Abstract
Immature zygotic embryo cultures of neem yielded highly regenerative cultures, with the response varying with the embryo stage at culture. Early dicotyledonous stage embryos were the most responsive followed by torpedo stage embryos. The embryo cultures differentiated three types of regenerants: somatic embryos (SEs), shoot buds and neomorphs. SEs exhibited morphological abnormalities such as pluricotyledony, fusion of cotyledons and absence of cotyledons. Although these SEs showed secondary embryogenesis, the occurrence of normal dicotyledonous embryos was extremely rare. On MS basal medium 3% of SEs developed a long tap root but a plumular shoot did not appear. However, it was possible to regenerate plantlets from immature zygotic embryo cultures of neem via neomorph formation and adventitious shoot bud formation. The transplantation survival of these plants was more than 80%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neem (Azadirachta indica A. Juss.), a very important multipurpose tree species, is propagated mostly through seeds, which have a very short span of viability (Ezumah 1986; Maithani et al. 1989; Sacande et al. 2001). This has restricted progress in germplasm collection, ex-situ conservation, establishment of plantations, species trials and tree improvement (Gamene et al. 1996). Tissue culture would not only overcome these limitations but also offers fascinating methods for large-scale production of plants in shorter duration devoid of seasonal constraints.
There are several reports on plant regeneration in tissue cultures of neem, and the most frequently used explant has been excised cotyledon or hypocotyl (Muralidharan and Mascarenhas 1989; Nirmalakumari et al. 1993; Shrikhande et al. 1993; Sarker et al. 1997; Su et al. 1997; Abubacker and Alagumanian 1999; Salvi et al. 2001). The majority of these publications have described regeneration via somatic embryogenesis, although in many cases the structures described as embryos do not appear like embryos or behave as such. In most other reports, a callus phase was involved, which may endanger genetic fidelity of the progeny. However, a few papers (Sarker et al. 1997; Salvi et al. 2001) reported direct shoot proliferation from seedling explants, but these papers lack details regarding the rate of shoot proliferation, frequency of rooting and transplantation survival. For many recalcitrant species, such as cereals, cotton and pines, immature zygotic embryos are the most suitable explants with which to establish regenerating cultures because of their juvenile nature (Bhojwani and Razdan 1996), and have been found to be the best starting material for induction of somatic embryogenesis in many perennial crops (Akhtar et al. 2000). Embryo culture is a well-established plant breeding tool in raising rare hybrids, which are normally lost due to post-zygotic sexual incompatibility (Bhojwani and Razdan 1996), and has been widely used for studying factors that control normal embryogenesis during sexual reproduction (Custers and Bergervoet 1990; Monnier 1990; Liu et al. 1993). However, in some interspecific hybridisations, embryos abort at much younger stages (Custers and den Nijs 1986), and it is likely that immature embryos at different stages of development are needed. So far, however, there has been no detailed report on in vitro zygotic embryo cultures of neem. In the investigation reported here, we have employed immature zygotic embryos at different stages of development and obtained various morphogenic responses in vitro. These responses were greatly influenced by the stage of the zygotic embryos at culture, information hitherto unreported in neem.
Materials and methods
Plant material and initiation of aseptic cultures
Immature fruits, from a 50-year-old neem tree growing in the botanical garden of the University of Delhi, were collected and thoroughly washed in 1% Savlon (Johnson and Johnson, New Brunswick, N.J.) solution for 5 min, followed by rinsing with sterile distilled water (SDW). All further manipulations were carried out under aseptic conditions in a laminar air-flow cabinet (Cleanair Atlantis, India). After rinsing in 90% ethanol for 30 s and two changes in SDW, the fruits were surface-sterilised in 0.1% mercuric chloride (HgCl2) solution for 8 min. The disinfected fruits were washed with SDW three times and dissected under a binocular microscope (Nikon SMZ-1, Japan), to excise the embryos. To identify the most responsive stage of embryos for morphogenesis, excised embryos at different stages of development—globular, heart shape, torpedo shape and early dicotyledonous stage (early dicotyledonous embryos were about 2.5 times smaller than fully developed dicotyledonous embryos) (Fig. 1)—were cultured on MS (Murashige and Skoog 1962) medium containing 3% sucrose and gelled with 0.8% agar (Qualigens, India). All plant growth regulators were added prior to autoclaving the medium at 1.06 kg cm−2 and 121°C for 15 min. The pH of the medium was adjusted to 5.8 prior to autoclaving. Four explants were cultured in 55×15 mm pre-sterilised, disposable Petri plates (Laxbro, India) containing 10 ml MS medium. Cultures were maintained in diffuse light (1,000–2,000 lx) with a 16 h photoperiod at 25°C±2°C and 50–60% relative humidity (RH). The Petri plates were sealed with Parafilm (American National Can, Greenwich, N.Y.).
MS medium supplemented with a range of growth hormones [6-benzylamino purine (BAP), 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3-acetic acid (IAA) and α-naphthaleneacetic acid (NAA)] was used for the induction of various regenerants, such as somatic embryos (SEs), neomorphs and shoot buds. At least 48 cultures were raised for each treatment and all experiments were repeated three times. Cultures were observed at weekly intervals and the regeneration in the cultures is expressed as a per cent response. Small shoots differentiated directly on zygotic embryos or on neomorphs, were detached from the parent tissue and transferred to a lower concentration of BAP (0.5 µM) for elongation.
Multiplication and rooting of shoots
Shoots regenerated directly from zygotic embryos and neomorphs were multiplied on MS medium supplemented with BAP (1 µM) + casein hydrolysate (CH) (250 mg l−1). For rooting, 4 cm long terminal portions of elongated shoots with 3–4 nodes were excised and cultured on MS with one-quarter strengths of the major salts and indole-3-butyric acid (IBA) (0.5 µM).
Cultures for shoot multiplication and rooting were grown in 150×25 mm glass culture tubes (Borosil, India), each containing 20 ml medium. All cultures were maintained at 25°C±2°C, 50–60% RH and diffuse light (1,000–2,000 lx) with a 16 h photoperiod. Twenty-four cultures were raised for each treatment and all experiments were repeated at least three times. Observations were recorded at weekly intervals and standard error of the mean was calculated (indicated by ±).
Transplantation
Rooted plants were washed to remove the agar, transferred to soilrite (Chowgule Industries, India) in hycotrays (Sigma, St. Louis, Mo.), placed in a glasshouse under high humidity and covered with clingfilm. After 10 days the clingfilm was removed; 3 weeks later the plants were transferred to soil in polythene bags and sprayed with a mixture of 0.1% urea (BASS, India) and Bavistin (BASS) (1:1). After another 4 weeks, the plants were transferred to pots and maintained in a polyhouse. After 3 months of transplantation, the plants were moved to a shaded area under natural conditions.
Histological studies
For histological studies, wax sections were cut. The materials were fixed in FAA (5:5:90 v/v/v formalin:acetic acid:70% ethanol) for 48 h and stored in 70% alcohol. The material was passed through a tertiary-butyl-alcohol series for dehydration, infiltrated with paraffin wax (melting point 60°C, Merck, Darmstadt, Germany) and finally embedded in pure paraffin wax. The paraffin blocks were mounted on wooden stubs, and 8–10 µm thick sections were cut using a Spencer Rotary Microtome (Buffalo, N.Y.) fitted with a steel knife. The sections were mounted on microslides, dewaxed and double stained with safranin (1%) and astra-blue (1%).
Results
Morphogenic responses varied considerably with the stage of embryo at culture. Globular embryos generally turned brown without showing any morphogenic response. Older embryos germinated, formed calli or differentiated three types of organised structures: shoots, SEs and neomorphs (abnormal structures with varied morphology) (Table 1). The same explant often differentiated more than one kind of regenerant. The frequency of differentiation of these structures varied with the stage of the zygotic embryo and composition of the culture medium.
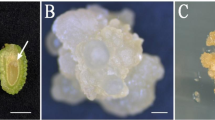
Maximum somatic embryogenesis and shoot bud differentiation occurred directly from the explant on MS +5 μM BAP medium, and the most responsive embryo stage was early dicotyledonous, followed by torpedo shape (Table 1). The former showed differentiation of shoots and SEs at higher frequency (57%) (Fig. 2A). The addition of 2,4-D (1 or 5 μM) alone, to MS medium induced only neomorph differentiation directly from the explant. Maximum neomorph formation (66%) occurred in cultures of torpedo shape embryos on MS +5 μM 2,4-D (Fig. 2B). In the presence of 2,4-D alone neither shoots nor SEs formed. On MS + BAP (5 μM), only torpedo shape embryos showed high frequency neomorph formation as well as somatic embryogenesis (40%) (Fig. 2C). In the combined presence of BAP (5 µM) +2,4-D (1 µM) in MS medium, early dicotyledonous embryos showed a fairly high degree of somatic embryogenesis and neomorph formation (50%) but these structures were differentiated from the callused explants (Fig. 2D). However, regeneration involving a callus phase runs the risk of introducing variability due to genetic instability of the callus cells. Therefore, only directly differentiated regenerants were utilised for micropropagation.
A Four-week-old culture of an early dicotyledonous embryo on Murashige-Skoog (MS) medium + 6-benzylamino purine (BAP) (5 µM), showing the differentiation of somatic embryos (SEs) (arrows) on one side and shoots (arrowheads) on the other side of the explant (×7.5). B Four-week-old culture of a torpedo shape embryo on MS + 2,4-dichlorophenoxyacetic acid (2,4-D) (5 µM), showing direct differentiation of neomorphs. Some of these structures also show cotyledon-like flaps. The portion of the explant in contact with the medium has proliferated into a brownish green callus (×8). C As in B, on MS + BAP (5 µM), showing differentiation of neomorphs (arrowheads) and SEs (arrows) at different sites on the same explant (×8). D Four-week-old culture of an early dicotyledonous stage embryo on MS + BAP (5 μM) + 2,4-D (1 μM), showing differentiation of SEs (SE) and neomorphs (NEO) from the callused explant. SEs show fused cotyledons (×8.6)
Three types of regenerants obtained in the cultures of zygotic embryos are described below:
Somatic embryos
SEs were loosely attached to the parent tissue. Irrespective of the treatment and the stage of zygotic embryo, SEs exhibited considerable morphological abnormalities, such as pluricotyledony (Fig. 3A, i), fusion of cotyledons (Fig. 3A, ii) and absence of cotyledons (Fig. 3A, iii). The occurrence of normal dicotyledonous embryos was extremely rare.
A SEs excised from cultures showing: i pluricotyledony, ii fusion of cotyledons, and iii absence of cotyledons (×12). B SEs cultured on MS basal medium for 4 weeks; very long tap root has developed in both the embryos but the plumular end has not formed a shoot (×2). C Eight-week-old SE culture on MS + BAP (1 µM) + indole-3-acetic acid (IAA) (0.5 µM), showing callusing of the explant and differentiation of secondary embryos from the callus (×1.5). D, E Cultures of SEs (5- and 8-week-old) on MS + gibberellic acid (GA3) (5.0 µM) + IAA (2.5 µM) showing secondary embryogenesis directly without any callus formation (×2.3)
SEs excised from the parent tissue were subjected to various treatments for germination as mentioned by Thorpe and Stasolla (2001). On MS basal medium, 3% of the embryos developed a long tap root in 4 weeks (Fig. 3B) but plumular shoots did not appear. Although SEs never germinated to form complete plantlets on any of the treatments tested, secondary embryogenesis occurred on MS basal medium supplemented with BAP + IAA, or GA3 + IAA.
On MS + BAP (1 μM) + IAA (0.5 μM), 100% SEs exhibited secondary embryogenesis with a mean number of 14 embryos per parent embryo. In this medium, secondary embryogenesis was preceded by callusing of the primary SEs (Fig. 3C). Primary SEs formed a brown, friable callus within 2 weeks and, after 5 weeks, secondary embryos differentiated from the callus. Additional embryos continued to appear up to 8 weeks. However, on GA3 (5.0 µM) + IAA (2.5 µM), secondary embryos differentiated directly from the primary SEs (Fig. 3D, E). Like primary SEs, secondary SEs exhibited morphological abnormalities, and failed to germinate on any of the treatments tested.
Neomorphs
The neomorphs were green, with smooth shiny surface and solid interior. Whereas some were spherical with no visible appendages, others showed notches like heart-shaped embryos (Fig. 2B–D) or developed foliar protuberances arising from their interior (Fig. 4A–D). In sections, the neomorphs appeared to be of epidermal origin and often showed provascular strands, suggesting that they could be suppressed embryos (Fig. 5A, B). This conclusion is further supported by the images shown in Fig. 4. Figure 4A shows a neomorph resembling a curled cotyledon; upon transfer to fresh medium, a shoot developed and its basal part callused. Stages in full shoot development from such neomorphs are shown in Fig. 4B–D. However, a clear cut radicle was never seen in these neomorphs.
A Four-week-old cultures of torpedo shape embryos on MS + BAP (5 μM), showing a neomorph resembling a curled cotyledon (×1.9). B Same, 3 weeks after transfer to fresh medium; neomorph has elongated and a shoot is emerging from inside. Note the presence of brown callus at the base of the explant (×2). C Same, after another 2 weeks, showing slightly advanced stage of shoot development and massive brown callus at the base (×2). D Same, 4 weeks after transfer on lower concentration of BAP (0.5 μM), showing elongation of the shoot and expansion of the neomorph (arrow). The shoot has elongated considerably, and few small shoots have developed at the base of the main shoot (×2)
A Section of a torpedo shape embryo cultured on MS + 2,4-D (5 μM), showing epidermal origin of a neomorph, with well-differentiated epidermis and compactly arranged internal cells (×470). B Neomorphs of various shapes. These structures are loosely attached to the explant and show provascular strands (×115). C Neomorphs from Fig. 2 B–D, showing differentiation of small shoots directly from the explant after 2 weeks of culture on MS + BAP (5 µM) (×2). D, E Same as C, after 8 weeks of culture; note further elongation of the shoots and basal callusing of the explant (×1.6). F Individual shoots were transferred to MS + 0.5 µM BAP. After 8 weeks, the shoot elongated (×1.6). G A shoot from F, rooted on one-quarter strength MS + 0.5 µM indole-3-butyric acid (IBA) (×1.6). H Hardened micropropagated plants, 8 months after transfer to soil (×0.1)
It was possible to regenerate full plants from the neomorphs via organogenesis. The excised neomorphs cultured on MS basal medium did not exhibit any morphogenesis. However, with the addition of BAP, numerous adventitious shoots differentiated directly from the neomorphs (Fig. 5C–E). In older cultures some of the shoots had elongated and considerable callusing has occurred at the base of neomorphs only (Fig. 5D, E). BAP was more effective for shoot differentiation at 5 μM than at 0.5 μM. At the higher level of cytokinin, 52% of cultures exhibited shoot differentiation compared to 29% at the lower level (Table 2).
Shoots
Whereas in almost all treatments, organogenesis occurred directly from zygotic embryos as well as from neomorphs, it was preceded by callusing of the explants on MS + BAP (5 µM) + 2,4-D (1 µM). However, to maintain genetic fidelity of the progeny, only directly differentiated healthy shoots were utilised. These shoots did not grow beyond 2 cm while attached to explants on regeneration medium. Therefore, individual shoots were excised from the parent tissue and transferred to MS + BAP (0.5 μM) for elongation. On this medium, 85% of cultures had developed shoots about 6 cm long within 8 weeks (Fig. 5F).
The shoots were multiplied, rooted and hardened by following the procedure described by Chaturvedi et al. (2003a, 2003b). Elongated healthy shoots were multiplied through axillary shoot proliferation on MS + BAP (1 µM) + CH (250 mg l−1) at a rate of 7- to 8-fold every 8 weeks. At each subculture, any axillary shoot that had grown fairly long was cut into single node segments and transferred to fresh medium for further multiplication. The number of propagules obtained at the end of a multiplication cycle was taken to be the rate of shoot multiplication. After 8 weeks on multiplication medium, more than 87% of the cultures developed shoots that were 8 cm long on average, each with seven to eight nodes.
Terminal 4-cm-long portions of shoots from 8-week-old cultures on MS + BAP (1 µM) + CH (250 mg l−1) were used for rooting. The remaining shoots were cut into single node segments and utilised for further multiplication. For rooting, one-quarter strength MS (major salts reduced to one-quarter strength) medium with 0.5 µM IBA was tested. On this medium, 81% of shoots had formed roots (average of six roots after 4 weeks; Fig. 5G). Transplantation survival of the micropropagated plants was more than 80% (Fig. 5H). The uniformity of the plants was confirmed by cytological analysis. Mitotic preparations were prepared from root-tips of 30 plants (selected randomly) regenerated from zygotic embryo cultures following the procedure described by Chaturvedi et al. (2003a, 2003b). All plants analysed showed a diploid number of chromosomes 2n=2×=24.
Discussion
In the present study we have established an efficient protocol for high frequency regeneration of neem using immature zygotic embryos as explants. As the developmental stage of embryos at the time of culture is an important determinant of their morphogenic response (Custers and Bergervoet 1990), in the present study zygotic embryos at different stages of development (globular, heart shape, torpedo shape and early dicotyledonous stage) were used to raise regenerable cultures.
The majority of publications on neem have described regeneration via somatic embryogenesis, although in many cases the structures described as embryos did not appear, and/or behave, like embryos. In the present study, high frequency differentiation into shoots, SEs and neomorphs was best observed in older embryo cultures. The most responsive embryo stage was early dicotyledonous followed by torpedo stage.
Muralidharan and Mascarenhas (1989) were probably the first to describe embryo-like structures in cultures of cotyledon segments of neem. These nodular structures, when separated from the parent explant, developed a tap root in addition to leafy appendages. The nodular structures never showed bipolar germination.
Shrikhande et al. (1993) reported in vitro plant regeneration via somatic embryogenesis in cultures of immature cotyledons. However, none of the structures resemble a bipolar embryo with closed radicular and plumular poles. Moreover, on germination medium, the so-called “embryos” developed only shoots. Most surprisingly, the authors have described mature embryos with suspensor. Mature embryos generally lack a suspensor, and at no stage can the suspensor be seen in macrophotographs as depicted in their paper. Su et al. (1997) were unable to confirm the results of Shrikhande et al. (1993) and observed only shoot bud formation.
Islam et al. (1993) also described the globular structures developed in cotyledon cultures as embryos and observed monopolar germination. However, we have described these embryo-like structures, which are similar to the “embryos” showing only monopolar shoot development in other reports (Muralidharan and Mascarenhas 1989; Islam et al. 1993; Shrikhande et al. 1993; Su et al. 1997; Sharma et al. 1999), as neomorphs. Although they are organised structures, in the present study such neomorphs cannot be compared with any of the normal organised structures; they could represent suppressed embryos. It was possible to regenerate full plants from most neomorphs via adventitious shoot bud differentiation on BAP-containing medium.
In the present study, SEs exhibited considerable morphological abnormalities, such as pluricotyledony, fusion of cotyledons and absence of cotyledons. The occurrence of normal embryos was extremely rare. Some other tree species where such abnormalities have been observed are Carya illinoinensis (Rodriguez and Wetzstein 1994), Feijoa sellowiana (Cruz et al. 1990), and Prunus avium (Garin et al. 1997). However, these SEs exhibited secondary embryogenesis. In this context, Merkle et al. (1990) remarked that secondary embryogenesis generally occurs when primary SEs fail to mature normally.
In summary, zygotic embryo cultures of neem yielded highly regenerative cultures, with the response depending on the stage of embryo at culture. These cultures could be an effective alternative method for micropropagation in neem. We were able to regenerate plantlets from these cultures of neem via neomorph formation and adventitious shoot bud formation. The transplantation survival of these plants was more than 80%. Uniformity of these plants was confirmed by cytological analysis, showing that all plants were diploid with the chromosome number 2n=2×=24.
Abbreviations
- BAP :
-
6-Benzylamino purine
- CH :
-
Casein hydrolysate
- 2,4-D :
-
2,4-Dichlorophenoxyacetic acid
- GA 3 :
-
Gibberellic acid
- IAA :
-
Indole-3-acetic acid
- IBA :
-
Indole-3-butyric acid
- NAA :
-
α-Naphthaleneacetic acid
- SE :
-
Somatic embryo
References
Abubacker MN, Alagumanian S (1999) In vitro organogenesis of various explants of Azadirachta indica A. Juss. (Neem). Plant Tissue Cult 9:177–180
Akhtar N, Kumari N, Pandey S, Ara H, Singh M, Jaiswal U, Jaiswal VS, Jain SM (2000) Somatic embryogenesis in tropical fruit trees. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants, vol 6. Kluwer, Dordrecht, pp 93–140
Bhojwani SS, Razdan MK (1996) Plant tissue culture: theory and practice. Elsevier, Amsterdam
Chaturvedi R, Razdan MK, Bhojwani SS (2003a) Production of haploids of neem (Azadirachta indica A. Juss.) by anther culture. Plant Cell Rep 21:531–537
Chaturvedi R, Razdan MK, Bhojwani SS (2003b) An efficient protocol for the production of triploid plants from endosperm callus of neem, Azadirachta indica A.Juss. J Plant Physiol 160:557–564
Cruz GS, Canhato JM, Abreu MAV (1990) Somatic embryogenesis and plant regeneration from zygotic embryos of Feijoa sellowiana Berg. Plant Sci 66:263–270
Custers JBM, Bergervoet JHW (1990) In vitro culture of embryos of Cucumis spp.: heart-stage embryos have a higher ability of direct plant formation than advanced stage embryos. Sex Plant Reprod 3:152–159
Custers JBM, den Nijs APM (1986) Effects of aminoethoxyvinylglycine (AVG), environment, and genotype in overcoming hybridization barriers between Cucumis species. Euphytica 35:639–647
Ezumah BS (1986) Germination and storage of neem (Azadirachta indica A. Juss.) seed. Seed Sci Technol 14:593–600
Gamene CS, Kraak HL, Van Piljen JG, de Vos CHR (1996) Storage behavior of neem (Azadirachta indica A. Juss.) seeds from Burkina Faso. Seed Sci Technol 24:441–448
Garin E, Grenier E, Grenier-De-March G (1997) Somatic embryogenesis in wild cherry (Prunus avium). Plant Cell Tissue Organ Cult 48:83–92
Islam R, Joarder N, Joarder OI, Hossain M, Rahman SM, Naderuzzaman (1993) Somatic embryogenesis and organogenesis in neem. In: Proceedings of World Neem Conference, Bangalore, pp 955–960
Liu W, Xu Z, Chua N (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5:621–630
Maithani GP, Bahuguna V, Rawat MMS, Sood OP (1989) Fruit maturity and interrelated effects of temperature and container on longevity of neem (Azadirachta indica) seed. Indian For 115:89–97
Merkle SA, Parrott WA, Williams EG (1990) Applications of somatic embryogenesis and embryo cloning. In: Bhojwani SS (ed) Plant tissue culture: applications and limitations. Elsevier, Amsterdam, pp 67–101
Monnier M (1990) Zygotic embryo culture. In: Bhojwani SS (ed) Plant tissue culture: applications and limitations. Elsevier, Amsterdam, pp 366–393
Muralidharan EM, Mascarenhas AF (1989) In vitro morphogenesis in Azadirachta indica A. Juss. and Eucalyptus citriodora Hook. f. Tissue culture and biotechnology of medicinal and aromatic plants, CIMAP, Lucknow, pp 49–55
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Nirmalakumari A, Ramaswamy NM, Sree Rangaswamy SR (1993) Tissue culture studies in neem (Azadirachta indica A. Juss.). In: Proceedings of the World Neem Conference, Bangalore, pp 981–992
Rodriguez APM, Wetzstein HY (1994) The effect of auxin type and concentration on pecan (Carya illinoinensis) somatic embryo morphology and subsequent conversion into plants. Plant Cell Rep 13:607–611
Sacande M, Golovina EA, Van Aelst AC, Hoekstra FA (2001) Viability loss of neem (Azadirachta indica) seeds associated with membrane phase behaviour. J Exp Bot 52:919–931
Salvi ND, Singh H, Tivarekar S, Eapen S (2001) Plant regeneration from different explants of neem. Plant Cell Tissue Organ Cult 65:159–162
Sarker RH, Islam MR, Hoque MI (1997) In vitro propagation of Neem (Azadirachta indica A. Juss.) plants from seedling explants. Plant Tissue Cult 7:125–133
Sharma AK, Sharma M, Chaturvedi HC (1999) Conservation of phytodiversity of Azadirachta indica A. Juss. through in vitro strategies. In: National symposium on role of plant tissue culture in biodiversity conservation and economic development. G.B. Pant Institute of Himalayan Environment and Development, Almora, pp 1–13
Shrikhande M, Thengane SR, Mascarenhas AF (1993) Somatic embryogenesis and plant regeneration in Azadirachta indica A. Juss. In vitro Cell Dev Biol 29P:38–42
Su WW, Hwang WI, Kim SY, Sagawa Y (1997) Induction of somatic embryogenesis in Azadirachta indica. Plant Cell Tissue Organ Cult 50:91–95
Thorpe TA, Stasolla C (2001) Somatic embryogenesis. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of angiosperms. Kluwer, Dordrecht, pp 219–236
Acknowledgements
Rakhi Chaturvedi thanks Professor S.P. Bhatnagar for his critical analysis of the manuscript and the Council for Scientific and Industrial Research (CSIR), India, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Harwood
Rights and permissions
About this article
Cite this article
Chaturvedi, R., Razdan, M.K. & Bhojwani, S.S. In vitro morphogenesis in zygotic embryo cultures of neem (Azadirachta indica A. Juss.). Plant Cell Rep 22, 801–809 (2004). https://doi.org/10.1007/s00299-004-0768-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-004-0768-0