Abstract
A simple and efficient garlic in vitro shoot regeneration protocol has been developed. This system uses axenic root tips cultivated from the beginning in the presence of light and does not require any change or refreshing of the original medium during the entire process. The application of light from the beginning of the culture process did not affect the callus formation rate but did significantly improve the explant regeneration ability. In a 2-month period it was possible to obtain up to 250 shoots per gram of callus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated garlic (Allium sativum L.) is a vegetatively propagated plant due to its sexual sterility. This characteristic limits the breeding of the species to clonal selection. Tissue culture and genetic transformation techniques have been shown to be efficient tools for the breeding of many crops and could be especially useful in the future improvement of this “genetically-closed” plant.
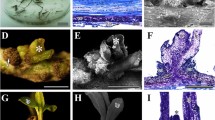
Typical garlic in vitro regeneration systems described in the literature include the classical two separate steps: a callus formation phase and a regeneration phase. These culture stages occur normally in different media. Moreover, callus induction usually occurs in the dark, while the subsequent regeneration phase takes place in the light. While garlic shoot regeneration from callus has been reported using several explant sources—young leaves (Nagasawa and Finer 1988), shoot tips (Kehr and Schaeffer 1976), basal plates (Koch et al. 1995) and flower organs (Suh and Park 1993)—root tips have shown to be the most advantageous explant to be used in a regeneration system (Haque et al. 1997; Myers and Simon 1998; Barandiaran et al. 1999a, 1999b; Robledo-Paz et al. 2000).
On the other hand, organogenic ability in garlic is dependent on the duration of the callus phase, especially if the medium contains 2,4-D (Novak 1980). In older cultures, regeneration level decreases and there is an increasing occurrence of abnormal plants. Consequently, regeneration through a system including a short or null callus stage is desirable to allow rapid regeneration, thereby avoiding genetic instability. In this way, Haque et al. (1997) regenerated plantlets by direct shoot bud organogenesis from root tips using the garlic cultivar White Roppen.
In the investigation reported here, we have established a garlic in vitro regeneration protocol using a single-step system: callus production and shoot regeneration occurred in the same dish in which the explant was originally inoculated. Using this system, we studied the effects of the presence or absence of light under three different in vitro culture parameters—callus formation rate, organogenic callus rate and number of shoots per organogenic callus.
Materials and methods
Plant material
Axenic root-tips were obtained from in vitro garlic plants cultured following the protocol of Barandiaran et al. (1999c). The accessions used were chosen to include three general groups of garlic—E213072 (Red type), E432095 (Chinese type) and E111300 (White type)—and were kindly provided by the Garlic Germplasm Bank of Cordoba (C.I.F.A.) (curator: F. Mansilla). The accession numbers correspond to those used in the European Allium Database (EADB).
Root culture
Root-tips (1 cm long) were cut off from micropropagated plants with a scalpel under sterile conditions. Explants were cultivated in petri dishes containing 25 ml of three different media: A3 (Barandiaran et al. 1999b), B4 (Barandiaran et al. 1999b) and H (Haque et al. 1997). A3 medium contains B5 salts and vitamins (Gamborg et al. 1968), 0.03 mg/l 2,4 D, 2 mg/l NAA and 3 mg/l BAP; B4 medium is identical to A3, except for the exclusion of 2,4 D. H medium contains MS salts (Murashige et al. 1962) with 0.2 mg/l NAA and 2.2 mg/l BAP. All three media were supplemented with 3% sucrose and 0.9% Bactoagar and adjusted to pH 5.7 prior to autoclaving. The media were neither changed nor refreshed during the culture period. Cultures were incubated in a growth chamber at 25±2°C for 2 months. Two different light conditions were tested: (1) the first set of cultures was subjected to a 16/8-h (light/dark) photoperiod with light supplied by Grolux fluorescent lights at an intensity of (55 μmol/m2 per second); (2) the second set of cultures was incubated in the dark. A control was included in the dark experiment in the callus induction stage and in the light experiment in the regeneration stage.
Experimental design and statistical analysis
The experimental design was completely randomised, with 21 treatments (genotype/medium/light condition) and three replications per treatment. One replication consisted of ten petri dishes with three explants each.
After 8 weeks of culture the following data were recorded: callus formation rate, organogenic callus rate and number of shoots per organogenic callus. Analyses of variance (ANOVA) were analysed using the statistix software.
Results and discussion
Garlic shoot regeneration using root tips as explants has been described in a number of reports (Haque et al. 1997; Myers and Simon 1998; Barandiaran et al. 1999a, 1999b; Robledo-Paz et al. 2000). As a general rule, callus formation takes place in the dark, and shoot regeneration takes place in the presence of light. In addition, different media are traditionally used at each stage of the process. An exception was the investigation of Haque et al. (1997) in which the cultures were incubated under constant cool-white fluorescent light for shoot initiation and two different media were used for shoot initiation and shoot proliferation. However, their objective was to develop a method for direct shoot regeneration, studying the effect of different media but not the effect of light on callus culture and shoot regeneration.
In our investigation, root tips were cultivated in three media, two of which (A3 and B4) only differ in the presence or absence of 2,4-D. The H medium also lacks 2,4-D and has a lower concentration of the auxin NAA (0.2 mg/l). The light conditions tested were: (1) light [16/8-h (light/dark) photoperiod] and (2) dark during the whole process. The controls were: (1) dark at the callus induction phase (light experiment) and (2) light at the regeneration phase (dark experiment).
Callus formation
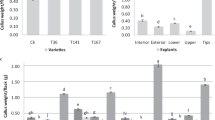
ANOVA on the results showed highly significant differences for callus formation rate among media (df=2; F=43.25; P≤0.001). However, callus formation rate did not show significant differences among light conditions and genotypes.
Media A3 and B4 gave the best results in callus formation, ranging from 41% to 67%, while the range obtained on H medium was between 2% and 37% (Fig. 1). H medium only produced routinely for the Red-type accession. The White and Chinese types showed the highest callus formation rates when root tips were cultured on A3 medium and in the presence of light (67% and 52%, respectively), while the Red type showed the best result when cultured on B4 medium and in the dark (56.7%) (Fig. 1).
Shoot regeneration
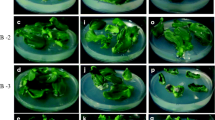
The ANOVA test showed highly significant differences for the percentage of calli that underwent shoot regeneration among genotypes (df=2; F=19.7; P≤0.001), media (df=2; F=30.15; P≤0.001) and light conditions (df=1; F=18.1; P≤0.001). No genotype showed shoot regeneration on H medium (Fig. 2). These data were not expected based on the results of Haque et al. (1997). The percentage of calli that underwent shoot regeneration was significantly higher when light was applied from the beginning of culture than in the control or the dark tests. The White accession showed a particular behaviour when cultured in B4 medium, resulting in higher organogenic levels in the dark than in the light and control tests (Fig. 2). As a general rule, root tips cultured on A3 medium and under light conditions showed the highest organogenic callus percentage (Figs. 3, 4). White- and Chinese-type accessions showed the best results on A3 medium when cultured under light conditions (50% and 28.3%, respectively), while the Red type presented them on B4 medium, also in the presence of light (15.2%) (Fig. 2).
The ANOVA test showed highly significant differences for the trait number of shoots per organogenic callus among genotypes (df=2; F=20.73; P≤0.001) and media (df=2; F=10.41; P≤0.001) and significant differences among light conditions (df=1; F=5.16; P≤0.05). All genotypes regenerated higher number of shoots when light was applied from the beginning than in the control or the dark tests (Fig. 5). The best medium for shoot regeneration in the White- and Chinese-type accessions was A3 (10% and 2.6%, respectively), while for Red type it was the B4 medium (1.3%).
Another trait studied was the number of shoots produced per gram of callus. Root tips from the White-type accession produced 250 shoots per gram callus in 2 months. This value is higher than that obtained by Robledo-Paz et al. (2000) (170 shoots per gram callus) and Barandiaran et al. (1999b) (16.2 shoots per gram callus) in a longer period of culture.
Our studies confirm the significant effect of genotype on callus formation and shoot regeneration. Interactions of accession × media and media × light conditions were significant in some cases. The Red-type accession showed the best results with respect to callus and shoot formation when cultured in a medium without 2,4-D (B4), whereas the White and Chinese types showed the best results when cultured in a medium with 2,4-D (A3). For callus formation, the Red-type accession showed the best results when cultured in the dark, which is in contrast to the other two accessions although the results are not statistically significant. The more efficient genotype in terms of shoots produced per explant in the one-step shoot regeneration system was the White type followed by the Chinese and Red types.
The culture of the explants from the beginning in the presence of light did not affect the number and quality of calli obtained. Furthermore, the use of light from the beginning significantly increased the organogenic potential of the explants. It was also found that culture in light is not essential for shoot regeneration. These conclusions contrast with the more traditional methods in which callus formation is carried out in the dark (Myers and Simon 1998; Barandiaran et al. 1999a, 1999b; Robledo-Paz et al. 2000). On the other hand, the use of a single medium from the beginning of the explant establishment, where no refreshment is needed, is an important novelty in the garlic regeneration protocols and contributes to a simplification of the system and a saving in costs.
Abbreviations
- BAP::
-
6-Benzylaminopurine
- 2,4-D::
-
2,4-Dichlorophenoxyacetic acid
- NAA::
-
α-Naphthaleneacetic acid
References
Barandiaran X, Martín N, Rodríguez-Conde MF, Di Pietro A, Martín J (1999a) Genetic variability in callus formation and regeneration of garlic (Allium sativum L.). Plant Cell Rep 18:434–437
Barandiaran X, Martín N, Rodríguez-Conde M, Di Pietro A, Martín J (1999b) An efficient method for callus culture and shoot regeneration of garlic (Allium sativum L.). HortScience 34:348–349
Barandiaran X, Martín N, Alba C, Rodríguez-Conde MF, Di Pietro A, Martín J (1999c) An efficient method for the in vitro management of multiple garlic accessions. In Vitro Cell Dev Plant 35:466–469
Gamborg OL, Miller RA, Ohyama K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:148–151
Haque MS, Wada T, Hattori K (1997) High frequency shoot regeneration and plantlet formation from root tip of garlic. Plant Cell Tissue Organ 50:83–89
Kehr AE, Schaeffer GW (1976) Tissue culture and differentiation of garlic. HortScience 11:422–423
Koch M, Tanami Z, Salomón R (1995) Improved regeneration of shoots from garlic callus. HortScience 30:378
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Myers JM, Simon PW (1998) Continuous callus production and regeneration of garlic (Allium sativum L.) using root segments from shoot tip-derived plantlets. Plant Cell Rep 17:726–730
Nagasawa A, Finer JJ (1988) Induction of morphogenic callus cultures from leaf tissue of garlic. HortScience 23:1068–1070
Novak FJ (1980) Phenotype and cytological status of plants regenerated from callus culture of Allium sativum L. Z Pflanzenzuecht 84:250–260
Robledo-Paz A, Villalobos-Arámbula VM, Jofre-Garfias AE (2000) Efficient plant regeneration of garlic (Allium sativum L.) by root-tip culture. In Vitro Cell Dev Plant 36:416–419
Suh S, Park H (1993) Rapid multiplication through immature bulbil cultures of garlic. J Kor Soc Hortic Sci 34:173–178
Acknowledgements
This work was partially funded by a national project (INIA, no. RF00-014) and a EU project (no. QLK1-CT-1999-00498).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Peña
Rights and permissions
About this article
Cite this article
Martín-Urdíroz, N., Garrido-Gala, J., Martín, J. et al. Effect of light on the organogenic ability of garlic roots using a one-step in vitro system. Plant Cell Rep 22, 721–724 (2004). https://doi.org/10.1007/s00299-003-0744-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-003-0744-0