Abstract
The sequence of events in the functional body pattern formation during the somatic embryo development in cowpea suspensions is described under three heads. Early stages of somatic embryogenesis were characterized by both periclinal and anticlinal cell divisions. Differentiation of the protoderm cell layer by periclinal divisions marked the commencement of somatic embryogenesis. The most critical events appear to be the formation of apical meristems, establishment of apical-basal patterns of symmetry, and cellular organization in oblong-stage somatic embryo for the transition to torpedo and cotyledonary-stage somatic embryos. Two different stages of mature embryos showing distinct morphology, classified based on the number of cotyledons and their ability to convert into plantlets, were visualized. Repeated mitotic divisions of the sub-epidermal cell layers marked the induction of proembryogenic mass (PEM) in the embryogenic calli. The first division plane was periclinally-oriented, the second anticlinally-oriented, and the subsequent division planes appeared in any direction, leading to clusters of proembryogenic clumps. Differentiation of the protoderm layer marks the beginning of the structural differentiation in globular stage. Incipient procambium formation is the first sign of somatic embryo transition. Axial elongation of inner isodiametric cells of the globular somatic embryo followed by the change in the growth axis of the procambium is an important event in oblong-stage somatic embryo. Vacuolation in the ground meristem of torpedo-stage embryo begins the process of histodifferentiation. Three major embryonic tissue systems; shoot apical meristem, root apical meristem, and the differentiation of procambial strands, are visible in torpedo-stage somatic embryo. Monocotyledonary-stage somatic embryo induced both the shoot apical meristem and two leaf primordia compared to the ansiocotyledonary somatic embryo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidation of explants is a frequent cause for plant recalcitrance (Anthony et al. 1999) and is most prevalent in the members of the family Leguminosae as they contain high levels of phenolics. After a period of uncertainty, about the tissue culture manipulations to alleviate the negative effects of this stress, modified regeneration methods are continuously reported in new species of Leguminosae (Popelka et al. 2004). Large-seeded grain legumes, excepting a few, are commonly regarded as recalcitrant to in vitro regeneration and propagation. Recent report on cowpea regeneration via somatic embryogenesis provides a better understanding on the sequence of morphological structures differentiated in culture (DOI 10.1007/s00299-005-0965-5). Somatic embryogenesis is a process by which a bipolar structure, resembling a zygotic embryo, develops from a non-zygotic cell without vascular connection with the original tissue. However, visualizing structures resembling the shape of an embryo is no proof since many embryos cannot convert into plantlets due to innumerable reasons like appropriate embryo maturation and absence of an organized shoot meristem (Nickle and Yeung, 1993; Wetzstein et al. 1993; Moon et al. 1994; Suhasini et al. 1996). Plantlet conversion frequency provides a quantitative assessment of functional shoot and root meristems. Thus, to demonstrate the presence of a well-organized meristem, longitudinal sections through somatic embryos or explants need to be examined. Apart from optimizing the regeneration system, a detailed histological study was undertaken to place the changes in growth and morphogenesis in different organs of the cultured embryo in the context of the structural and anatomical features of the embryo at the time of culture in cowpea.
Materials and methods
Plant material and histology
For histological studies, embryogenic calli and different developmental stages of somatic embryos from cell suspensions (DOI 10.1007/s00299-005-0965-5) were collected periodically during the experimental period that was fixed in formalin: acetic acid: alcohol (FAA, 5:5:90 by vol.), for 48h at room temperature, dehydrated in ethanol, n-propanol, and n-butanol series, and embedded in paraffin following the standard procedures (Jensen 1962). Serial sections cut at 5–7 μm thickness on a rotary microtome equipped with a steel knife were stained in 0.05% toluidine blue and mounted. Slides were examined under bright field optics in a Zeiss Photomicroscope III, and sections were photographed using Kodak T-Max 100 film.
Results and discussion
The growth and development of higher plants are characterized by the cell division, expansion, and differentiation along two axes: the apical-basal and the radial. The latter is clearly evident in dicotyledonous species as the concentric rings of cell layers in the stem, hypocotyl, and root. An increase in size across this axis can arise by the generation of new cell layers following divisions in the vascular cambium. The establishment of the apical-basal axis is a critical event in somatic embryogenesis (Yeung and Stasolla 2000). Several investigators have emphasized a close resemblance in the developmental pattern of zygotic and somatic embryos, although the cytological and developmental characteristics at the early stages of somatic embryo development are still uncertain (Dodeman et al. 1997). The use of histology to study the ontogeny of plant development in plant tissue culture systems is reviewed by Yeung (1999). We studied the cellular ontogeny of somatic embryos induced in cell suspension cultures of cowpea. The events of morphogenesis are grouped under three headings: (i) early stages of somatic embryogenesis described as the process of induction of proembryogenic mass (PEMs) from the sub-epidermal cells of the explant (Fig. 1), (ii) late stages of somatic embryogenesis described as induction of different stages of somatic embryos from the PEMs following the activation of repeated cell division of embryogenic single cells (Fig. 2) and (iii) the plantlet conversion described as the final stages of vascular patterning for induction of two meristems—namely, shoot apical and root apical meristems (Figs. 3 and 4).
a–i Early structural events observed in primary leaf explants cultured in callus induction medium Legend: a leaf explant with single layer of epidermis and distinct parechymatous cells, b mitotic divisions in the sub-epidermal cells followed by epidermal cell expansion, c periclinally and anticlinally oriented division planes in the epidermal cell layer with rapidly dividing sub-epidermal cells, d–e induction and proliferation of embryogenic calli (5–7 d of culture) beneath the sub-epidermal cell layer, f formation of dense mass of proembryogenic cells (10 d of culture) clustering distinct from the mother cells, g–i different developmental stages of somatic embryos (15 d of culture) from semi-solid calli
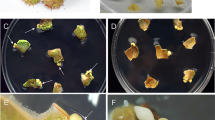
a–f Apical-basal pattern formation of somatic embryos induced in cell suspensions Legend: a globular-stage somatic embryo. Inner cells divide periclinally to differentiate ground and vascular tissue, b, c gradual establishment of polarity associate with differentiation of procambial cell layer (pc), d oblong-stage somatic embryo with distinct procambial cell layer (pc), e advanced globular embryo with established polarity. The two meristems are separated by a linear array of juvenile cells, f torpedo-stage somatic embryo with visible three embryonic cell domains (arrow heads). The apical domain, derived from the upper tier, has been partitioned into cotyledon (cot) primordial and shoot apical meristem (sam). The basal domain derived from the lower tier, formed the hypocotyls (h) and root (r), g–h close-view of torpedo-stage somatic embryos with three distinct embryonic cell domains; protoderm cells (p), ground meristem (gm), procambium cells (pc), shoot apical meristem (sam), root apical meristem (ram), i–k abnormal somatic embryos in cell suspensions
a–e Induction of shoot primordia from mature cotyledonary-stage somatic embryo Legend: a mature monocotyledonary somatic embryo before germination, b germinating somatic embryo, c closer view of shoot apical meristem (sam) with two distinct leaf primordia (lp) (400x), d ansiocotyledonary somatic embryo, e lack of sam in ansiocotyledonary somatic embryo which fails to initiate lp
a–c Vascular patterning of hypocotyledonary axis in germinating somatic embryos Legend: a three-tissue system namely dermal, ground, and vascular are visible, b circular arrangement of conjoint type of vascular bundles, c close-view of conjoint type of vascular bundles. ep epidermis, c collenchyma, vb vascular bundle, p pith, px protoxylem, mx metaxylem, ph phloem
Early stages of somatic embryogenesis
The primary leaf explant remained green during the first wk of culture and became swollen and yellowish, later. On the day of inoculation, a single layer of epidermis with distinct parenchyma cells was noticed (Fig. 1a). Longitudinal sections of the primary leaf explants cultured on callus induction medium revealed rapid mitotic divisions in sub-epidermal cells followed by expansion of epidermal cells (Fig. 1b). The cytoplasm-rich, sub-epidermal cells divided, first. The epidermal cells also began to divide concomitant with the mitotic activities of sub-epidermal cells. The initial division was restricted to a few number of epidermal cells. The first division plane was periclinal (Fig. 1b), the second anticlinal (Fig. 1c), and the subsequent division occurred in random plane, which resulted in small clusters of highly meristematic cells. Cell division in this region may be related just to the need for the epidermis to expand to accommodate the proliferation of internal cells.
Beneath the layer of broad patches of dividing (epidermal and sub-epidermal) cells, by d 7, small proembryogenic cell clumps were formed which gradually protruded above the surface of leaf explant as a mass of embryogenic callus (Fig. 1d). A similar pattern of somatic embryogenesis was observed in immature zygotic embryos of soybean (Hartweck et al. 1988), and in the cotyledons of mungbean (Mendoza et al. 1993). However, histological observations by Loiseau et al (1998) and Fernando et al. (2000) showed the sub-epidermal origin of globular structures, with enclosed epidermis, from the cotyledonary mesophyll. The structural variations observed in the embryogenic structures are probably related to the polar accumulation of an endogenous hormone, which may induce the somatic embryos along the periphery as observed by Souter and Lindsey (2000). Although extensive work has been carried out in vitro on PEM induction in various legumes (Lakshmanan and Taji 2000) to-date, the availability of structural details on the cellular origin and development of PEM is scarce especially from the leaf sections (Suhasini et al. 1996). Though the precise origin of somatic embryos has been traced in a few cases, the pathways of their origin varied considerably.
The PEMs consist of small, dense cytoplasmic cells but highly vacuolated. An increase in both the size and number of PEMs could be noticed by d 10 (Fig. 1e). These clumps further underwent cycles of growth and fragmentation in the same callusing medium resulting in the formation of dense mass of embryogenic calli after 15d of culturing. These embryogenic cells differentiated into nearly spherical globules with recognizable epidermis. The distinctive feature of embryogenic cells is to get separated from the surrounding mother cell leading to the formation of initial stages of somatic embryo (Fig. 1f–h). Actively dividing cells at the periphery of embryos react strongly with the stain than the metabolically inert, inner cell layers (Fig. 1i). The connection to the initial explant was small at the globular-stage that completely disappeared at the end of embryo development (Fig. 1f–i). Thus, torpedo-stage somatic embryos exhibited a closed vascular system (Fig. 1i). The observed pattern of PEM produced by simultaneous mitotic divisions of sub-epidermal cells confirmed the multicellular origin of somatic embryos as reported by Williams and Maheswaran (1986). An interesting observation is that the growth centers formed just beneath the epidermis rapidly multiply to induce clumps of PEMs. These further divide and differentiate to early stages of somatic embryos only after dissociating from the mother cell. The embryos remain independent with well-developed vasculature in advanced stages of somatic embryos. However, somatic embryos were induced in a heterogenous cell population and PEMs formed from various initial cell types, as observed by video cell tracking in carrot (Toonen et al. 1994). But, Nomura and Komamine (1985) showed that the isolated small, cytoplasm-rich carrot cells, after an unequal first division, have the ability to develop into somatic embryos. Thus, the formation of asymmetrically dividing cells is dependent on the concentration of 2,4-D (Pasternak et al. 2002) and genotype (Bogre et al. 1990). The type of division also determines further development (Feher et al. 2003).
Late stages of somatic embryogenesis
One of the interesting features of somatic embryogenesis is the transition of different stages of somatic embryos. Since the general morphology of somatic embryo is similar to that of zygotic counterpart, little attention has been paid to understand the somatic embryo development beyond the PEM (Yeung 1995). Repeated cycles of cell division and fragmentation of embryogenic cells in suspensions lead to the induction of early stages of somatic embryos after 2wk period.
Longitudinal sections of somatic embryos at different stages formed independently in embryogenic suspensions showed the typical structure of somatic embryos (Fig. 2). The induction of globular stage somatic embryos marked the beginning of structural differentiation (Fig. 2a). Histogenesis begins with the formation of a protoderm covering the globular somatic embryo that strongly reacts with the stain. The outer cell layer tends to divide more rapidly in an anticlinal direction, while the inner layers preferentially divide in a periclinal direction to form ground and vascular tissue (Fig. 2b). Axial elongation of inner isodiametric cells of the globular embryo leads to the formation of longitudinal extensions near the basal end of the embryo, which in turn, leads to the formation of oblong-stage embryo (Fig. 2d). Procambial cell differentiation and polarity establishment were observed at oblong-stage of embryo development (Fig. 2d, e). A change in size and shape of cells along the growth axis of the procambium is an important step in morphogenesis. Later, the ground meristem of the oblong stage embryo begins to vacuolate leading to the formation of a notch region in the protoderm cell layer. This induces the formation of torpedo-stage somatic embryos (Fig. 2f). In this transition-stage, three major embryonic tissues are visible (Fig. 2g): protoderm (p), ground meristem (gm), and procambium (pc). Subsequently, partitioning of cotyledon and shoot primordia in the upper layer of torpedo-stage somatic embryos takes place. The hypocotyl is composed of a layer of epidermal cells, enclosing two layers of cells of the cortex, a layer of cells of the endodermis, frequently a layer of cells of the pericyle, and a central core of 20–30 procambial strands. Asynchronous induction of different stages of somatic embryos was observed in suspension culture. Globular somatic embryos were observed together with later stages as described by Dornelas et al. (1992) and Fernando et al. (2002). Anatomical examination of the abnormal stages of somatic embryos showed an irregular vascularization along the inner cell layers. Abnormalities were induced irrespective of the stage of somatic embryos. The different stages of somatic embryos at which abnormalities were induced from the protoderm cells are shown in Fig. 2i–k. Cell division in the outer and inner layers is irregular leading to failure in the establishment of polarity. Active cell division observed only in the outer layer of cells led to the induction of secondary embryos. The most frequent abnormality associated with an inhibition in the normal development of somatic embryos in culture is the secondary embryogenesis. Sagare et al. (1995) in chickpea and Fernando et al. (2002) in soybean reported this type of abnormality. In addition, different types of abnormalities are also associated with cotyledonary-stage somatic embryos.
Plantlet conversion
The next important morphogenic event in embryogenesis is the formation of cotyledons and well-defined shoot apical meristem. Longitudinal and cross sections of mature cotyledonary-stage somatic embryos revealed the final stages of patterning at cellular level (Fig. 3). One of the most common abnormalities observed at this stage is the variable number of cotyledons in mature somatic embryos. Somatic embryos with single cotyledon are classified as monocotyledonary somatic embryo. It was observed that ansiocotyledonary somatic embryos presented only a single prominent cotyledon, the other being small and rudimentary. This was unable to germinate into a complete plantlet. The somatic embryos characterized as monocotyledonary, in the initial developmental stages, differentiated the shoot apical meristem (Fig. 3) followed by extension of hypocotyledonary axis in the germination medium (half-strength B5 +3% maltose +2500 mgl−1 KNO3 +0.05 mgl−1 TDZ). The reason for the failure maybe attributed to the pronounced vacuolation in the apical notch region. In addition, establishment of polarity is regarded as an important event for germination (Souter and Lindsey 2000). Mature monocotyledonary somatic embryos, obtained after the maturation phase germinated to complete plantlets with well-established apical dome cells as observed in Arachis hypogaea by Chengalrayan et al. (1997). In contrast, ansiocotyledonary-stage somatic embryos (Fernando et al. 2002) failed to initiate the functional meristem for the establishment of complete plantlets. The anatomical features of these embryos showed improper development of apical meristem and protoderm cells along the hypocotyledonary axis. Thus, the absence of a well-developed shoot meristem may be responsible for the lack of conversion of somatic embryos into plantlets (Suhasini et al. 1996).
Longitudinal sections of the mature monocotyledonary somatic embryos revealed an organized pattern of differentiation leading to the formation of shoot primordia (Fig. 3c). These embryos contain a well-defined meristematic dome. The cells within the meristematic dome are tightly packed with dense cytoplasm, and small vacuoles at the apical notch of the shoot meristem. The sub-epidermal cells begin to divide mainly in the periclinal direction. On d 5, shoot and leaf primordia begin to initiate from the protoderm cell layer in the development of a complete plantlet. Shoot apical meristem consists of four layers of small cells overlying the procambial strands of the hypocotyls and positioned between the two cotyledons. Just below the shoot apical meristem, the procambial strands of the hypocotyls divide into two groups, each extending into a cotyledon. This anatomical feature was clearly noticed in monocotyledonary-stage somatic embryo (Fig. 3a–c) of cowpea. However, in ansiocotyledonary-stage of somatic embryo an irregular differentiation of meristematic dome with no apical meristem region was observed. Cell at the periphery (ansio and monocotyledonary stages) initiated rudimentary leaf primordia along the protoderm cell layer of the meristematic dome (Fig. 3e). Thus, both the cotyledons differ in their cellular patterning at the organogenic region of the apical meristems rather than in the distal region. However, the cellular patterning in the procambium and ground tissues of both the cotyledons are similar. The procambium is composed of narrow, elongated prosenchymatous, meristematic cells that give rise to vascular tissue. The ground meristem is composed of large, thin-walled cells (Fig. 4). Transverse sections of the hypocotyledonary axis from the mature monocotyledonary somatic embryos, after 7d of germination, revealed the presence of three distinguishable tissue systems: the dermal, ground, and vascular systems. The dermal tissues are composed of single layer of closely arranged rectangular cells, the epidermis. The ground tissue contains circular collenchyma cells and the cambium forms the large proportion of vascular tissue in the vascular region. The conjoint type of vascular bundle is observed beneath the phloem and pericycle (Fig. 4b). Vascular bundles are arranged in the form of ring with the protoxylem towards the centre and metaxylem towards the periphery. Pith formed the central part of the hypocotyledonary axis which was enclosed by the vascular tissues (Fig. 4c).
Results obtained, thus, implicate the major role of auxin (2,4-D) in embryogenesis, providing the positional information for the co-ordination of correct cellular patterning from the globular stage. Auxin has proved a difficult molecule to localize in tissues, being highly diffusible and occurring in both active and inactive, conjugated forms (Normanly and Bartel 1999). Shoot meristem and leaf primordia regarded as the main site of synthesis with the polar auxin transport system hold the key for many responses. However, both intrinsic and extrinsic signals help to establish polarity in the early plant embryo development. The establishment of auxin transport system is a prerequisite for patterning events in the apical region of the embryo at the beginning of the transition from globular to heart-stage embryo (Yeung 1995). It is required for hypocotyl and root formation later in the development and maintenance. Once the meristems in the root and shoot have established, their sustainability is determined by the expression of a number of signaling systems that regulate the expression, which is yet to be fully understood.
References
Anthony JM, Senaratna T, Dixon KW, Sivasithamparam K, Bunn E (1999) In vitro regeneration of recalcitrant Australian plants.In Vitro Cell Dev Biol 35–53
Bogre L, Stefanov I, Abraham M, Somogyi I, Dudits D (1990) Differences in the responses of 2,4-D treatment between embryogenic and non-embryogenic lines of alfalfa. In: Nijkamp HJJ, van derPlas LHW, van Aartrijk J (eds) Progress in plant cellular and molecular biology. Kluwer Academic, Dordrecht, The Netherlands, pp 427–436
Chengalrayan K, Mhaske VB, Hazra S (1997) High-frequency conversion of abnormal peanut somatic embryos. Plant Cell Rep 16:783–786
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48(313):1493–1509
Dornelas MC, Vieera MLC, Appezzato-da-Gloria B (1992) Histological analysis of organogenesis and somatic embryogenesis induced in immature tissues of Stylosanthes scabra. Ann Bot 70:477–482
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228
Fernando JA, Vicira MLC, Geraldi IO, Appezzato-da-Gloria B (2002) Anatomical study of somatic embryogenesis in Glycine max (L.) Merrill. Braz Arch Biotechnol 45(3):277–286
Hartweck LM, Lazzeri PA, Cui D, Collins GB, Williams EG (1988) Auxin-orientation effects on somatic embryogenesis from immature soybean cotyledons. In Vitro Cell Dev Biol 24:821–828
Johansen DA (1940) Plant Microtechnique. McGraw-Hill, New York
Lakshmanan P, Taji A (2000) Somatic embryogenesis in leguminous plants. Plant Biol 2:136–148
Loiseau J, Michaux-Ferriere N, Deunff YL (1998) Histology of somatic embryogenesis in pea. Plant Physiol Biochem 36(9):683–687
Mendoza AB, Kazumi H, Nishimura T, Futsuhara Y (1993) Histological and scanning electron microscopic observations on plant regeneration in mungbean cotyledons cultured in vitro. Plant Cell Tissue Organ Cult 32:137–143
Moon YH, Kim SK, Choi SB (1994) Plant regeneration of soybean cultivars via somatic embryogenesis. J Plant Biol 37:333–341
Nomura K, Komamine A (1985) Identification and isolation of single cells that produce somatic embryos at a high frequency in a carrot suspension culture. Plant Physiol 79:988–991
Normanly J, Bartel B (1999) Redundancy as a way of life—IAA metabolism. Curr Opin Plant Biol 2:207–213
Pasternak T, Miskolczi P, Ayaydin F, Meszaros T, Dudits D, Feher A (2002) Exogenous auxin and cytokinin dependent activation of CDKs and cell division in leaf protoplast-derived cells of alfalfa. Plant Growth Regul 32:129–141
Popelka JC, Terryn N, Higgins TJV (2004) Gene technology for grain legumes: can it contribute to the food challenge in developing countries? Plant Sci 167:195–206
Sagare AP, Suhasini K, Krishnamurthy KV (1995) Histology of somatic embryo initiation and development in chickpea (Cicer arietinum L.). Plant Sci 109:87–93
Souter M, Lindsey K (2000) Polarity and signaling in plant embryogenesis. J Exp Bot 51(347):971–983
Suhasini K, Sagare AP, Krishnamurthy KV (1996) Study of aberrant morphologies and lack of conversion of somatic embryos of chickpea (Cicer arietinum). In Vitro Cell Dev Biol Plant 32(6):6–10
Toonen MAJ, Hendriks T, Schmidth EDL, Verhoeven HA, van Kammen A, de Vries SC (1994) Description of somatic embryo-forming single cells in carrot suspension cultures employing video cell tracking. Planta 194:565–572
Wetzstein HY, Baker CM (1993) The relationship between somatic embryo morphology and conversion in peanut Arachis hypogaea L. Plant Sci 92:81–89
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behavior of cells as an embryogenic group. Ann Bot 57(4):443–462
Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis. In: Thorpe TA (ed) Embryogenesis in plants. Kluwer Academic, Dordrecht, Boston, London, pp 205–247
Yeung EC (1999) The use of histology in the study of plant tissue culture systems—some practical comments. In Vitro Cell Dev Biol Plant 35:137–143
Yeung EC, Stasolla C (2000) Somatic embryogenesis—apical meristem formation and conversion. Korean J Plant Tissue Cult 27:253–258
Acknowledgement
We gratefully acknowledge Dr. Kapil Deo Singh for helpful discussion.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by P. Debergh
Rights and permissions
About this article
Cite this article
Ramakrishnan, K., Gnanam, R., Sivakumar, P. et al. Developmental pattern formation of somatic embryos induced in cell suspension cultures of cowpea [Vigna unguiculata (L.) Walp]. Plant Cell Rep 24, 501–506 (2005). https://doi.org/10.1007/s00299-005-0966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0966-4