Abstract
Arabinogalactan proteins (AGPs) represent a class of proteoglycans implicated in the development and differentiation of cells and tissues both in planta and in vitro. Here we report that AGP-rich extracts isolated from media of embryogenic and non-embryogenic suspension cultures of sugar beet (Beta vulgaris L.) are able to enhance the organogenesis of guard protoplast-derived callus and to increase the number of shoots formed, in comparison to control cultures. Immunocytochemical detection of carbohydrate antigens in the extracts revealed the presence of epitopes that typify both AGP and pectin, the latter being frequently bound to AGPs or, in some cases, even contributing to the polysaccharide structure of proteoglycan molecules. The most abundant epitopes proved to be those recognized by the JIM13, LM2, and MAC207 antibodies, whereas some others could be found only in relatively small or trace amounts—these included epitopes recognized by JIM16, JIM5, and LM6. Surprisingly, the JIM4- and JIM8-binding epitopes that are expressed in the course of in vitro morphogenetic processes of many species could not be detected at all in sugar beet AGPs. This is the first report of the improvement of sugar beet protoplast-derived callus organogenesis by exogenous AGP-rich extracts, an achievement that will have great impact on the biotechnological applications of protoplast technology in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arabinogalactan proteins represent a class of proteoglycans that is widespread throughout the plant kingdom, having been found in every species, plant organ, and tissue examined so far (Nothnagel 1997; Showalter 2001). These molecules are involved in the regulation of several important events related to plant growth and development, including cell expansion (Willats and Knox 1996; van Hengel and Roberts 2002; Park et al. 2003; Lee et al. 2005), cell proliferation (Serpe and Nothnagel 1994), differentiation of specific tissue or cell types (Majewska-Sawka and Nothnagel 2000), formation and differentiation of vegetative and floral organs (Lu et al. 2001; Acosta-Garcia and Vielle-Calzada 2004; Wiśniewska and Majewska-Sawka 2006), and sexual reproduction (Sun et al. 2004a). AGPs have also been proven to play an important role in the course of in vitro development of plant cells and tissues, as perturbation of endogenous molecules by phenylglycoside dye ([β-d-Glc]3; Yariv et al. 1967) frequently results in severe disturbances of cell physiology. The observed effects depend on the length of treatment with (β-d-Glc)3, the applied concentration of the reagent, and the developmental phase. The cessation of the growth and mitotic divisions of suspension cells is the earliest disturbance observed (Serpe and Nothnagel 1994; Langan and Nothnagel 1997), whereas more stringent treatment may even lead to the induction of programmed cell death, as reported for Arabidopsis and tobacco cells (Gao and Showalter 1999; Chaves et al. 2002). In embryogenic carrot cultures, (β-d-Glc)3 perturbed morphogenesis so that either shoot primordia were malformed, while root formation was not affected, or overall growth was inhibited, depending on the developmental phase at which phenylglycoside was added (Thompson and Knox 1998). In Cichorium, somatic embryogenesis was completely blocked by treatment with 250 μM Yariv, the effect being reversible upon transfer of the cells to the control medium (Chapman et al. 2000a).
A slight perturbation of the AGP level in Arabidopsis suspension cell culture affected the expression of a number of genes, mostly related to the wounding response, as well as genes encoding cell wall proteins and signal transduction components (Guan and Nothnagel 2004).
The developmental pathway of cells or tissues in vitro might also be changed by supplementation of the culture medium with exogenous AGPs. Arabinogalactan proteins isolated from carrot seeds proved to be effective in increasing the number of embryos formed (Kreuger et al. 2000) and in re-induced embryogenic processes in cell cultures that had lost their embryogenic potential (Kreuger and van Holst 1993). A similar effect was observed when AGPs isolated from embryogenic cultures were added to non-embryogenic cells (van Hengel et al. 2001).
Aside from model plants such as carrot, tobacco, and Arabidopsis, only a very few species of agronomic importance have been studied in terms of a possible role for AGPs in the regulation of development in vitro. In Picea alba, the molecules isolated from embryogenic cells were able to improve the embryo maturation process (Egertsdotter and von Arnold 1995). In cotton, the number of embryos formed in cultures of two elite cultivars increased significantly when exogenous AGPs were added to the medium (Poon et al. 2004).
Here we report a beneficial effect of AGPs isolated from suspension culture media on the development and differentiation of sugar beet guard cell protoplasts, and in consequence on in vitro shoot regeneration in this important agronomic species.
Materials and methods
Shoot cultures
The diploid, male-fertile line of sugar beet (no. 0170) was obtained from the Sugar Beet Breeding Company (KHBC), Breeding Station in Straszków, Poland. Shoot tips of flowering plants were excised, sterilized with 5% calcium hypochlorite for 20 min, rinsed several times with sterile distilled water, and placed on Murashige and Skoog medium (MS; Murashige and Skoog 1962) supplemented with 4.4 μM benzyloaminopurine (BAP). After numerous shoots had been initiated from axillary buds, they were separated and placed on MS medium with 0.44 μM BAP and 0.11 μM naphthalene acetic acid (NAA) for shoot growth. Shoots were transferred to the same medium every 2 weeks, and the 2-week-old leaves were used for protoplast isolation.
Non-embryogenic suspension cultures
Non-embryogenic suspension cultures were initiated from white, friable callus that developed at the base of some shoot tip explants, placed on MS with 4.4 μM BAP. Callus was subcultured on the same medium every 4 weeks, and after the cultures were established, the portions of tissue were put into liquid MS medium containing 4.4 μM BAP, agitated on a rotary shaker (140 rpm), and transferred to fresh media once a week. Three-month-old suspensions, 7 days after the last subculture, were used as a source of conditioned medium and media-derived AGP-rich extracts.
Embryogenic suspension cultures
The cultures were initiated from unpollinated sugar beet ovules cultured in liquid MS supplemented with 4.4 μM BAP, and agitated on a rotary shaker (140 rpm). After numerous embryos and embryo-like structures had developed, they were transferred to fresh medium.
Protoplast isolation and culture
Excised leaves were preplasmolized overnight in 0.45 M sorbitol dissolved in salt solution (CPW), prepared as described by Frearson et al. (1973). The epidermis from the abaxial leaf surface was peeled off with fine forceps and placed in an enzyme solution composed of 1.9% cellulase RS, 0.1% macerozyme R-10 (both from Yakult Honsha Co. Ltd, Japan), and 0.003% pectolyase Y-23 (Seishin Pharmaceutical Co. Ltd, Japan) dissolved in CPW with 0.45 M sorbitol. The digestion took place for 8–12 h, with gentle agitation (20 rpm), at 26°C in the dark.
The protoplasts were separated from undigested tissue debris by filtration through 20 μm nylon mesh, and centrifuged at 1,400–1,500 rpm for 12 min. The protoplast pellet was gently resuspended and washed in CPW salts with 0.45 M sorbitol and then centrifuged. The washing step was repeated twice. Finally, the protoplasts were immobilized in 2.4% calcium alginate and cultured in liquid MS medium supplemented with 2 μM BAP, 5 μM NAA, 100 μM n-propyl gallate, and 0.35 M sucrose (the control). Several variations of the culture medium were tested with the aim of improving protoplast development and the differentiation of protoplast-derived calli into shoots:
-
1.
Four millilitres MS (as in the control) supplemented with 400 μl of conditioned medium collected from non-embryogenic suspensions of the same genotype, 7 days after the last subculture.
-
2.
Four millilitres MS (as in the control) supplemented with AGPs isolated from culture medium of non-embryogenic suspensions, at 0.5; 1.0; 2.5; 5.0; 10.0, and 20.0 μg/ml.
-
3.
Four millilitres MS (as in the control) supplemented with AGPs isolated from culture medium of embryogenic suspensions, at 0.5; 1.0; 2.5; 5.0; 10.0, and 20.0 μg/ml.
Shoot regeneration
After the callus microcolonies had formed and reached the size of several cells, the alginate beads were transferred to the regeneration media solidified with 1.1% agarose. They were initially kept in the dark for 2–3 weeks, and developing callus was then transferred onto fresh media and maintained under light.
The results of our previous studies suggested that both reduced sucrose content (1.5%) and the presence of TIBA in the medium significantly promoted sugar beet organogenesis, so we explored the possibility of further improving the process by comparing the effects of two different TIBA concentrations. Subsequently, the results were compared with those obtained for standard medium containing NAA (Table 1). Callus subcultures were performed every 3 weeks, and up to five subcultures were carried out for each experiment. Emerging shoots were transferred to MS medium without growth regulators.
Isolation of AGPs from conditioned media
AGPs were isolated from conditioned media derived from four identically treated non-embryogenic, efficiently proliferating suspension cell cultures and from two identically treated embryogenic cultures, all of the same sugar beet genotype (no. 0170). Each batch of conditioned medium was obtained from a 3-month-old suspension culture, but as these cultures were initiated on different dates, the media were collected at different times. Culture media were filtered through 40 μm nylon mesh, and centrifuged at 4,000 rpm for 20 min to remove cells and cell debris. The AGPs present in the media were precipitated with 60 μM (β-d-Glc)3 Yariv reagent [1,3,5-tri-(p-glycosyloxyphenylazo)-2,4,6-trihydroxybenzene] by incubation for 48 h at 4°C, in the presence of 1% NaCl and 0.02% NaN3. The AGP-Yariv complexes were centrifuged at 2,500 rpm for 20 min, the supernatant was discarded, and the pellet was resuspended in distilled water. The resulting mixture was centrifuged, the supernatant was saved, and the pellet was resuspended in aliquots of distilled water until the red color was washed out of the pellet. All the supernatants were pooled, supplemented with 1% NaCl and 0.02% NaN3, and incubated for 48 h at 4°C. Following centrifugation, the Yariv reagent was separated from the AGP by washing in acetone: DMSO (3:1) followed by AGP precipitation at −20°C in the presence of 1% NaCl for at least 2 h. The washing and precipitation with NaCl were repeated several times, until the Yariv-derived red color was completely washed out of the AGPs. Finally, the AGP pellet was dissolved in distilled water, dialyzed for 4 days at 4°C (molecular mass cut-off: 12.000 Da, Sigma Aldrich, St Louis, USA), and stored in aliquots at −20°C until used.
Rocket and crossed electrophoreses of AGPs
Isolated AGPs were quantified by rocket electrophoresis, using a known concentration of gum arabic as the standard (Sigma Aldrich, St Louis, USA), according to the method described by Komalavilas et al. (1991). The 1% agarose gel contained 15 μM Yariv reagent for AGP precipitation. Electrophoresis was performed in Tris-glycine buffer (25 mM Tris, 200 mM glycine, pH 8.4) for several hours, until the rockets were well developed. The concentration of AGP in the samples was estimated in relation to the peak area of gum arabic.
The molecules were further characterized by two-dimensional crossed electrophoresis (van Holst and Clarke 1986). AGPs (17 μg) were initially separated in 1% agarose gels with Tris-boric acid-EDTA buffer (90 mM Tris, 90 mM boric acid, 2 mM EDTA, pH 8.3), and then run in the second dimension in a 1% agarose gel in Tris-glycine buffer, pH 8.4, supplemented with 15 μM Yariv reagent, which precipitated the AGPs as they moved towards the anode. For both rocket and crossed electrophoresis, de-staining of gels to remove unbound Yariv reagent was performed by overnight washing in 1% NaCl. As a result, characteristic precipitation profiles were obtained for each sample and R f values were determined for visible peaks.
Dot-blots
AGPs were also characterized in terms of the binding capacity of several antibodies. Since AGPs frequently co-precipitate with some pectic domains and may share common epitopes with pectic polysaccharides, we used both anti-AGP (MAC207, LM2, JIM4, JIM8, JIM13, JIM14, JIM15, JIM16) and anti-pectic antibodies (JIM5, JIM7, LM5, LM6) characterized in Table 2. The antibodies JIM4, JIM14, JIM15, JIM16, and LM2 were obtained from Dr. P. Knox, University of Leeds, UK; JIM5, JIM7, JIM8, and JIM13 antibodies from Dr. K. Roberts, John Innes Center, UK; and LM5, LM6, and MAC207 are commercial antibodies (PlantProbes, UK). Antibody binding tests were performed by the dot-blot method. Briefly, 2 μl of AGP sample in four dilutions (1.0, 0.5, 0.25, and 0.125 μg/μl) were dotted onto nitrocellulose paper (Hybond Trans-Blot Transfer Medium, Bio-Rad) and air-dried. The nitrocellulose sheets were then blocked in TBST buffer (10 mM Tris, 150 mM NaCl, 0.05% Tween-20, pH 8.0) supplemented with 1% BSA and 1% dry, non-fat milk for 2 h at room temperature. The incubation with primary antibodies (all diluted 1:250 in TBST with 1% BSA and 1% milk) was carried out for 12 h at room temperature and followed by four washings in TBST. The blots were subsequently incubated for 2.5 h with secondary anti-rat antibody coupled to alkaline phosphatase (Sigma Aldrich, St Louis, USA) that was diluted 1:2500 (vol/vol) in TBST with 1% milk. After several washings in TBST buffer and AP buffer (100 mM Tris, 100 mM NaCl, 5 mM MgCl2, pH 8.5), the blots were incubated with BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) in AP buffer for 18 min. The reaction was stopped by dipping the blots in distilled water. Controls were performed by omitting the incubation with primary antibodies.
Statistical analysis
Student’s t-test was used to establish the significance of two factors: (1) the effect of exogenous AGPs on the efficiency of shoot formation, and (2) the influence of regeneration medium composition on the efficiency of shoot formation. P values ≤0.05 were considered significant.
Results
Protoplast culture and shoot regeneration
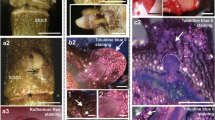
The digestion of epidermal strips in enzyme solution resulted in the release of numerous guard cell-derived protoplasts and relatively few protoplasts derived from epidermal pavement cells; mesophyll protoplasts appeared only sporadically. Within 1 week of culture, protoplasts changed their round shape into more elongated or irregular shapes and soon started to divide (Fig. 1a, b). After the next week, callus microcolonies composed of several cells had already formed (Fig. 1c). Whole alginate beads were then transferred to regeneration media solidified with agarose. Callus tissues were initially cultured in the dark (Fig. 1d), and later under light conditions. Shoot formation occurred either in the first subculture in the light or during the following three subcultures (Fig. 1e, f).
Media containing 4.4 μM BAP and 2.2 μM NAA induced organogenesis only sporadically, whereas removing auxin and simultaneously supplementing the media with the anti-auxin TIBA greatly improved the process (Table 1). The beneficial effect of TIBA was evident at 2.0 μM but was much more pronounced at 4.0 μM TIBA. In control cultures, 2.0 μM TIBA enhanced shoot formation about twofold compared with standard medium with NAA, whereas 4.0 μM TIBA improved organogenesis about 20-fold. The effects at both concentrations were statistically significant (Table 1).
Further improvement was achieved by adding AGP-rich extracts to protoplast media during the initial 2 weeks of culture. The effects of proteoglycans on protoplast development and callus-derived shoot differentiation were examined for two different AGPs, namely, those isolated from media conditioned by embryogenic and non-embryogenic cultures, and for six different AGP concentrations. Regardless of the origin of the AGPs, their beneficial biological effects included faster protoplast development, more efficient cell division, and a higher ratio of callus growth, but these effects were obvious only when 2.5 μg ml−1 AGP was added (Fig. 2a–d). Comparing the efficiency of shoot formation on medium with 4.0 μM TIBA, the proteoglycans from non-embryogenic cultures stimulated the process almost threefold in relation to the control variant (17.71% vs. 6.82%), whereas AGPs from embryogenic cultures proved to be even more efficient, leading to an approximately sevenfold increase in shoot number (47.22% vs. 6.82%).
AGPs at a concentration of 1.0 μg ml−1 did not cause visible changes in protoplast development (Table 1), and the same was true of 0.5 and 5.0 μg ml−1 AGP, as well as for the addition of various conditioned media (Table 1). Relatively high AGP concentrations of 10.0 and 20.0 μg ml−1 had either no effect or caused a slight decrease in the protoplast development ratio (data not shown).
Characterization of extracts from the conditioned media
Extracts were obtained from conditioned media derived from 3-month-old embryogenic (EC) and non-embryogenic suspensions (NEC) collected 1 week after the last subculture. The aqueous solutions of AGPs were examined in terms of (1) pattern of electrophoretic profiles and (2) the presence of structural carbohydrate epitopes that typify proteoglycans and pectins. The position and number of peaks visible in the electrophoretic profiles seem to be somewhat related to the morphogenetic properties of tissues cultured in vitro, and the expression of definite AGP epitopes in early stages of organ or embryo differentiation is considered to be an indicator of the cells’ developmental pathway, at least for some species.
The concentration of sugar beet AGP in the six extracts (from four non-embryogenic and two embryogenic cultures) was estimated by rocket electrophoresis.
The crossed-electrophoresis profiles obtained for all AGP extracts were similar both in terms of peak number and peak position in the gel; only one peak was observed in all cases, with an R f value ranging between 0.55 and 0.65 (Fig. 3a, b). Gum arabic AGPs showed two peaks, with R f = 0.50 and 0.70 (Fig. 3c).
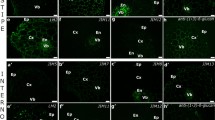
Extracts from non-embryogenic and embryogenic suspension cells of sugar beet showed both similarities and differences in terms of the presence and relative abundance of particular carbohydrate epitopes that typify arabinogalactan-proteins and pectins. Both extracts were characterized by the widespread occurrence of epitopes that bind JIM13, MAC207 and LM2 antibodies (Fig. 4a, b, h), whereas epitopes that bind JIM16, JIM5, and LM6 were obviously less abundant (Fig. 4g, k, l).
Immunodetection of carbohydrate epitopes in AGP extracts by dot-blotting and antibody binding: JIM13 (a), MAC207 (b), JIM8 (c), control (d), JIM14 (e), JIM15 (f), JIM16 (g), LM2 (h), JIM7 (i), LM5 (j), JIM5 (k), LM6 (l). Lines 1–4 correspond to AGPs from non-embryogenic media, lines 5–6 to AGPs from embryogenic media, and line 7 to gum arabic. Numbers 0.125, 0.25, 0.5 and 1.0 indicate the concentrations of AGP (μg/μl)
Media from embryogenic cultures proved to be extremely rich in epitopes that bind two anti-pectin antibodies, JIM7 and LM5 (Fig. 4i, j), and they also displayed the presence of JIM14 and JIM15 epitopes (Fig. 4e, f); these four events differentiated them from non-embryogenic cultures. The epitopes recognized by the JIM8 (Fig. 4c) and JIM4 antibodies could not be detected in any of the AGPs tested. Control blot does not show any sign of staining (Fig. 4d).
Discussion
We have tested the biological activity of two different AGP extracts, including one derived from conditioned media of embryogenic cultures and a second from non-embryogenic medium, and found that both displayed a strong beneficial effect on sugar beet organogenesis from protoplast-derived callus. The concentration of the added AGPs was an important factor, as a significant enhancement of shoot regeneration was proven only for cultures growing in the presence of 2.5 μg ml−1AGP. Other concentrations examined by us, i.e. 0.5; 1.0; 5.0; 10.0 and 20.0 µg ml−1 had either no effect or even decreased the ratio of protoplast differentiation. The results of our experiments agree with those obtained with carrot, in which the biological effect is dependent on both the concentration and type of exogenous proteoglycans (Kreuger and van Holst 1993, 1995).
Some arabinogalactan proteins either induce or stimulate the processes of embryo formation and maturation, and the most effective in this respect are proteoglycans isolated from seeds or conditioned media (Kreuger and van Holst 1993, 1995; Egersdotter and von Arnold 1995).
The addition of 10 μg ml−1 AGPs isolated from carrot seeds or from media of non-embryogenic cultures to poorly embryogenic cultures of this species greatly increased the number of embryos formed, in comparison to the control (Kreuger and van Holst 1993). Moreover, carrot seed AGPs were also shown to act positively on cyclamen embryogenesis (Kreuger et al. 1995). AGPs from Norway spruce seed extracts, added to non-maturing embryo cultures in concentrations of 5, 10, or 20 μg ml−1, caused the enlargement of embryogenic regions and improved further embryo development (Egersdotter and von Arnold 1995).
The embryogenesis-enhancing and embryogenesis-inhibiting AGPs epitopes, characterized by their ability to bind ZUM 18 and ZUM 15 antibodies, were identified for carrot (Toonen et al. 1997). Whereas ZUM 18 AGPs showed the most pronounced effect at a concentration of 0.2 μg ml−1, the unfractionated AGPs had to be applied at 10 μg ml−1 to cause a similar response (Kreuger and van Holst 1995).
The electrophoretic properties of AGP molecules are postulated to be important indicators of their biological properties, and two features are considered especially significant: (1) the molecules’ R f values and (2) the number of peaks present in the profile. AGPs from embryogenic cultures of several species show one or two peaks of relatively high R f values compared with non-embryogenic cells. Highly embryogenic carrot cells synthesize AGP composed of two subclasses characterized by R f values of 0.4 and 0.6, whereas the loss of embryogenic capacity is accompanied by the accumulation of a different AGP, with an R f of 0.3 (Kreuger and van Holst 1993). Similarly, the embryogenic callus of Euphorbia pulcherrima contains AGPs characterized by an R f of 0.2, and a much more abundant class of molecules with an R f of 0.4. Tissues lacking embryogenic properties produce molecules characterized by an R f of 0.3 (Saare-Surminski et al. 2000). We found no such differences, however, between AGPs from sugar beet embryogenic and non-embryogenic cultures, as both showed only one peak of almost identical R f. Nevertheless, the relatively fast electrophoretic migration of sugar beet AGPs (R f between 0.55 and 0.65) seems to be important.
Expression of particular AGP epitopes has been shown to occur in either specific cells or tissues of the embryo or in conditioned media in which embryogenic suspensions have been growing (Kreuger and van Holst 1993, 1995; Egersdotter and von Arnold 1995; Toonen et al. 1996; Borderies et al. 2004). Some data indicate that the globular stage of several plant species is characterized by a specific pattern of AGP localization. For example, JIM13-, JIM16-, and LM2-responding epitopes occur in the surface cell layer designated as the future protoderm of Cichorium embryos (Chapman et al. 2000a; b); JIM13 epitopes appear in proembryogenic mass cells in Picea abies and disappear later on (Filonova et al. 2000); and LM2 typifies protoderm of Euphorbia pulcherrima (Saare-Surminski et al. 2000), whereas JIM4 binding epitopes were found in young carrot and maize embryos (Stacey et al. 1990; Samaj et al. 1999). JIM8 has been postulated to label carrot cells that are predestined to enter somatic embryogenesis (Toonen et al. 1997), and molecules produced by these cells are thought to provide a signal required for the progression of embryo formation (McCabe et al. 1997). The absence of the JIM8 epitope from media collected from sugar beet cultures is an unexpected result, as the antibody recognizing this epitope was produced by immunization with sugar beet protoplast membrane (Pennell et al. 1991).
Some of the epitopes mentioned above have also been detected in non-embryogenic cells and media (Knox et al. 1991; Smallwood et al. 1996). Similarly, in sugar beet the expression of JIM8 and JIM13 has been described in non-embryogenic suspension cells (Butowt et al. 1999), as well as in leaf tissue and in poorly regenerating mesophyll protoplasts (Majewska-Sawka and Münster 2003). This apparent discrepancy in terms of AGP epitopes expression pattern may be explained by the fact that the biological activity of particular AGP molecules probably depends not only on the presence of the structural domains that are detected by the specific antibody, but also on other conformational and compositional features that change dynamically in the course of AGP biogenesis as a result of the cleavage, transfer, assembly, and reassembly of carbohydrate monomers, dimmers, or oligomers. Moreover, the involvement of AGPs in a specific developmental process should also be considered not only in the context of developmental time-point, but also in the context of definite plant species and type of tissue studied.
More recently, new species have been added to the list of those, for which improvement of somatic embryogenesis has been achieved by the addition of exogenous AGPs or β-arabinogalactans (β-AG). In cotton, the increased efficiency of embryo formation by the elite cultivars Coker 315 and Siokra 1–4 was reported as a consequence of incorporation of 1–2 μg ml−1 of AGPs derived from a highly embryogenic genotype (Poon et al. 2004). Interestingly, the addition of pure β-AG added to the media at the concentration of 10 or 20 μg ml−1 increased the efficiency of embryo formation from cotyledonary callus of Vigna radiata from 36.65 to 50.0% and 76.5%, respectively (Das and Pal 2004). The differentiation of microspore-derived embryos has also been improved in maize and wheat cultures by the addition of exogenous AGP or β-AG (Borderies et al. 2004; Letarte et al. 2006).
To our knowledge, the effects of exogenous AGPs on protoplasts have been studied only in carrot. Addition of 1–3 μg ml−1 of seed proteoglycans resulted in a 30-fold increase in the number of somatic embryos formed from protoplast-derived callus (van Hengel et al. 2001). Similar enhancement was also observed when AGPs from an embryogenic suspension were added to the carrot protoplast media.
It is now well established that AGPs contribute to intra- and intercellular signaling, so several postulated mechanisms of activity have been put forward. The most commonly considered relates to the presence of the glycosylphosphatidylinositol anchor (GPI) that is involved in binding numerous AGPs to the cell surface (Youl et al. 1998; Schultz et al. 1998; Svetek et al. 1999). After cleavage by phospholipase and eventual breakdown to phosphatidylinositol and inositol phosphoglycan, the GPI anchor itself or its components could act as signaling molecules (Gaspar et al. 2001; Schultz et al. 2004; Sun et al. 2004b). On the other hand, signaling via interaction with other transmembrane proteins has also been proposed (Schultz et al. 1998).
The AGP extracts used in this study have been shown to possess some pectic domains that are able to bind the JIM7 and LM5 antibodies. Two possible reasons for this would confirm the prior observations of other authors. The first is that co-purification of AGPs and pectins during AGP isolation could occur, either via Ca+2-mediated interaction between negatively charged uronic acids of the two compounds or through the interaction of positively charged amino acids forming the protein core of the AGPs with the uronic acid of pectins (Showalter 2001). The second relates to the recent studies confirming the presence of specific pectic epitopes in the AGP molecules, which seems to be satisfactorily proven (Showalter 2001; Immerzeel et al. 2004, 2006). This suggests that oligogalacturonides released from pectin domains could also affect embryogenesis. However, given that two AGP-rich extracts of sugar beet differed significantly in their pectic epitope contents while having identical biological effects at the same concentrations, we postulate that the observed effects are mainly AGP-dependent.
Another result of our studies indicated that the enhancement of sugar beet organogenesis might be achieved by modifying the regeneration media. We have proven by statistical methods that supplementing the media with the anti-auxin TIBA at a concentration of 2–4 μM has a strong beneficial effect on organogenesis from guard cell protoplast-derived callus. The positive influence of TIBA on regeneration of sugar beet shoots from different explants has been previously reported (Tétu et al. 1987; Jacq et al. 1992; Kulshreshtha and Coutts 1997), so our data contribute to the number of explant types and genotypes that are highly responsive to anti-auxin treatment.
To sum up, the data presented in this paper are the first to show that exogenous AGPs may have a strong effect on the development of sugar beet guard cell protoplasts and organogenesis of protoplast-derived callus. This could have a strong impact on possible biotechnological applications of protoplast technology for the genetic improvement of this important crop.
References
Acosta-Garcia G, Vielle-Calzada JP (2004) A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 16:2614–2628 doi:10.1105/TPC.104.024588
Borderies G, le Bechec M, Rossignol M, Laffitte C, Le Deunff E, Beckert M, Dumas C, Matthys-Rochon E (2004) Characterization of proteins secreted during maize microspore culture: arabinogalactan proteins (AGPs) stimulate embryo development. Eur J Cell Biol 83:205–212 doi:10.1078/0171-9335-00378
Butowt R, Niklas A, Rodriguez-Garcia MI, Majewska-Sawka A (1999) Involvement of JIM13-and JIM8-responsive carbohydrate epitopes in early stages of cell wall formation. J Plant Res 112:107–116
Chapman A, Blervacq AS, Vasseur J, Hillbert JL (2000a) Arabinogalactan-proteins in Cichorium somatic embryogenesis: effect of β-glucosyl Yariv reagent and epitope localisation during embryo development. Planta 211:305–314 doi:10.1007/s004250000299
Chapman A, Helleboid S, Blervacq AS, Vasseur J, Hilbert JL (2000b) Removal of the fibrillar network surrounding Cichorium somatic embryos using cytoskeleton inhibitors: analysis of proteic components. Plant Sci 150:103–114 doi:10.1016/S0168-9452(99)00185-5
Chaves I, Regalado AP, Chen M, Ricardo CP, Showalter AM (2002) Programmed cell death induced by (β-d-galactosyl)3 Yariv reagent in Nicotiana tabacum BY-2 suspension-cultured cells. Physiol Plant 116:548–553 doi:10.1034/J.1399-3054.2002.1160414.X
Clausen MH, Willats WGT, Knox JP (2003) Synthetic methyl hexagalacturonate hapten inhibitors of anti-homologalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res 338:1797–1800 doi:10.1016/S0008-6215(03)00272-6
Das S, Pal A (2004) Differential regeneration response in two cotyledon types of Vigna radiata: histomorphological analysis and effect of β-arabinogalactan. J Plant Biochem Biotechnol 13:101–106
Egertsdotter U, von Arnold S (1995) Importance of arabinogalactan proteins for the development of somatic embryos of Norway spruce (Picea abies). Physiol Plant 93:334–345 doi:10.1111/J.1399-3054.1995.TB02237.X
Filonova LH, Bozhkov PV, von Arnold S (2000) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51:249–264 doi:10.1093/JEXBOT/51.343.249
Frearson EM, Power JB, Cocking EC (1973) The isolation, culture and regeneration of Petunia leaf protoplasts. Dev Biol 33:130–137
Gao M, Showalter AM (1999) Yariv reagent treatment induces programmed cell death in Arabidopsis cell cultures and implicates arabinogalactan protein involvement. Plant J 19:321–331 doi:10.1046/J1365-313X.1999.00544.X
Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ (2001) The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Mol Biol 47:161–176 doi:10.1023/A:1010683432529
Guan Y, Nothnagel EA (2004) Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in Arabidopsis cell cultures. Plant Physiol 135:1346–1366 doi:10.1104/PP.104.039370
van Hengel AJ, Roberts K (2002) Fucosylated arabinogalactan-proteins are required for full root cell elongation in Arabidopsis. Plant J 32:105–113 doi:10.1046/J.1365-313X.2002.01406.X
van Hengel AJ, Tadesse Z, Immerzel P, Schols H, van Kammen A, de Vries SC (2001) N-acetyloglucosamine and glucosamine containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol 125:880–1990
van Holst GJ, Clarke AE (1986) Organ-specific arabinogalactan-proteins of Lycopersicon peruvianum (Mill) demonstrated by crossed electrophoresis. Plant Physiol 80:786–789
Immerzeel P, Schols HA, Voragen AGJ, de Vries SC (2004) Different arabinogalactan proteins are present in carrot (Daucus carota) cell culture medium and in seeds. Physiol Plant 122:181–189 doi:10.1111/J.1399-3054.2004.00395.X
Immerzeel P, Eppink M, de Vries S, Schols A, Voragen AGJ (2006) Carrot arabinogalactan proteins are interlinked with pectins. Physiol Plant 128:18–28 doi:10.1111/J.1399-3054.2006.00712.X
Jacq B, Tetu T, Sangwan RS, de Laat A, Sangwan-Noreel BS (1992) Plant regeneration from sugarbeet (Beta vulgaris L.) hypocotyls cultured in vitro and flow cytometric nuclear DNA analysis of regenerants. Plant Cell Rep 11:329–333 doi:10.1007/BF00233359
Jones L, Seymour GB, Knox JP (1997) Localization of petic galactan in tomato cell walls using a monoclonal antibody specific to (1→4)-β-D-galactan. Plant Physiol 113:1405–1412
Knox JP, Linstead PJ, Peart J, Cooper C, Roberts K (1991) Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J 1:317–326
Komalavilas P, Zhu JK, Nothnagel EA (1991) Arabinogalactan-proteins from the suspension culture medium and plasma membrane of rose cells. J Biol Chem 266:15956–15965
Kreuger M, van Holst GJ (1993) Arabinogalactan proteins are essential in somatic embryogenesis of Daucus carota L. Planta 189:243–248 doi:10.1007/BF00195083
Kreuger M, van Holst GJ (1995) Arabinogalactan-protein epitopes in somatic embryogenesis of Daucus carota L. Planta 197:135–141 doi:10.1007/BF00239949
Kreuger M, Postma E, Brouwer Y, van Holst GJ (1995) Somatic embryogenesis of Cyclamen persicum in liquid medium. Physiol Plant 94:605–612 doi:10.1111/J.1399-3054.1995.TB00974.X
Kreuger M, van Hengel A, de Vries S (2000) Effect of AGPs and chitinases on somatic embryogenesis. In: Nothnagel EA, Bacic A, Clarke A (eds) Cell and developmental biology of arabinogalactan-proteins. Kluwer/Plenum Publishers, Dordrecht/New York, pp 109–121
Kulshreshtha S, Coutts RHA (1997) Direct somatic embryogenesis and plant regeneration from mature sugar beet (Beta vulgaris L.) zygotic cotyledons. Plant Growth Reg 22:87–92 doi:10.1023/A:1005889429586
Langan KJ, Nothnagel EA (1997) Cell surface arabinogalactan-proteins and their relation to cell proliferation and viability. Protoplasma 196:87–98
Lee KJD, Sakata Y, Mau SL, Pettolino F, Bacic A, Quatrano RS, Knight CD, Knox JP (2005) Arabinogalactan proteins are required for apical cell extension in the moss Physcomitrella patents. Plant Cell 17:3051–3065 doi:10.1105/TPC.105.034413
Letarte J, Simion E, Miner M, Kasha KJ (2006) Arabinogalactan-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell Rep 24:691–698 doi:10.1007/s00299-005-0013-5
Lu H, Chen M, Showalter AM (2001) Developmental expression and perturbation of arabinogalactan-proteins during seed germination and seeding growth in tomato. Physiol Plant 112:442–450 doi:10.1034/J.1399-3054.2001.1120319.X
Majewska-Sawka A, Münster A (2003) Cell-wall antigens in mesophyll cells and mesophyll-derived protoplasts of sugar beet: possible implication in protoplast recalcitrance. Plant Cell Rep 21:946–954 doi:10.1007/s00299-003-0612-Y
Majewska-Sawka A, Nothnagel EA (2000) The multiple roles of arabinogalactan proteins in plant development. Plant Physiol 122:3–9
McCabe PF, Valentine TA, Forsberg LS, Pennel RI (1997) Soluble signals from cells identified at the cell wall establish a developmental pathway in carrot. Plant Cell 9:2225–2241
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–479 doi:10.1111/J.1399-3054.1962.TB08052.X
Nothnagel EA (1997) Proteoglycans and related components in plant cells. Inter Rev Cytol 174:195–291
Park MH, Suzuki Y, Chono M, Knox JP, Yamaguchi I (2003) CsAGP1, a gibberellin-responsive gene from cucumber hypocotyls, encodes a classical arabinogalactan protein and is involved in stem elongation. Plant Physiol 131:1450–1459 doi:10.1104/PP.015628
Pennell RI, Janniche L, Kjellbom P, Scofield GN, Peart JM, Roberts K (1991) Developmental regulation of plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant Cell 3:1317–1326
Poon S, Clarke A, Heath R (2004) Improving the efficiency of embryogenesis in elite cotton cultivars. In: 12th Australian Cotton Conference Proceedings, pp 733–737
Saare-Surminski K, Preil W, Knox JP, Lieberei R (2000) Arabinogalactan proteins in embryogenic and non-embryogenic callus cultures of Euphorbia pulcherrima. Physiol Plant 108:180–187 doi:10.1034/J.1399-3054.2000.108002180.X
Samaj J, Baluska F, Bobak M, Volkmann D (1999) Extracellular matrix surface network of embryogenic units of friable maize callus contains arabinogalactan-proteins recognized by monoclonal antibody JIM4. Plant Cell Rep 18:369–374 doi:10.1007/s002990050588
Schultz C, Gilson P, Oxley D, Youl J, Bacic A (1998) GPI-anchors on arabinogalactan-proteins: implications for signaling in plants. Trends Plant Sci 3:426–431 doi:10.1016/S1360-1385(98)01328-4
Schultz CJ, Ferguson KL, Lahnstein J, Bacic A (2004) Post-translational modifications of arabinogalactan-peptides of Arabidopsis thaliana. Endoplasmic reticulum and glycosylphosphatidylinositol-anchor signal cleavage sites and hydroxylation of proline. J Biol Chem 279:45503–45511 doi:10.1074/JCB.M407594200
Serpe MD, Nothnagel EA (1994) Effects of Yariv phenylglycosides on Rosa cell suspensions: evidence for the involvement of arabinogalactan proteins in cell proliferation. Planta 193:542–550 doi:10.1007/BF02411560
Showalter AM (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58:1399–1417 doi:10.1007/PL00000784
Smallwood M, Yates EA, Willats WGT, Martin H, Knox JP (1996) Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 198:452–459 doi:10.1007/BF00620063
Stacey NJ, Roberts K, Knox JP (1990) Patterns of expression of the JIM4 arabinogalactan-protein epitope in cell cultures and during somatic embryogenesis in Daucus carota L. Planta 180:285–292 doi:10.1007/BF00194009
Sun W, Kieliszewski MJ, Showalter AM (2004a) Overexpression of tomato LeAGP-1 arabinogalactan-protein promotes lateral branching and hampers reproductive development. Plant J 40:870–881 doi:10.1111/J.1365-313X.2004.02274.X
Sun W, Zhao ZD, Hare MC, Kieliszewski MJ, Showalter AM (2004b) Tomato LeAGP-1 is a plasma membrane-bound, glycosylphosphatidylinositol-anchored arabinogalactan-protein. Physiol Plant 120:319–327 doi:10.1111/J.0031-9317.2004.0236.X
Svetek J, Yadav MP, Nothnagel EA (1999) Presence of a glycosylphosphatidylinositol lipid anchor on rose arabinogalactan proteins. J Biol Chem 274:14724–14733
Tétu T, Sangwan RS, Sangwan-Norreel BS (1987) Hormonal control of organogenesis and somatic embryogenesis in Beta vulgaris callus. J Exp Bot 38:506–517 doi:10.1093/JXB/38.3.506
Thompson HJM, Knox JP (1998) Stage-specific responses of embryogenic carrot cell suspension cultures to arabinogalactan protein-binding β-glucosyl Yariv reagent. Planta 205:32–38 doi:10.1007/s004250050293
Toonen MAJ, Schmidt EDL, Hendriks T, Verhoeven HA, van Kammen A, de Vries SC (1996) Expression of the JIM8 cell wall epitope in carrot somatic embryogenesis. Planta 200:167–173 doi:10.1007/BF00208305
Toonen MAJ, Schmidt EDL, van Kammen A, de Vries SC (1997) Promotive and inhibitiory effects of diverse arabinogalactan proteins on Daucus carota L. somatic embryogenesis. Planta 203:188–195 doi:10.1007/s004250050181
Willats WGT, Knox JP (1996) A role for arabinogalactan-proteins in plant cell expansion: evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J 9:919–925 doi:10.1046/J.1365-313X.1996.9060919.X
Willats WGT, Steele-King CG, Marcus SE, Knox JP (1999) Side chains of pectic polysaccharides are regulated in relation to cell proliferation and cell differentiation. Plant J 20:619–628 doi:10.1046/J.1365-313X.1999.00629.X
Willats WGT, Limberg G, Buchholt HC, van Alebeek G-J, Benen J, Christensen TMIE, Visser J, Voragen A, Mikkelsen JD, Knox JP (2000) Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327:309–320 doi:10.1016/S0008-6215(00)00039-2
Wiśniewska E, Majewska-Sawka A (2006) Cell wall polysaccharides in differentiating anthers and pistils of Lolium perenne. Protoplasma 228:65–71
Yariv J, Lis H, Katachalski E (1967) Precipitation of arabic acid and some seed polysaccharides by glycosylphenylazo dyes. Biochem J 105:1C–2C
Yates EA, Valdor JE, Halsam SM, Morris HR, Dell A, Mackie W, Knox JP (1996) Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 2:31–39 doi:10.1093/GLYCOB/6.2.131
Youl JJ, Bacic A, Oxley D (1998) Arabinogalactan-proteins from Nicotiana alata and Pyrus communis contain glycosylphosphatidylinositol membrane anchors. Biochemistry 95:7921–7926
Acknowledgments
This study was supported by State Committee for Scientific Research, project no 3P06A03025. The authors thank Dr. Paul Kretchmer (kretchmer@sfedit.net) at San Francisco Edit for his assistance in editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.J. Rose.
Rights and permissions
About this article
Cite this article
Wiśniewska, E., Majewska-Sawka, A. Arabinogalactan-proteins stimulate the organogenesis of guard cell protoplasts-derived callus in sugar beet. Plant Cell Rep 26, 1457–1467 (2007). https://doi.org/10.1007/s00299-007-0348-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0348-1