Abstract
Mammalian Bax is known to cause cell death when expressed in plants. We examined transgenic plants expressing both Bax and organelle-targeted green fluorescent protein to determine the cellular changes that occur during Bax-induced cell death. The mitochondria changed morphologically from being bacilli-shaped to being round, eventually becoming swollen. Mitochondria streaming also stopped. The chloroplasts lost membrane function and their contents leaked out, followed by the disruption of the vacuole. Light was not essential for Bax-induced ion leakage or organelle disruption. These results indicate that Bax induces temporal and spatial cell death events at the organelle level in the plant. A heterologous system, using Bax, would therefor be available to investigate cell death, which is commonly conserved in animals and plants
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investigations on programmed cell death (PCD) in animal systems have revealed pathways in which proteins of the Bcl-2 family play a key role. The Bcl-2 family includes pro- (e.g., Bax, Bak, Bid) and anti-apoptotic (e.g., Bcl-2, Bcl-xl, Ced-9) proteins that appear to control the initiation of apoptosis by means of the mitochondria (Gross et al. 1999). When Bax is translocated from the cytosol to the outer membrane of the mitochondria it induces the release of proteins, such as cytochrome c, to the cytosol, and consequently triggers apoptosis (Liu et al. 1996; Jürgensmeier et al. 1998).
Comparative genomic studies have revealed that the Bcl-2 family-like proteins are absent in yeasts and plants (Aravind et al. 1999). However, when Bax is expressed, it has been shown to be able to kill plant (Lacomme and Cruz 1999; Kawai-Yamada et al. 2001) and yeast cells (Zha et al. 1996; Jürgensmeier et al. 1997), while the expression of the anti-apoptotic proteins Bcl-xl, Bcl-2, and Ced-9 has been shown to protect tobacco plants from cell death induced by UV irradiation and paraquat treatment and from the HR upon tobacco mosaic virus (TMV) infection and attack by fungal pathogens (Mitsuhara et al. 1999; Dickman et al. 2001). As a means to study plant defense mechanisms, several investigators have been using the Bax gene as an inducer of cell death. For example, AvrPtoB, an effector protein conserved among diverse genera of plant pathogens, inhibits cell death initiated by Bax (Abramovitch et al. 2003). In addition, overexpression of AtBI-1, a plant homolog of mammalian BI-1, inhibits Bax-induced cell death in Arabidopsis (Kawai-Yamada et al. 2001) as well as fungal elicitor-induced cell death in cultured rice cells (Matsumura et al. 2003). These investigations revealed that plants have mechanisms similar to those operating in animal apoptosis. Bax-induced plant cell death is a potentially useful heterologous system for studying the evolution of the regulation of cell death. The ROS that result from external and internal stresses trigger cell death in plants (Levine et al. 1994; Pennell and Lamb 1997; Orozco-Cardenas and Ryan 1999). We recently observed the generation of ROS in transgenic Arabidopsis plants harboring the mouse Bax gene (Kawai-Yamada et al. 2004). Baek et al. (2004) demonstrated that when protoplasts expressing Bax are treated with the anti-oxidant N-acetyl-l-cysteine (NAC), Bax-mediated ROS production and the cell death phenotype are strongly suppressed. In fact, Bax-induced cell death in yeast cells has been observed to be suppressed by genes conferring anti-oxidant activities (Kampranis et al. 2000; Levine et al. 2001; Pan et al. 2001). Despite these results, detailed information on Bax-mediated cellular changes in plants is still not available. In the present study, we analyzed the temporal and spatial events that occur at the organelle level in plant cells under Bax-induced cell death. On the basis of our results, we conclude that the vulnerability of organelles to Bax is an orchestrated mechanism that occurs prior to the consequential death of the plant cell. This heterologous system provides a useful basis for discussing the cell death mechanism that is commonly preserved in both animals and plants.
Materials and methods
Plant materials
The Bax transgenic line was obtained by transforming Arabidopsis thaliana ecotype Col-0 with the DEX-inducible vector pTA7002 (Aoyama and Chua 1997) containing mouse Bax (Kawai-Yamada et al. 2001). Double-transgenic plants possessing mitochondria (mt)-GFP or plastid (pt)-GFP and Bax were generated using pollen from the Bax transgenic plants to fertilize the flowers of mt-GFP or pt-GFP plants (Niwa et al. 1999). The F2 population that grew on the medium containing hygromycin (20 μg ml−1) was used for further analysis. Transgenic plants were grown at 23°C under continuous light. Tobacco suspension-cultured cells BY-2 were grown in modified MS medium (Murashige and Skoog 1962) enriched with 0.2 mg l−1 2,4-D and maintained at 28°C under continuous darkness. Arabidopsis cell suspension cultures were derived from Bax transgenic Arabidopsis plants (Kawai-Yamada et al. 2001) and maintained in MS medium supplemented with 3% sucrose and 0.1 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D). The cells were transferred to fresh medium every 7 days, and experiments were carried out 3 days after the transfer.
Cell fractionation
Three-week-old Bax transgenic plants were homogenized using a pestle and mortar in 0.4 M mannitol, 25 mM MOPS buffer (pH 7.8), 1 mM EGTA, 4 mM cysteine, and 0.2% (w/v) bovine serum albumin. The homogenate was filtered through Miracloth (Calbiochem, San Diego, Calif.), then the filtrate was subsequently centrifuged at 2,000 g for 10 min at 4°C. The supernatant was transferred to a new tube and re-centrifuged at 10,000 g for 15 min at 4°C to obtain a pellet containing mitochondria. The supernatant was re-centrifuged at 100,000 g for 60 min at 4°C and the resulting supernatant was used as a cytosolic fraction. The pellet containing mitochondria was resuspended in 0.4 M mannitol, 10 mM Tricine (pH 7.2), and 1 mM EGTA. These fractions were stored at –80°C.
Immunological detection
Total protein extraction and Western blot analysis were performed as described in Kawai et al. (1995). Proteins (20 μg) were electrophoresed on sodium dodecyl sulfate (SDS)-polyacrylamide gels (15%), blotted onto a polyvinylidene difluoride (PVDF) membrane (Immobilon, Millipore), and then treated with anti-Bax antibody (06-499; Upstate Biotechnology, Charlottesville, Va.) or anti-VDAC antibody (PM035; GTMA). Bax and VDAC proteins were visualized with the enhanced chemiluminescence (ECL) system according to the manufacturer’s instructions (Amersham Pharmacia, Piscataway, N.J.).
Yeast strain and expression
Expression vector pTS909-Bax-GFP was constructed by ligating the SalI-tagged coding sequence of mouse Bax cDNA into the SalI site of a cassette vector pTS910-GFP. The Bax-GFP fragment was cut out with HindIII/SalI and inserted into the HindIII/SalI site of pTS909 possessing a 2-μm replicon. The Saccharomyces cerevisiae strain BF264-15 Dau (MATα ade1 his2 leu2-3, 112 trp1-1a ura3; Lew et al. 1991) was transformed with plasmid pTS909-Bax or pTS909-Bax-GFP by the lithium acetate method. The plasmid pTS909 was also introduced into yeast as a control. Tryptophane+ (Trp+)-transformants were streaked either on a synthetic dropout (SD)-glucose plate or on a SD-galactose plate and incubated at 30°C for 3 days.
Transient expression of Bax-GFP in tobacco BY-2 cells
To express the GFP-tagged Bax in tobacco suspension cells BY-2, we cloned the SalI-tagged coding sequence of mouse Bax amplified by PCR into the SalI site of a GFP cassette plasmid (pUC18-GFP) containing a CaMV 35S promoter and a nopaline synthase (NOS) terminator (Niwa et al. 1999). Plasmid DNA (5 μg) was introduced into 4-day-old tobacco BY-2 cells by particle bombardment with a helium-driven particle accelerator (PDS/1000; Bio-Rad, Hercules, Calif.). The applied bombardment parameters were 1,100 psi (1 psi =6.89 kPa) of bombardment pressure, 1.0-μm gold particles, a distance of 12 cm from the macrocarrier to the leaf pieces and suspension cells, and a decompression vacuum of 28 in Hg. The bombarded samples were incubated at 28°C in continuous darkness.
Microscopic observations
Microscopic observation was carried out using a fluorescent microscope (Nikon Eclipse-600 and a 100× 1.4 numerical aperture objective) and a confocal laser scanning microscope system (MicroRadiance MR/AG-2; Bio-Rad). BY-2 cells or 3-week-old transgenic Arabidopsis leaves treated with or without DEX were placed on glass slides and examined with an argon ion laser (488 nm) in order to observe the GFP and with a green HeNe laser (543 nm) to observe chlorophyll autofluorescence, Mito-RFP, and Mito Tracker Red.
To visualize active mitochondria that maintained a transmembrane potential, BY-2 cells were stained with the fluorescent probe Mito Tracker Red (500 nM, Molecular Probes, Invitrogen, Carlsbad, Calif.) for 15 min as described in Arimura and Tsutsumi (2002). For the control of mitochondrial localization, Mito-RFP was co-transfected to BY-2 cells. Mito-RFP contains a mitochondrial-targeting signal derived from the Arabidopsis ATPase σ-subunit (Niwa et al. 1999) connected with RFP (Clontech, Palo Alto, Calif.). The chimera gene was under 35S CaMV promoter regulation.
For electron microscopic analysis, leaf sections obtained from 3-week-old plants treated with 10 μM DEX were fixed in 2.5% glutaraldehyde in cacodylate buffer (pH 7.2), and then treated with OsO4. The substituted samples were embedded in Spurr’s resin, and thin serial sections were prepared with an Ultracut UCT (Leica, Wien, Austria). Sections were stained with uranylacetate and lead citrate (UA/Pb) and observed under the electron microscope (Hitachi H-7600; Hitachi High-Technologies, Shinjyuku-ku, Japan).
Evans blue staining
Cell cultures treated with or without DEX were incubated for 15 min with 0.5% Evans blue and then washed extensively to remove excess and unbound dye with distilled water. Dye bound to dead cells was solubilized in 50% methanol with 1% SDS for 30 min at 50°C and quantified by absorbance at 600 nm.
Ion leakage measurement
Leaf discs from 1-month-old Arabidopsis plants were floated on distilled water or solutions containing various chemicals. Electrolyte leakage was monitored using an electrical conductivity meter (Horiba B-173, Japan).
Results
Bax targets mitochondria and disrupts the mitochondrial transmembrane potential
Bax is known to translocate to mitochondria during the initiation of apoptosis in animal cells (Gross et al. 1999). Lacomme and Cruze (1999) reported that the GFP-tagged C-terminal region of Bax, which was ectopically expressed using a virus vector, co-localized with Mito Tracker Red in Nicotiana benthamiana leaf trichomes. In this study, we determined the cellular localization of the full-length Bax protein in transgenic plants. Following DEX treatment to induce Bax gene expression, several cellular fractions (total, cytosol, and mitochondria-rich) were isolated for Western blot analysis. As shown in Fig. 1a, the level of Bax in total protein extracts increased after 1 day of DEX treatment. A similar pattern was found for the mitochondria-rich fraction. In contrast, the level of Bax protein decreased in the cytosol fraction after 2 days of treatment. VDAC (voltage-dependent anion channel), a mitochondrial outer membrane protein, was not detected in the cytosolic fraction, indicating that there was no mitochondrial contamination in the cytosol fraction.
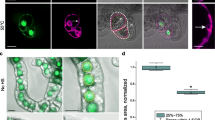
Cellular localization of Bax protein. a Immunodetection of Bax protein in a transgenic Arabidopsis plant. Following treatment of the transgenic plants harboring Bax (3 week-old) with 10 μM DEX for up to 3 days, total, cytosol and mitochondrial-enriched fractions were isolated and used for Western blot analyses with antibodies against Bax and voltage-dependent anion channel (VDAC). b Effects of GFP-tagged Bax (Bax-GFP) on Bax-induced lethality in yeast. Expression vectors (pTS909-Bax and pTS909-Bax-GFP) or control plasmid (pTS909) were transformed into yeast strain BF264-15Dau (Xu and Reed 1998). The transformants were streaked on glucose (Glu)- or galactose (Gal)-containing medium lacking tryptophane. Photographs were taken after a 3-day incubation at 30°C. c Distribution of Bax-GFP fluorescence in plant cells. Tobacco suspension cells were co-transfected with mitochondria-targeted red fluorescent protein (Mito-RFP) and GFP, or Bax-GFP by the particle accelerator as described in the Materials and methods. The transfected cells cultured for 1 day were observed under a confocal laser scanning microscope (MicroRadiance MR/AG-2; Bio-Rad) at a 488-nm excitation wavelength to detect GFP (green) and a 543-nm for RFP (red). Magnified images of Bax-GFP-expressing cells are shown in the lower panels. d Loss of mitochondrial membrane potential after Bax expression. BY2 cells transiently expressing Bax-GFP (1 day after transfection) were stained with a mitochondrial membrane potential indicator, Mito Tracker red and observed under a confocal laser scanning microscope
To further analyze the localization of Bax in plant cells, we first constructed yeast expression vectors that contained cDNAs encoding Bax (pTS909-Bax) or Bax-GFP (pTS909-Bax-GFP) under the control of a GAL1 promoter. These plasmids were transformed into the wild-type yeast strain BF264-15 Dau, and the ability of the fusion protein to induce cell death was studied. As a control study, the empty pTS909 (vector) plasmid was transformed into the same yeast strain. As shown in Fig. 1b, yeast cells transformed with pTS909-Bax or pTS909-Bax-GFP markedly lost viability when yeast cells were plated on galactose-containing medium. In contrast, cells transformed with the empty vector pTS909 survived. This result indicates that the GFP-tagged Bax retains the ability to induce cell death in yeast.
We then expressed Bax-GFP in plant cells. The vector, pUC18-35 S-Bax-GFP (Bax-GFP), was constructed and introduced into tobacco BY-2 suspension cells by means of particle bombardment. Under confocal laser scanning microscopy, the fluorescent signal of Bax-GFP was observed in cytosolic and punctuated structures that co-localized with the fluorescent signals of Mito-RFP (a marker for mitochondria) (Fig. 1c). Bombardment of the control plasmid pUC18-35S-GFP (GFP) resulted in a cytosolic localization. This result indicates that Bax protein is localized to the mitochondria in plant cells. To visualize those functional mitochondria retaining a transmembrane potential, we used Mito Tracker Red, a vital mitochondrial fluorescent probe. In cells expressing GFP, Mito Tracker Red clearly stained the mitochondria, while Bax-GFP-expressing cells did not show the fluorescence of Mito Tracker Red (Fig. 1d). These results suggest that mitochondrial localization of Bax causes the loss of mitochondrial membrane potential.
Early morphological changes of mitochondria after the Bax expression
To analyze the behavior of the organelles during Bax-induced cell death, we visualized mitochondria and chloroplasts using GFP. Double-transgenic plants possessing Bax (DEX-inducible; Kawai-Yamada et al. 2001) and organelle-targeted-GFP [mt- or pt- targeted GFP (S65T) under the control of the 35S promoter; Niwa et al. 1999] were obtained. When 3-week-old seedlings harboring the DEX-inducible Bax gene (either mt-GFP/Bax or pt-GFP/Bax plants) were transferred to a medium containing 10 μM DEX, massive cell death was observed at the whole-plant level within 4 days. In contrast, this did not occur in the control lines (mt-GFP/pTA, pt-GFP/pTA) (Fig. 2a). Immunoblot analysis revealed that the Bax proteins were detectable 24 h after initiation of the DEX treatment in the mt-GFP/Bax and the pt-GFP/Bax plants (Fig. 2b). Mesophyll cells of the mt-GFP/pTA and the pt-GFP/pTA plants (3-week-old) were observed with or without 10 μM DEX for 3 days under a confocal laser scanning microscope. As shown in Fig. 2c, the GFP signal in the mt-GFP plants was seen as bacilliform shapes that moved. On the other hand, pt-GFP co-localized with the red autofluorescence derived from chlorophylls, and these fluorescent patterns were not affected by the DEX treatment.
Phenotypes associated with the expression of Bax. a Phenotypes of plants possessing mt-GFP or pt-GFP, and Bax. Three-week-old seedlings grown in the absence of DEX were transferred to a medium containing 0 μM (−) or 10 μM (+) DEX and cultured at 23°C for 4 days. The mt-GFP/pTA and pt-GFP/pTA lines were used as negative controls. b Accumulation of Bax protein as a function of time. Three-week-old seedlings grown on non-inductive medium were transferred onto the inductive medium containing 5 μM DEX and cultured at 23°C. Proteins were isolated at selected time points (0, 1, and 2 days) after initiation of the DEX treatment. Twenty micrograms of total proteins was used for Western blot analysis. c Distribution of mt-GFP and pt-GFP fluorescence in double-transgenic plant cells. Three-week-old seedlings were transferred onto the medium containing 0 μM (−) or 10 μM (+) DEX and cultured at 23°C for 4 days. These plant cells were observed under a confocal laser scanning microscope at a 488-nm excitation wavelength for GFP (green) and a 543-nm for chlorophyll autofluorescence (red), as described in the Materials and methods
Using mt-GFP/Bax plants, we observed mitochondrial morphology during Bax-induced cell death (Fig. 3a). In the absence of DEX treatment (day 0), the mitochondria assumed a bacilliform shape (Fig. 3a; a′, f′), and most were actively moving around, possibly due to cytoplasmic streaming. Within 1 day after initiation of the DEX treatment, the morphology of the mitochondria had changed dramatically and assumed a round shape (Fig. 3a; b′, g′). At this stage most of the mitochondria were immobile, with only a few moving slowly. By the second day of DEX treatment, mitochondria swelling was observed and cytoplasmic streaming had stopped completely [Fig. 3a; c′, h′; see electronic supplementary material (movie)]. Irregular clumping of the mitochondria was noted at 3 days (Fig. 3a; d′, i′), and by the 4th day, mt-GFP no longer fluoresced and the chlorophyll autofluorescence had declined dramatically (Fig. 3a; e′, j′).
Morphological changes in the mitochondria and chloroplasts during Bax-induced cell death. a Morphological changes in the mitochondria. Three-week-old mt-GFP/Bax seedlings were transferred to the medium containing 10 μM DEX and cultured at 23°C. Mesophyll cells were examined using a confocal laser scanning microscope at each time point (0, 1, 2, 3, and 4 days). The merged images of mt-targeted GFP (green) and autofluorescence of chlorophyll (red) are shown. f′–j′ are magnified images of a′-e′. b Distribution of pt-GFP during Bax-induced cell death. Mesophyll cells of pt-GFP/Bax plants were examined as described in 3a. The merged images of pt-targeted GFP (green) and autofluorescence of chlorophyll (red) are shown. f′-o′ are magnified images of a′-e′. Yellow signals are areas where the green and red signals co-localize. * Vacuole
Chloroplast disruption followed by vacuolar rupture
The pt-GFP (green) signal and the autofluorescence of the chloroplasts (red) had completely co-localized at day 0 (Fig. 3b; a′, f′, k′). However, after 1 day of DEX treatment, pt-GFP was no long uniformly distributed within the chloroplast (Fig. 3b; b′, g′, l′). This tendency was observed in most of the chloroplasts approximately 20 h following initiation of the DEX treatment. At 2 days post-DEX treatment, pt-GFP had leaked out of the chloroplasts and spread into the cytosol (Fig. 3b; c′, h′, m′). However, we were still able to confirm the existence of the vacuole at this time. At 3 days post-DEX treatment, the pt-GFP had spread and could be detected throughout the cell (Fig. 3b; d′, i′, n′). This widespread distribution of the pt-GFP indicated that the tonoplast had ruptured between day 2 and day 3. By the fourth day of DEX treatment, chlorophyll autofluorescence had decreased and eventually disappeared (Fig. 3b; e′, j′, o′).
Ultrastructural analysis
Electron microscopic analysis was carried out to examine the subcellular morphology of Bax-expressing cells. As shown in Fig. 4a, living cells were characterized by a rigid cell wall and well-structured chloroplasts tightly pressed against the plasma membrane. However, broken chloroplasts and plasmolysis were apparent in mesophyll cells of plants expressing Bax (Fig. 4b). Electron microscopy also confirmed that the mitochondria became round in shape and aggregated following Bax expression (Fig. 4d). Relative to the control (Fig. 4e), the lamella structure of the chloroplasts of the Bax-expressing cells had loosened within a day (Fig. 4f). With 2 days of Bax expression, the inner structure of chloroplast had been destroyed (Fig. 4g).
Electron micrographs of Bax-expressing Arabidopsis mesophyll cells. Mesophyll cells obtained from plants treated with 10 μM DEX for 1 day (f), 2 days (g), 3 days (d), or 4 days (b) were examined under an electron microscope. a, c, e Control cells, b, d, f, g Bax-expressing cells. a, b Whole cell, c, d mitochondria, e, f, g chloroplast
Chlorosis is light-dependent in Bax-expressing plants
Light is required for lesion formation in response to pathogens (Peever and Higgins 1989; Guo et al. 1993), lesion-mimic mutants ( Jabs et al. 1996; Genoud et al. 1998), and transgenic plants that form spontaneous HR-like lesions ( Elkind et al. 1990; Chamnongpol et al. 1996). To examine the influence of light in Bax-induced plant cell death, we treated seedlings or detached leaves of Bax plants with 5 μM DEX under complete dark or light conditions. As shown in Fig. 5a, DEX-treated leaves showed an apparent chlorosis after a 3- to 4-day exposure to light. Such discoloration was not observed in leaves kept in the dark. Immunological Western blot analysis confirmed the expression of Bax protein in plants grown under dark or light conditions (Fig. 5b).
Effects of light on Bax-induced chlorosis. a Suppression of Bax-induced chlorosis under the dark condition. Three-week-old plants grown on MS medium under continuous light (100 μmol m−2 s−1) were transferred to a medium containing 5 μM DEX and subsequently grown under light or dark conditions. The photographs were taken 1–4 days following initiation of the DEX treatment. b Immunological detection of Bax protein in the Bax transgenic plants treated with (+) or without (−) 5 μM DEX under dark or light conditions for 24 h. Ten micrograms of total proteins was used for Western blot analysis. c Bax-induced ion leakage under dark or light conditions. Detached leaves of Bax transgenic plants were incubated with (+) or without (−) 5 μM DEX in distilled water at 23°C under light or dark conditions. Electrical conductivity of the solution was measured with a conductivity meter (n=3; relative value). d Bax-induced chloroplast and tonoplast disruption under the dark condition. Three-week-old transgenic plants containing Bax and plastid tagged-GFP (pt-GFP/Bax) were treated with 10 μM DEX under the dark condition. Compare with 3b images under the light condition. As a control, a transgenic plant with an empty vector and plastid targeted-GFP (pt-GFP/pTA) was treated similarly. Images were obtained using a confocal laser scanning microscope at the following wavelengths: 448 nm for pt-GFP and 568 nm for the chlorophylls. Merged images are shown in the lower panel
Ion leakage was used to monitor plant cell death (Mitsuhara et al. 1999; Rizhsky et al. 2002). We previously reported that the ectopic-expression of Bax triggers the leakage of ions in plants grown in light (Kawai-Yamada et al. 2004). The leaf discs of plants expressing the Bax protein showed an obvious ion leakage even when kept in the dark (Fig. 5c), while the control samples (−DEX) did not show any ion leakage regardless whether they were kept under dark or light conditions. To investigate the behavior of the chloroplasts and vacuole, we used both transgenic plants harboring Bax and pt-GFP genes. Both in the presence (Fig. 3b) and absence (Fig. 5d) of light, a massive leakage of pt-GFP from the chloroplasts to the cytosol occurred and the vacuole was disrupted within 3 days post-DEX treatment.
To further investigate light-mediated Bax-induced chlorosis, p-benzoquinone (BQ) was used as a quencher of singlet oxygen and triplet chlorophyll, which are generated by the photo-reaction in chloroplasts (Fujimori and Livingston 1957; Feierabend and Winkelhusener 1982). Leaf discs of plants expressing Bax were incubated in distilled water with or without BQ. Following a 4-day incubation in continuous light, the leaf samples treated with BQ remained green (Fig. 6a). However, BQ treatment did not inhibit ion leakage in plants expressing Bax (Fig. 6b).
a Suppression of Bax-induced chlorosis by BQ treatment. Leaf discs obtained from Bax transgenic plants (3 weeks old) were treated with 5 μM DEX solution with (+) or without (−) 1 mM BQ for 4 days at 23°C. b Bax-induced ion leakage in BQ-treated leaflets. Three leaf discs obtained from Bax transgenic plants were treated with DEX (0 μM or 5 μM) and BQ (0–2.0 mM) under light condition at 23°C, and the electrical conductivity of the solution was measured with a conductivity meter (n=3). c Immunological detection of the Bax protein under the control of a DEX-inducible system. Three-day-old suspension-cultured cells were treated with 0 μM (−) or 10 μM (+) DEX and then cultured at 23°C for 3 days. Proteins were isolated at each time point (0, 1, 2 and 3 days) after the DEX treatment. Twenty micrograms of the total protein was used for Western blot analysis. d Induction of cell death by Bax. Cell death of suspension cells was measured by Evans blue staining each day (0, 1, 2 and 3 days) following the treatment with DEX, as described in the Materials and methods. Three-day-old suspension cells of transgenic lines (pTA and Bax) were treated with 0 μM (−) or 10 μM (+) DEX
In addition, we studied the effects on the chloroplast using cultured cells. Arabidopsis suspension cells transformed with the Bax gene were generated and grown in the dark. The Bax protein was detected immunologically (Fig. 6c). Bax protein levels in DEX-treated suspension cells rose within 1 day and remained constant for 2 days. We then evaluated the rate of dead cells in Bax-expressing cell suspensions using Evans blue. As shown in Fig. 6d, Bax transgenic lines showed a remarkable increase in cell death upon Bax expression. DEX treatment had no effects on the basal level of Evans blue staining in the control cells. These results indicate that light is essential for leaf chlorosis, which is the last step of plant cell death, but it is not essential for ion leakage or organellar disruption.
Discussion
There exists a great deal of controversy surrounding our understanding of the basic mechanism of cell death in plants and metazoa (see Lam et al. 2001). The obvious similarity in the evolutionary aspects of PCD in distantly related organisms, however, provides an insight into how cell survival is sustained through intracellular organelles. In relation to this, recent advancements in gene cloning and the development of heterologous suicide systems have warranted the studies on possible universal mechanisms in cell death (Zha et al. 1996; Laccome and Cruz 1999; Dickman et al. 2001; Kawai-Yamada et al. 2004).
Bax, a pro-apoptotic member of the Bcl-2 family, is a crucial protein in the PCD pathways of animal systems. Bax localizes in the cytosol of healthy cells and translocates to the surface of mitochondria upon the initiation of apoptosis (Nechushtan et al. 2001). The mitochondrial localization of Bax allows proteins such as cytochrome c to move from the mitochondria to the cytosol, which eventually leads to the initiation of apoptosis (Jürgensmeier et al. 1998). Interestingly, the overexpression of Bax in yeast and plants causes lethality even though these organisms do not have Bcl-2-related proteins naturally (Zha et al. 1996; Jürgensmeier et al. 1997; Lacomme and Cruz 1999; Kawai-Yamada et al. 2001). Furthermore, anti-apoptic proteins Bcl-2, Ced-9, and Bcl-xl can increase the resistance to stress caused by fungal pathogens, UV-B irradiation, or paraquat (Mitsuhara et al. 1999; Dickman et al. 2001). These results imply that plants and metazoa share a similar cell-death pathway.
We reported recently that in transgenic Arabidopsis, Bax-induced cell death was accompanied by the generation of ROS, a metabolic process probably originating in the mitochondria (Kawai-Yamada et al. 2004). In the present study, we demonstrated that the full-length Bax protein fused with GFP was localized on plant mitochondria and that this preceded the disruption of the mitochondrial transmembrane potential, the latter being known as a typical feature of early apoptosis in metazoa (Finucane et al. 1999). Lacomme and Cruz (1999) used a virus vector system and demonstrated that a fusion protein consisting of the transmembrane domain of Bax and GFP co-localizes with the signal of Mito Tracker Red in the leaf trichome cell of Nicotiana benthamiana. In the Arabidopsis protoplast system, Baek et al. (2004) showed the importance of the C-terminal region of the Bax protein to localize mitochondria. Our results support these data and prove that dysfunction of mitochondria depends on Bax localization.
Using double-transgenic Arabidopsis plants possessing Bax and organelle-targeted GFP, we observed that the mitochondria underwent morphological changes following Bax expression: the original bacilliform shape changed into a round form and eventually the mitochondria became swollen. It has recently been reported that the fragmentation of tubular mitochondria into short punctiform structures is a common early feature of apoptotic mammalian cells (Vieira et al. 2002; Breckenridge et al. 2003). Normal HeLa cells display worm-like-shaped mitochondria, whereas cells in the process of Bax- or etoposide-induced death have punctiform mitochondria (Desagher and Martinou 2000). Changes in the morphology of the mitochondria have been reported in yeast as well as in mammalian cells (Dimitrova et al. 2004). Karbowski et al. (2004) showed that mitochondria fusion is blocked during Bax-induced apoptosis and that permeabilization of the outer mitochondrial membrane occurs within the same time range. Fannjiang et al. (2004) reported that the mitochondrial fission proteins Dnm1, Mdv1, and Fis1 regulate cell death in yeast. In this study, we also demonstrated that mitochondrial punctation occurs during Bax-induced cell death. This morphological change may be the one common feature of cell death that is widely maintained. On the other hand, during the apoptosis of human fibrosarcoma cells, mitochondrial aggregation around the nucleus was observed to occur earlier than release of cytochrome c (Haga et al. 2003). However, our results suggest that mitochondrial aggregation is one of the later events (after 3 days) that occur during Bax-induced plant cell death. An actin polymerization inhibitor causes mitochondrial aggregation in tobacco cells (Van Gestel et al. 2002), suggesting that Bax-induced mitochondrial aggregation in plant may be associated with actin filament dysfunction.
Interestingly, light is required for the HR in rice leaves infected with Xanthomonas (Guo et al. 1993) and tomato plants treated with the AAL toxin (Moussantos et al. 1993) or FB1 (Stone et al. 2000). In addition, when tobacco leaves infiltrated with Pseudomonas solanacearum were incubated in the dark, the HR was not induced and the tissue reaction, a spreading of necrotic lesions, resembled a compatible interaction (Lozano and Sequeria 1970). Although the reason for light-dependent augmentation of lesion formation is poorly understood, light can enhance the oxidative burst (Allen et al. 1999). Phytochrome signaling (Genoud et al. 1998) or photosynthesis (Gray et al. 1997) is also required for some lesion phenotypes. It is possible that light is important for the oxidative burst and photo-bleaching in the case of the HR. The results of our analysis of cell suspensions cultured in the dark be an indication that light is not essential for Bax-induced cell death in plants. Exposure to light is a prerequisite for the chlorosis of plants expressing Bax. Nevertheless, ion leakage, the disruption of the chloroplasts and of tonoplasts, and consequential DNA degradation (data not shown) are events entirely separate from light-induced chlorosis. Bax induction is likely to cause two mechanisms: first, the accumulation of ROS, which is generated in Bax-associated mitochondria, triggers cell death by a light-independent process; second, chlorophyll is broken down through a light-dependent photo-breaching mechanism.
In mammalian cells, Bax causes Ca2+ release from the endoplasmic reticulum to the cytosol, which results in a subsequent increase of mitochondrial Ca2+ (Scorrano et al. 2003). In addition, Ca2+ is also a prominent modulator of the mitochondrial permeability transition. We observed that cytoplasmic streaming in the mitochondria ceased as a result of Bax and that this occurred at almost same time as the transformation of the mitochondria into a punctiform. Cytoplasmic streaming, which requires actomyosin systems, has been found to be inhibited by cytosolic Ca2+ increase and by a depletion of ATP (Kikuyama and Tazawa 1982; Kuroda 1999). Similar to the process that occurs in mammalian cells, Bax may down-regulate cytoplasmic streaming by increasing cytosolic Ca2+ levels in the plant cells.
In conclusion, we observed that Bax caused the following physiological changes in the cell: the loss of mitochondrial membrane potential, the cessation of cytoplasmic streaming, and consequential organelle destruction, including the loss of membrane permeability. We propose here that an orchestrated organelle vulnerability is the prerequisite for Bax-induced cell death in plants.
Abbreviations
- BQ:
-
p-Benzoquinone
- CaMV:
-
Cauliflower mosaic virus
- DEX:
-
Dexamethazone
- GFP:
-
Green fluorescent protein
- HR:
-
Hypersensitive response
- PCD:
-
Programmed cell death
- RFP:
-
Red fluorescent protein
- ROS:
-
Reactive oxygen species
References
Abramovitch RB, Kim YJ, Chen S, Dickman MB, Martin GB (2003) Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J 22:60–69
Allen LJ, MacGregor KB, Koop RS, Bruce DH, Karner J, Brown AW (1999) The relationship between photosynthesis and a mastoparan-induced hypersensitive response in isolated mesophyll cells. Plant Physiol 119:1233–1241
Aoyama T, Chua NH (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11:605–612
Aravind L, Dixit VM, Koonin EV (1999) The domains of death: evolution of the apoptosis machinery. Trends Biochem Sci 24:47–53
Arimura S, Tsutsumi N (2002) A dynamin-like protein (ADL2b), rather than FtsZ, is involved in Arabidopsis mitochondrial division. Proc Natl Acad Sci USA 99:5727–5731
Baek D, Nam J, Koo YD, Kim DH, Lee J, Jeong JC, Kwak SS, Chung WS, Lim CO, Bahk JD, Hong JC, Lee SY, Kawai-Yamada M, Uchimiya H, Yun DJ (2004) Bax-induced cell death of Arabidopsis is meditated through reactive oxygen-dependent and -independent processes. Plant Mol Biol 56:15–27
Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160:1115–1127
Chamnongpol S, Willekens H, Langebartels C, Montagu M, Inzé D, Camp W (1996) Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J 10:491–503
Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377
Dickman MB, Park YK, Oltersdorf T, Li W, Clemente T, French R (2001) Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc Natl Acid Sci USA 98:6957–6962
Dimitrova I, Toby GG, Tili E, Strich R, Kampranis SC, Makris AM (2004) Expression of Bax in yeast affects not only the mitochondria but also vacuolar integrity and intracellular protein traffic. FEBS Lett 566:100–104
Elkind Y, Edwards R, Mavandad M, Hedrick SA, Ribak O, Dixon RA, Lamb CJ (1990) Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci USA 87:9057–9061
Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, Hill RB, Basanez G, Hardwick JM (2004) Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev 18:2785–2797
Feierabend J, Winkelhusener T (1982) Nature of photooxidative events in leaves treated with chlorosis-inducing herbicides. Plant Physiol 70:1277–1282
Finucanes DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR (1999) Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem 274:2225–2233
Fujimori E, Livingston R (1957) Interaction of chlorophyll in its triplet state with oxygen carotene, etc. Nature 180:1036–1038
Genouds T, Millar AJ, Nishizawa N, Kay SA, Schafer E, Nagatani A, Chua NH (1998) An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10:889–904
Gray J, Close PS, Briggs SP, Johal GS (1997) A novel suppressor of cell death in plants encoded by the Lls1 gene of maize. Cell 89:25–31
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13:1899–1911
Guo A, Reimers PJ, Leach JE (1993) Effect of light on incompatible interactions between Xanthomonas oryzae pv oryzae and rice. Physiol Mol Plant Pathol 42:413–425
Haga N, Fujita N, Tsuruo T (2003) Mitochondrial aggregation precedes cytochrome c release from mitochondria during apoptosis. Oncogene 22:5579–5585
Jabs T, Dietrich RA, Dangl JR (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273:1853–1856
Jürgensmeier JM, Krajewski S, Armstrong RC, Wilson GM, Oltersdorf T, Fritz LC, Reed JC, Ottilie S (1997) Bax- and Bak-induced cell death in the fission yeast Schizosaccharomyces pombe. Mol Biol Cell 8:325–339
Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acid Sci USA 95:4997–5002
Kampranis SC, Damianova R, Atallah M, Toby G, Kondi G, Tsichlis PN, Makris AM (2000) A novel plant glutathione S-transferase/peroxidase suppresses Bax lethality in yeast. J Biol Chem 275:29207–29216
Karbowski M, Arnoult D, Chen H, Chan DC, Smith CL, Youle RJ (2004) Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J Cell Biol 164:493–499
Kawai M, Uchimiya H (1995) Biochemical properties of rice adenylate kinase and subcellular location in plant cells. Plant Mol Biol 27:943–951
Kawai-Yamada M, Jin L, Yoshinaga K, Hirata A, Uchimiya H (2001) Mammalian Bax-induced plant cell death can be down-regulated by overexpression of Arabidopsis Bax Inhibitor-1. Proc Natl Acid Sci USA 98:12295–12300
Kawai-Yamada M, Ohori Y, Uchimiya H (2004) Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax, hydrogen peroxide and salicylic acid -induced cell death. Plant Cell 16:21–32
Kikuyama M, Tazawa M (1982) Ca2+ ion reversibly inhibits the cytoplasmic streaming of Nitella. Protoplasma 113:241–243
Kuroda K (1990) Cytoplasmic streaming in plant cells. In: Boutne GH (ed) International review of cytology. Academic Press, New York, pp 267–307
Lacomme C, Cruz SS (1999) Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acid Sci USA 96:7956–7961
Lam E, Kato N, Lawton M (2001) Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411:848–853
Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79:583–593
Levine A, Belenghi B, Damari-Weisler H, Granot D (2001) Vesicle-associated membrane protein of Arabidopsis suppresses Bax-induced apoptosis in yeast downstream of oxidative burst. J Biol Chem 276:46284–46289
Lew DJ, Dulic V, Reed SI (1991) Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66:1197–1206
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Lozano JC, Sequeria L (1970) Differentiation of races of Pseudomonas salanacearum by a leaf infiltration technique. Phytopathology 60:833–838
Matsumura H, Nirasawa S, Kiba A, Urasaki N, Saitoh H, Ito M, Kawai-Yamada M, Uchimiya H, Terauchi R (2003) Overexpression of Bax inhibitor suppresses the fungal elicitor-induced cell death in rice (Oryza sativa L) cells. Plant J 33:425–434
Mitsuhara I, Malik KA, Miura M, Ohashi Y (1999) Animal cell-death suppressors Bcl-x(L) and Ced-9 inhibit cell death in tobacco plants. Curr Biol 15:775–778
Moussatos V, Witsenboer H, Hille J, Gilchrist D (1993) Behaviour of the disease resistance gene Asc in protoplasts of Lycopersicon esculentum mill. Physiol Mol Plant Pathol 43:255–263
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nechushtan A, Smith CL, Lamensdorf I, Yoon S-H, Youle RJ (2001) Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol 153:1265–1276
Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic, S65T-type green fluorescent protein in living plants. Plant J 18:455–463
Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96:6553–6557
Pan L, Kawai M, Yu LH, Kim KM, Hirata A, Umeda M, Uchimiya H (2001) The Arabidopsis thaliana ethylene-responsive element binding protein (AtEBP) can function as a dominant suppressor of Bax-induced cell death of yeast. FEBS Lett 508:375–358
Peever TL, Higgins VJ (1989) Electrolyte leakage, lipoxygenases and lipid peroxidation induced in tomato leaf tissue by specific and non specific elicitors from Cladosporium fulvum. Plant Physiol 90:867–875
Pennell RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9:1157–1168
Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodemel S, Inzé D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32:329–342
Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139
Stone JM, Heard JE, Asai T, Ausubel FM (2000) Simulation of fungal-mediated cell death by fumonisin B1 and selection of fumonisin B1-resistant (fbr) Arabidopsis Mutants. Plant Cell 12:1811–1822
Van Gestel K, Köhler RH, Verbelen JP (2002) Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J Exp Bot 53:599–667
Vieira HL, Boya P, Cohen I, El Hamel C, Haouzi D, Druillenec S, Belzacq AS, Brenner C, Roques B, Kroemer G (2002) Cell permeable BH3-peptides overcome the cytoprotective effect of Bcl-2 and Bcl-X(L). Oncogene 21:1963–1977
Xu Q, Reed JC (1998) Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Cell 1:337–346
Zha H, Fisk HA, Yaffe MP, Mahajan N, Herman B, Reed JC (1996) Structure-function comparisons of the proapoptotic protein Bax in yeast and mammalian cells. Mol Cell Biol 16:6494–6508
Acknowledgements
We thank Drs. J.C. Reed, N.H. Chua, T. Aoyama, and Y. Kikuchi, and Dr. W. Uchida and Dr. H. Ogawa for their help and gift of materials. Critical reading of this manuscript by Ms. M. Uchimiya and Dr. P. Gallois is appreciated deeply. This research was supported by Research for the Future from the Japan Society for the Promotion of Science
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Toriyama
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yoshinaga, K., Arimura, Si., Hirata, A. et al. Mammalian Bax initiates plant cell death through organelle destruction. Plant Cell Rep 24, 408–417 (2005). https://doi.org/10.1007/s00299-005-0948-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0948-6