Abstract
Transgenic herbicide tolerant Acacia sinuata plants were produced by transformation with the bar gene conferring phosphinothricin resistance. Precultured hypocotyl explants were infected with Agrobacterium tumefaciens strain EHA105 in the presence of 100 μM acetosyringone and shoots regenerated on MS (Murashige and Skoog, 1962, Physiol Plant 15:473–497) medium with 13.3 μM benzylaminopurine, 2.6 μM indole-3-acetic acid, 1 g l−1 activated charcoal, 1.5 mg l−1 phosphinothricin, and 300 mg l−1 cefotaxime. Phosphinothricin at 1.5 mg l−1 was used for the selection. Shoots surviving selection on medium with phosphinothricin expressed GUS. Following Southern hybridization, eight independent shoots regenerated of 500 cocultivated explants were demonstrated to be transgenic, which represented transformation frequency of 1.6%. The transgenics carried one to four copies of the transgene. Transgenic shoots were propagated as microcuttings in MS medium with 6.6 μM 6-benzylaminopurine and 1.5 mg l−1 phosphinothricin. Shoots elongated and rooted in MS medium with gibberellic acid and indole-3-butyric acid, respectively both supplemented with 1.5 mg l−1 phosphinothricin. Micropropagation of transgenic plants by microcuttings proved to be a simple means to bulk up the material. Several transgenic plants were found to be resistant to leaf painting with the herbicide Basta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acacia sinuata (Lour.) Merr (A. concinna (Willd.) DC.) is a multipurpose leguminous tree that occurs widely in the tropical forests throughout India and South Asia. Acacia species, including Acacia sinuata, are grown in agro-silvopastoral systems for fuel, timber, shelterbelts, and soil improvement (Jamal and Huntsinger 1993). The dry pods of the tree called “shikakai” are important raw materials for cosmetics and agro-based industries. Saponins present in the dry pods are used for the semi-synthesis of steroidal drugs (Vaidyaratnam 1994).
The growing demand for pulp and timber products has led to the improvement of some trees at the genetic level with the help of gene transfer techniques (Tzfira et al. 1998). Owing to the long generation time lag caused by seed germination and flowering most of the forest-tree species have been left unaltered in relevance to molecular breeding. Desirable traits such as herbicide and insect resistance are not readily available within the breeding population of A. sinuata. The former trait would be useful in weed control and could allow establishment of tree plantations in the weed infected areas for superior plantation management (Bishop-Hurley et al. 2001; Polpin and Arce-Johnson 2005). Herbicide resistance genes have been widely used in crop biotechnology for weed control (Lörz et al. 1998; Meilan et al. 2000).
Stable genetic transformation methods have been developed for many forest-tree species and economically important traits such as resistance to a virus, insect, and herbicide have been engineered (Herschbach and Kopriva 2002). Transformation of legume trees was reported for Acacia mangium (Xie and Hong 2002) and Robinia pseudoacacia (Han et al. 1993; Igasaki et al. 2000; Zaragoza et al. 2004). After the first successful recovery of transgenic poplar plants expressing a bacterial aroA gene for herbicide tolerance (Fillatti et al. 1987) consistent and stable expression of the bar gene was reported in Picea abies and Pinus radiata (Brukhin et al. 2000; Charity et al. 2005). In the present communication, we described the use of PPT as a selection agent to produce transgenic A. sinuata resistant to the herbicide Basta. In addition, we demonstrated a method to increase the number of Basta resistant transgenic plants through an in vitro micropropagation system using “micro cuttings” derived from transgenic plants.
Materials and methods
Plant material
Seeds of A. sinuata were procured from the Institute of Forest Genetics and Tree Breeding, Coimbatore, Tamil Nadu, India, from elite trees. Seeds from each tree were pooled and retained as single lot. Seed sterilization was performed using a protocol described by Vengadesan et al. (2000). Aseptic seed germination was carried out on Murashige and Skoog (1962) medium (MS) containing 87.6 mM sucrose and 0.8% agar (Agar agar, type-1, Himedia, Mumbai, India) or 0.15% gellan gum (Gelrite Sigma, St. Louis, USA). All media were autoclaved at 121°C for 15 min. All cultures were maintained at 25±2°C with a 16 h photoperiod, at a photon flux of 15 μmol m−2 s−1 fluorescent white light. Hypocotyl segments (10 mm long) were excised from 7-day-old in vitro grown seedlings and used as explant source.
Regeneration system
A previously developed unpublished regeneration system based on the production of shoots and roots from in vitro derived hypocotyl explants was used. Hypocotyl explants were cultured on MS medium supplemented with 13.3 μM 6-benzylaminopurine (BA), 2.6 μM Indole-3-acetic acid (IAA), and activated charcoal (Sigma, St. Louis, MO) (1 g l−1) (regeneration medium). Explants were transferred to fresh regeneration medium at 20-day intervals. Elongation of the shoots was performed on elongation medium [MS medium with 1.7 μM gibberellic acid (GA3)]. Shoots were rooted on rooting medium [MS medium with 2.5 μM Indole-3-butyric acid (IBA)]. All cultures were maintained in the growth room under conditions mentioned above.
Phosphinothricin (PPT) toxicity
Prior to the gene transfer studies, sensitivity of the hypocotyl explants to phosphinothricin was determined by culturing the explants on the regeneration medium with 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, and 2 mg 1−1 phosphinothricin (Duchefa Biochemie, The Netherlands) for 20 days. The explants were transferred to fresh regeneration medium at 20-day intervals. The experiment was repeated five times with 100 hypocotyl segments per treatment. Subsequently, a selective concentration of PPT was determined and used in transformation. A control (without PPT) was also maintained.
Agrobacterium tumefaciens strain, plasmid, and culture
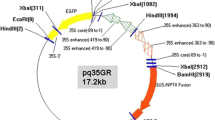
Genetic transformation was performed using the A. tumefaciens EHA105 strain harboring the binary vector pME504 (Fig. 1) that carries a neomycin phosphotransferase (nptII) gene under the control of nos promoter, and the bar and uid-A genes separately under the control of CaMV 35S promoter (Edelman et al. 2000). Bacterial cultures were grown in 5 ml YEP medium (Chilton et al. 1974) containing 50 mg l−1 kanamycin and 10 mg l−1 tetracycline (both from Sigma, St. Louis, USA). Five milliliter of bacterial culture was transferred to 25 ml of YEP medium, and then grown for 36 h at 200 rpm (Orbitek, Chennai, India). For the transformation, the culture was centrifuged at 2996×g for 10 min, and the cells resuspended in MS liquid medium (pH 5.6) with 100 μM acetosyringone (Fluka, Germany) to a final density of 0.6–0.8 OD600 nm. The bacteria were incubated at 28°C for an hour before being used for infection of plant cells.
Transformation and selection of hypocotyl segments and plant regeneration
Hypocotyl explants were precultured on regeneration medium for 5 days. Transformation of hypocotyl segments was performed by pipetting 20 μl of the A. tumefaciens culture onto the ends of hypocotyl segments, without further wounding. The bacterial culture was left for 20 min and later blotted with sterile Whatman filter paper. About 100 explants were cocultivated horizontally on regeneration medium for 72 h, with five explants per plate (15 mm × 90 mm, Borosil, India). Each experiment consisted of 100 explants and was repeated four times (five experiments in total). After cocultivation the explants were washed twice in sterile distilled water followed by half strength MS liquid medium with 300 mg 1−1 cefotaxime (Höechst, Mumbai, India), blotted dry, and transferred to the regeneration medium with 300 mg 1−1 cefotaxime and 1.5 mg 1−1 PPT (selection medium) as the selection agent. After 4 weeks of initial culture explants with regenerated shoots were transferred three times at 20-day intervals to fresh medium of the same composition.
GUS histochemical activity assay
GUS activity assay of the putative transformed shoots (from cocultivated hypocotyl explants after first transfer), microcuttings, and leaves from microshoots was performed as described by Jefferson et al. (1987). The samples were immersed in a substrate solution composed of 0.1 M NaHPO4 buffer (pH 7.0), 0.5 mM K3[Fe(CN)6], 0.5 mM K4[Fe(CN)6], 10 mM EDTA, 800 mg l−1 X-Gluc, 0.06% (v/v) Triton X-100, and incubated at 37°C for 8 h. Subsequently the tissues were washed in 70% ethanol and were photographed with a Nikon E400 microscope with HIII photographic unit (Nikon Co., Tokyo, Japan). The number of GUS-expressing shoots was recorded, as well as the number of shoots arising from microcuttings.
DNA extraction and Southern hybridization analysis
DNA was extracted from the pinnate leaves of GUS positive shoots using the DNeasy Plant Mini kit (Qiagen GmbH, Germany). About 15 μg of the DNA was digested overnight using the restriction enzyme Sacl (MBI Fermentas) and fractionated on a 0.8% agarose gel, and blotted onto Hybond N+ membrane (Amersham Biosciences, UK). A 520 bp amplified fragment (using the primers 5′ATCGTCAACCACTACATCGAGAC; 5′CCAGCTGCCAGAAACCCACGTC) of the bar sequence from the plasmid pME504 was labeled by the ECL Random prime labeling and detection system (Amersham Biosciences, UK). Further hybridization, washing, and detection were performed according to the manufacturer's instructions.
Propagation of putative transformants through microcuttings
Transformed shoots that were confirmed by Southern analysis were excised under aseptic conditions into nodal cuttings (each cutting with a single node). Each node was cultured for further multiplication of transgenic shoots as described by Vengadesan et al. (2003) on medium with 6.6 μM BA and 1.5 mg 1−1 PPT. Elongation and rooting of microshoots were performed on elongation medium and rooting medium, respectively in the presence of 1.5 mg 1−1 PPT. Nodal cuttings from individual shoots were maintained separately. Shoots grown in the presence of the selection agent were chosen for hardening in a green house.
In vivo assay for Basta resistance
Basta leaf painting assay was performed on 3-week old plants growing in the green house for Basta resistance with a solution containing 0.1 mg/ml Basta (0.01%) and 0.1% Tween-20. The effect of the herbicide was assessed after 7, 15, and 30 days based on the survival of the hardened plants.
Experimental design and statistical analysis
Results of several transformation experiments (of 100 explants each) have been averaged and results were expressed here as a percentage response, along with the standard error of the mean derived from the individual experiments. Data were analyzed using one-way ANOVA and significant differences were determined at the 0.05 probability level by Scheff's test. Statistical analysis of data was performed (Sokal and Rholf 1981) using J.M.P 4.0 (SAS Institute 1996) statistical software.
Results and discussion
Regeneration of plants from hypocotyl explants
After 15 days on regeneration medium, hypocotyl explants produced shoots and roots from the cut ends without intervening callus. During the direct adventitious shoot formation, the explants turned green and produced green nodular structures at the apical ends. Simultaneously, root initials originated from the opposite ends of the hypocotyls. Shoots or roots were regenerated by 68.0±3.0% of explants, 55.0±3.4% of explants regenerated only shoots, and 12.0±2.1% of explants regenerated both shoots and roots. Explants placed on the regeneration medium produced 4–5 shoots/explant (data not shown). The direct organogenesis from hypocotyl explants of A. sinuata is beneficial because it accelerates adventitious shoots production and avoids problems related to somaclonal variation (Confalonieri et al. 2003).
Selection using phosphinothricin (PPT)
Our experiments showed that complete inhibition of regeneration of both shoots and roots occurred at 1.5 mg 1−1 PPT. Percent response (percentage of explants regenerating shoots on regeneration medium containing PPT) of the explants was significantly different (p<0.001) at various PPT concentrations (Fig. 2). Phosphinothricin above 1.5 mg 1−1 led to progressive necrosis of the meristematic areas and subsequent shoot death. PPT is the active ingredient of the glufosinate-ammonium herbicide (Basta). Glufosinate ammonium inhibits the action of glutamine synthetase, resulting in the accumulation of lethal levels of ammonia in susceptible plants. Thus, the herbicidal role of Basta is due to the combined effects of ammonium toxicity and inhibition of photosynthesis. The enzyme phosphinothricin acetyltransferase, encoded by the bar gene, inactivates the active ingredient phosphinothricin by acetylation of its free NH2 group, thereby neutralizing its toxic effect on plant tissue. There are a few reports of selection of transformed tree species explants with PPT. De Block (1990) used different concentrations of phosphinothricin (5, 10, and 20 mg 1−1) to select transformed hybrid aspen and poplar plants. Resistant poplar colonies were selected using 5 mg 1−1 phosphinothricin (Chupeau et al. 1994), following electroporation of protoplasts. In Norway spruce the use of Basta (1 mg l−1) for continued expression of the bar gene in the transgenic plants was reported (Brukhin et al. 2000) and black locust transformants were selected on 4 mg 1−1 PPT (Zaragoza et al. 2004).
Toxicity of PPT. Explants were cultured on regeneration medium containing PPT (0–2 mg l−1). Explants were transferred to a fresh medium of the same composition three times at 20-day intervals. Results are from five individual experiments with 100 explants per selection process per experiment. The mean was calculated for each experiment and then the mean ± SE of the different experiments was calculated. Bars represent standard error. Column bars with different letters are significantly different at p<0.001 as determined using Scheff's test
Plant transformation and regeneration
Following 72 h cocultivation, and after 3 weeks of culture on selection medium explants started to regenerate either shoots or shoots and roots from the cut ends of the hypocotyl explants. Shoot regeneration was completely inhibited in the presence of 1.5 mg l−1 PPT (Fig. 2). The response varied among the regenerating hypocotyl explants. On average 12±2.3% of the hypocotyl explants responded and produced three shoots/explant. Shoot initiation took place from the apical cut ends of a hypocotyl. Some explants (7.0±2.0%) developed three shoots and two roots from the opposite cut ends (Fig. 4A). In every experiment, there was a significant difference (p<0.001) in the number of explants producing either shoots or shoots and roots (Fig. 3).
Shoot, shoot and root regeneration from hypocotyl explants after cocultivation with EHA105 pME504. Shoot, shoot and root regeneration from hypocotyl explants after cocultivation with Agrobacterium EHA 105 pME504 and selection on 1.5 mg l−1 PPT. Results are from five separate experiments with 100 explants per experiment
Continuous culture of regenerating explants on the selection medium (1.5 mg l−1 PPT) reduced the number of shoots regenerating from the explants, indicating that PPT-based selection was sufficiently stringent and only transformed cells survived. At the end of a third transfer 10.0±3.5% of the explants survived the selection pressure producing 10 shoots (Fig. 3).
GUS activity assay
GUS assays were performed on regenerated shoots after first transfer on selection medium. GUS expression was observed in both shoots and roots (Fig. 4D). Examining a total of 57 shoots that regenerated from 500 cocultivated explants after the first transfer, 46 shoots (80.7±6.0%) showed uniform GUS expression in various plant parts. However, in 11 shoots (19.3±5.0%) GUS activity was observed in the form of scattered blue dots/sectors in the regenerated shoots indicating their chimeric nature (data not shown). A high occurrence of escapes (63%) was also reported in black locust when PPT was used as the selection agent (Zaragoza et al. 2004). GUS-expressing shoots were subjected to Southern analysis and if possible, further propagated by micro cuttings. Nodal cuttings, as well as the shoots arising from them (Fig. 4E), and leaves (Fig. 4F) also revealed GUS activity.
Regeneration of herbicide-tolerant A. sinuata plants. A Cocultivated hypocotyl explants with regenerated putatively transformed shoots and roots. Bar: 2 mm. B Micro cuttings obtained from transformed shoots. Bar: 2 mm. C Elongated and rooted micro shoot. Bar: 3 mm. D GUS activity in the shoots and roots after first transfer of hypocotyl explants in selection medium. Bar: 3 mm. E GUS activity in micro shoots obtained from micro cuttings. Bar: 3 mm. F GUS activity in leaves of transformed shoots obtained from micro shoots. Bar: 3 mm. G Healthy plants 30 days after leaf painting with herbicide Basta (0.1 mg/ml). H Wilted shoots of the non-transformed control plants after Basta painting
Southern analysis
Southern analysis of putative transgenic A. sinuata was performed to prove integration of the transgene into the plant genome, and to determine the T-DNA copy number. Since the distance between the SacI restriction enzyme site and the left border is 4 kb (Fig. 1), fragments hybridizing should be greater than 4 kb. It is evident from the hybridization analysis (Fig. 5, lanes 3–10) that the eight plants analyzed were independent transformants harboring multiple T-DNA copies. Transgenic plants 3 and 5 showed three hybridization fragments indicating that three copies of T-DNA inserted, while plant 2 (lane 4) showed a single band confirming insertion of a single copy of T-DNA. Plants 4, 6, and 8 had two bands confirming insertion of two T-DNA copies, while plant 7 (lane 9) showed four fragments confirming insertion of four T-DNA copies. No hybridization occurred with DNA isolated from control plants (nontransformed, lane 2). The final transformation frequency, based on the Southern blot analysis, was eight independent transformation events per 500 initial explants, i.e., 1.6% of explants regenerated transformants. We have confirmed the integration of T-DNA by the analysis of hybridization fragments but will have to wait another 2–3 years to confirm transmission to progeny from the T0 transformed trees. Morphological examination of the first transformants after 1 year in the field indicated a normal phenotype compared to seed-derived controls.
Southern hybridization analysis of putatively transformed A. sinuata plants. Genomic DNA was extracted from leaves of eight independent transformation events (lane 3–10). Up to 15 μg per lane of genomic DNA was digested with Sacl, fractionated with 0.8% agarose gel and blotted onto a nylon membrane. The blot was hybridized to a 520 bp bar fragment as the probe (Dig-labeled). Plasmid DNA (pME504) (1 ng) was also digested with Sacl and labeled as positive control (lane 1). The negative control (lane 2) is DNA from untransformed leaf tissue
Successful expression of a transgene depends on its stability and expression in the progenies. In the present study, seven among the eight independent transformation events have been shown to carry multiple copies of the T-DNA. Multiple copies of the transgene are prone for transgene inactivation, silencing, and they are likely to cause a high frequency of insertional mutagenesis. However, in recent years several methods have been proposed to overcome this problem through cotransformation, site specific homologous recombination, generating transposon mediated single copy gene delivery methods, and the “agrolistic” method (Hansen and Chilton 1996; Koprek et al. 2001). These methods can be employed in the future for producing single copy transgene inserts in this tree species.
In vitro propagation of PPT resistant shoots through micro cuttings
After confirmation of the transgenic status of the in vitro grown shoots through Southern hybridization, further propagation was performed through micro cuttings. Transformed shoots were cloned on 1.5 mg l−1 PPT containing medium without cefotaxime. The subculture process was slow and approximately 6 weeks were required for the shoots to elongate (Fig. 4B). From the initial eight different GUS expressing shoots about three axillary buds per shoot could be propagated as microshoots. All the 24 micro cuttings survived on MS medium containing 6.6 μM BA and 1.5 mg l−1 PPT and produced 24 micro shoots after 4 weeks. These micro shoots after rooting and hardening resulted in 20 plants. Thus, selective propagation of shoots with the desired character was achieved using micro cuttings and the number of PPT resistant plants increased two-fold. Propagation of micro cuttings in the presence or absence of PPT did not show any significant difference (p<0.001) in the regeneration response (data not shown).
Rooting of the micro shoots was a problem as the shoots were small and delicate. The elongation and rooting process took 5 weeks and the roots were fragile (Fig. 4C). Similar problems were encountered during transformation studies in citrus (Peña et al. 1995). Nevertheless, clones from each separate transformant survived the rooting and hardening process.
Assay for Basta resistance
Bar gene expression in the transformed plants was further confirmed through the leaf painting test using the herbicide Basta. Of the 20 plants surviving hardening, 17 were resistant to commercial Basta herbicide (0.01%) (data not shown). Undamaged plants following leaf painting were assumed to be stable transformants (Fig. 4G). Control (nontransformed) plants turned yellow, bleached, and showed necrosis (Fig. 4H). Screening for Basta resistance by foliar spray in black locust had been discussed by Zaragoza et al. (2004). Similar test was performed in soybean to select for herbicide resistant transgenic plants (Zeng et al. 2004).
Herbicide resistance would be very useful in trees especially during establishment, where competition from weeds is the greatest (Shin et al. 1994). Developing herbicide resistant plants would allow the use of more efficient herbicides without concern for plant health in forestry, especially for commercial and higher intensity plantation systems (Confalonieri et al. 2003; Tang and Newton 2003; Polpin and Arce-Johnson 2005). To our knowledge it is for the first time that an Acacia species has been genetically engineered for herbicide tolerance. The efficiency of regeneration was low perhaps owing to the use of the powerful selection agent PPT. However, we showed the feasibility of the technique of nodal micro cuttings for enhanced transgenic plant production in this woody legume.
Abbreviations
- BA:
-
6-Benzylaminopurine
- CaMV:
-
Cauliflower mosaic virus
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- GA3 :
-
Gibberellic acid
- GUS:
-
β-Glucuronidase
- NPT II:
-
Neomycin phosphotransferase
- PPT:
-
Phosphinothricin
References
Bishop-Hurley SL, Zabkiewicz RJ, Grace L, Gardner RC, Wagner A, Walter C (2001) Conifer genetic engineering: transgenic Pinus radiata (D. Don) and Picea abies (Karst) plants are resistant to the herbicide Buster. Plant Cell Rep 20:25–243
Brukhin V, Clapham D, Elfstrand M, von Arnold S (2000) Basta tolerance as a selectable and screening marker for transgenic plants of Norway Spruce. Plant Cell Rep 19:899–903
Charity JA, Holland L, Grace LJ, Walter C (2005) Consistent and stable expression of the nptII, uidA and bar genes in transgenic Pinus radiata after Agrobacterium tumefaciens-mediated transformation using nurse culture. Plant Cell Rep 23:606–616
Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nester EW (1974) Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumours. Proc Natl Acad Sci USA 71:3672–3676
Chupeau MC, Pautot V, Chupeau Y (1994) Recovery of transgenic trees after electroporation of poplar protoplasts. Trans Res 3:13–19
Confalonieri M, Balestrazzi A, Bisoffi A, Carbonera D (2003) In vitro culture and genetic engineering of Populus spp: synergy for forest tree improvement. Plant Cell Tissue Organ Cult 72:109–138
De Block M (1990) Factors influencing the tissue culture and the Agrobacterium tumefaciens mediated transformation of hybrid aspen and poplar clones. Plant Physiol 93:1110–1116
Edelman M, Perl A, Flaishman M, Blumental A (2000) Transgenic Lemnaceae. Eur Patent Appl 102:1552
Fillatti JJ, Sellmer J, McCown B, Haissig B, Comai L (1987) Agrobacterium mediated transformation and regeneration of Populus. Mol Gen Genet 206:192–199
Han KH, Keathley DE, Davis JM, Gordon MP (1993) Regeneration of a transgenic woody legume (Robinia pseudoacacia L. black locust) and morphological alterations induced by Agrobacterium rhizogenes-mediated transformation. Plant Sci 88:149–157
Hansen G, Chilton M (1996) “Agrolistic” transformation of plant cells: integration of T-strands generated in planta. Proc Natl Acad Sci USA 93:14978–14983
Herschbach C, Kopriva S (2002) Transgenic trees as tools in tree and plant physiology. Tree 16:250–261
Igasaki T, Mohri T, Ichikawa H, Shinohara K (2000) Agrobacterium tumefaciens-mediated transformation of Robinia pseudoacacia. Plant Cell Rep 19:448–453
Jamal A, Huntsinger L (1993) Deterioration of a suitable agro-silvo-pastoral system in the Sudan: the gum gardens of Kardofan. Agrofor Syst 23:23–38
Jefferson RA, Kavanaugh TA, Bevan EW (1987) GUS fusion: Glucuronidase as a selective and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Koprek T, Rangel S, McElroy D, Louwerse JD, Williams-Carrier RE, Lemaux PG (2001) Transposon-mediated single-copy gene delivery leads to increased transgene expression stability in barley. Plant Physiol 125:1354–1362
Lörz H, Becker D, Lutticke S (1998) Molecular wheat breeding by direct gene transfer. Euphytica 100:219–223
Meilan R, Han KH, Ma CP, James RR, Eaton JA, Stanton BJ, Hoien E, Crockett RP, Strauss SH (2000) Development of glyphosate-tolerant hybrid cotton woods. Tappi J 83:164–166
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Peña L, Cervera M, Juarez J, Ortega C, Pina JA, Duran-Vila N, Navarro L (1995) High efficiency Agrobacterium-mediated transformation and regeneration of citrus. Plant Sci 104:183–191
Polpin MJ, Arce-Johnson P (2005) Transgenic trees for a new era. In Vitro Cell Dev Biol Plant 41:91–101
SAS (Statistical Analysis Systems Institute) (1996) SAS (Statistical Analysis Systems Institute) A guide to Statistical and Data Analysis Using JMP® and JMP IN® Software. SAS Institute, North Carolina, USA
Shin DI, Podila GK, Huang Y, Karnosky DF (1994) Transgenic larch expressing genes for herbicide and insect resistance. Can J For Res 24:2059–2067
Sokal PR, Rholf FC (1981) Biometry. Freeman WH, San Francisco, CO, p 776
Tang W, Newton RJ (2003) Genetic transformation of conifers and its application in forest biotechnology. Plant Cell Rep 22:1–5
Tzfira T, Zuker A, Altman A (1998) Forest-tree biotechnology: genetic transformation and its application to future forests. Trends Biotechnol 16:439–446
Vaidyaratnam PS (1994) Indian medicinal plants: a compendium of 500 species, vol 1. Orient Longman Ltd., Madras, pp 33–35
Vengadesan G, Ganapathi A, Prem Anand R, Ramesh Anbhazhagan V (2000) In vitro organogenesis and plant formation in Acacia sinuata (Lour.) Merr. Plant Cell Tissue Organ Cult 61:23–28
Vengadesan G, Ganapathi A, Prem Anand R, Selvaraj N (2003) In vitro propagation of Acacia sinuata (Lour.) Merr. from nodal segments of 10-year-old tree. In Vitro Cell Dev Biol Plant 39:409–414
Xie DY, Hong Y (2002) Agrobacterium-mediated genetic transformation of Acacia mangium. Plant Cell Rep 20:917–922
Zaragoza C, Munoz-Bertomeu J, Arrillaga I (2004) Regeneration of herbicide tolerant black locust transgenic plants by SAAT. Plant Cell Rep 22:832–838
Zeng P, Vadnais DA, Zhang Z, Polacco JC (2004) Refined glufosinate selection in Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill]. Plant Cell Rep 22:478–482
Acknowledgements
The senior author sincerely thanks Dr. Victor Gaba, Department of Virology, Institute for Plant Protection, ARO, Volcani Center, Israel for critical reviewing of this manuscript. The author also thanks Mr. Danny Shavit, Shavit Professional Scientific Photography, PSP Ltd., P.O.B.83, Bet Dagan 50250, Israel for his valuable assistance in photography.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Jouanin
Rights and permissions
About this article
Cite this article
Vengadesan, G., Amutha, S., Muruganantham, M. et al. Transgenic Acacia sinuata from Agrobacterium tumefaciens-mediated transformation of hypocotyls. Plant Cell Rep 25, 1174–1180 (2006). https://doi.org/10.1007/s00299-006-0176-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-006-0176-8