Plant J 2:165–175
Skaria M (2000) A microbudding technique for biological indexing and ultra-high density planting of citrus. Proc Fourteenth IOCV Conf, pp 411–413
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985) Identification of the signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Tomaz ML, Mendes BMJ, Mourão Filho FAA, Demétrio CGB, Jansakul N, Rodriguez PM (2001) Somatic embryogenesis in Citrus spp. carbohydrate stimulation and histodifferentiation. In Vitro Cell Dev Biol Plant 37:446–452
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Kamo.
Rights and permissions
About this article
Cite this article
Dutt, M., Grosser, J.W. An embryogenic suspension cell culture system for Agrobacterium-mediated transformation of citrus. Plant Cell Rep 29, 1251–1260 (2010). https://doi.org/10.1007/s00299-010-0910-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-010-0910-0