Abstract
The psoriatic arthritis impact of disease (PSAID) questionnaire has been developed to measure disease impact on patients with psoriatic arthritis. It was aimed to evaluate its validity and reliability in association with sociodemographic and clinical factors and compare it with disease activity and patient-reported outcome measures in a Turkish psoriatic arthritis population. A prospective observational study was conducted to validate the Turkish version of the PSAID. All consecutive patients with psoriatic arthritis were evaluated between January 2019 and October 2019. Demographic and clinical features were recorded. The PSAID and patient-reported outcome measures were applied to all patients. Interclass and intra-class correlation analyses were performed. Convergent validity and correlation coefficients were used for validity analyses. There were 80 patients with a mean age of 50.2 ± 9.9 years. Cronbach’s α value of the PSAID and intra-class correlation were 0.799 and 0.984, respectively. The total median PSAID score was 4.7. Pain, fatigue, ability to work, functional capacity and feeling of discomfort were the five highest-scoring subscales. There was satisfactory internal consistency for each subscale of the PSAID. As disease severity increased from low to high, the PSAID scores significantly increased. There were acceptable correlations between the PSAID and other patient-reported outcome measures. The PSAID is shown to be a reliable and valid questionnaire in Turkish patients with psoriatic arthritis. Good correlation with disease activity and patient-reported outcome measures represent an opportunity to use the PSAID in clinical practice to tailor individualized treatment choices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patient-reported outcome measures (PROMs) have been regarded as important instruments to provide insight into patients’ perceptions of health about chronic diseases and individual priorities [1]. Besides, they have been regarded as reliable indicators for baseline status of the disease and any change during treatment, and important endpoints in both clinical trials and long-term observational studies [2, 3]. Although many of PROMs are used in chronically diseased patients, selection of PROMs and evaluation of their outcomes are challenging. Due to the presence of limits for the time and resources of both patients and physicians, their widespread use in studies and clinical trials seems to be infeasible [1, 4].
Psoriatic arthritis (PsA) as a chronic inflammatory disease is seen in an average of 30% of patients with psoriasis [5]. Due to the involvement of skin and joint involvements, impaired physical and psychosocial health-related quality of life is an essential issue in these patients [3].
In relation to chronic diseases, there have been several different PROMs with their proposed advantages and disadvantages. Among these, the psoriatic arthritis impact of disease (PSAID) questionnaire that has been endorsed by the European League against Rheumatism is unique for being a disease-specific PROM for PsA [2]. With the use of the PSAID, it is possible to measure the impact of PsA on diverse aspects of disease on patients’ lives including fatigue, functional capacity, sleep, skin problems and coping [3,4,5]. In previous studies, it has also been shown that the PSAID is significantly correlated with disease activity measures [1, 6].
However, the results of such PROMs have been reported to be influenced by several socio-demographic variables and associated comorbid situations [1]. After its development, the PSAID has been validated in different countries or populations [3, 5, 7,8,9]. A good validity of the PSAID with the Disease Activity in Psoriatic Arthritis (DAPSA) has been also shown [4]. Thus, further studies on the validation and reliability of the PSAID on different subsets of PsA based on the severity grades of DAPSA have not been performed in Turkey yet.
In this study, we aimed to construct validity and reliability of the Turkish version of the PSAID, to evaluate sociodemographic and clinical factors on PSAID and compare it with other disease activity measures and established PROMs.
Materials and methods
Study
This study was a prospective observational study to perform internal validation of the Turkish version of the PSAID questionnaire in PsA patients. We took consent of Laure Gossec for our study to use the Turkish version of the PSAID questionnaire published at the website of EULAR (https://pitie-salpetriere.aphp.fr/psaid/questionnaire.php). Ethics committee approval was obtained for this study (Numune Training and Research Hospital Ethics Committee, E-2018-2371). The study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. Written informed consent was taken from the participants or from the legally authorized representatives for the participants who were illiterate or vulnerable.
Participants
All consecutive patients with PsA who admitted to or followed at the Physical Medicine and Rehabilitation Clinic, Numune Education and Research Hospital and Department of Rheumatology, Faculty of Medicine, Ankara University, Ankara, Turkey were evaluated in the study between January 2019 and October 2019. Diagnosis of PsA according to the Classification for Psoriatic Arthritis (CASPAR) criteria, ages between 18 and 75 years and absence of chronic inflammatory disease besides PsA were the inclusion criteria [4, 10]. Cognitive disorders that prevent to reply to the questions in the scales and the questionnaires, skin disorders other than psoriasis, pregnancy, and refusal to participate in the study were the exclusion criteria.
Variables
Demographic data including age (year), sex, weight in kilograms and height in meters, education level as illiterate, primary, secondary or college and university, employment status, presence of comorbid disease, smoking status as ever or never and disease duration (year) were recorded. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). The drugs used for PsA were searched and grouped as conventional synthetic disease-modifying antirheumatic drugs (DMARD), biological DMARD and non-steroidal agents.
At the inclusion of the study, data were collected at a single time point. The pain was assessed using a 0–100 mm visual analog scale (VAS) indicating 0 = “no pain” and 100 = “the most severe pain”. Three self-reported questions about the patient global experience of PsA were scored on a Likert scale as Patient Global Assessment (PGA).
Physical examination
Typical psoriatic nail dystrophy (pitting, onycholysis, subungual hyperkeratosis, splinter hemorrhage), the involvement of PsA as axial or peripheral disease and presence of erosive arthritis were prospectively recorded. For peripheral joint assessment, tender (68 joints) and swollen (66 joints) joint counts were calculated separately according to the measures used in clinical trials for rheumatoid arthritis and psoriasis [10, 11]. The Leeds Dactylitis Index (LDI) using a dactylometer was measured quantitatively as showing higher scores with worse dactylitis. To assess enthesitis in PsA patients, the Leeds Enthesitis Index (LEI) was used with a score range of 0–6 [10]. Higher LEI scores were regarded as greater enthesitis burden.
Evaluation of disease activity
Erythrocyte sedimentation rate (ESR) (mm/h) and C-reactive protein (CRP) (mg/dL) were measured as acute phase reactants. ESR was examined with Western blot technique and 0–20 mm/h accepted as normal range. CRP was examined with immuno-turbidimetric method and > 5 mg/L accepted as positive value.
Disease-related characteristics such as duration of the disease, since the diagnosis and disease activity score as the Psoriasis Areas and Severity Index (PASI) were reported. Using clinical and laboratory data of the patients, the clinical DAPSA (cDAPSA) was calculated for each patient [10]. For assessment of cDAPSA, higher scores were regarded as a reflection of more severe disease activity (range 0–164). The patients were categorized into four different disease activities based on the scores of cDAPSA as remission (REM) ≤ 4, low disease activity (LDA) > 4 and ≤ 13, moderate disease activity (MDA) > 13 and ≤ 27, and high disease activity (HDA) > 27 [12]. The PASI scale was applied for quantitative assessment of all psoriasis lesional burden with a score range of 0–72 [10]. Besides, the physicians reported their global assessments of disease activity in an identical manner to PGA (range 0–100).
Patient-reported outcome measures
The PSAID questionnaires with both physical and psychological domains were applied to all patients. The PSAID final value was calculated as described with a range from 0 to 10, where 10 represents the worst health score [2, 6]. All patients were requested to answer or fill the following scales or questionnaires: Turkish version of Health Assessment Questionnaire (HAQ) was used to assess the physical function of the patients with a score range of “0” as “no difficulty” to “3” as “unable to do” [13]. Psoriatic Arthritis Quality of Life (PSAQoL) as a PsA-specific health related quality of life instrument was employed. Its range was from 0 to 20 as with higher scores indicating the worse health-related quality of life situations. For measurement of disability due to different dermatological conditions, Turkish validated form of Dermatology Life Quality Index (DLQI) was used with a score range from 0 to 30 [14]. Higher scores were used to represent a greater impact of the dermatological conditions on quality of life. Using the Turkish validated form of Hospital Anxiety and Depression Scale (HADS-A), the anxiety subscale score was calculated from 0 (best) to 21 (worst). A total HADS-A score was 21, and higher scores represented higher levels of anxiety [15]. Turkish version of Nottingham Health Profile (NHP) as a measure of a patient’s perceived emotional, social, and physical health was applied to the patients [16, 17]. Its score was evaluated based on the range from 0 (best health state) to 100 (worst health state). Four subscales as behavioral disengagement (COPE1), self-blame (COPE2), substance use (COPE3) and denial (COPE 4) from the brief COPE were used to explore the predictors of distress in PsA patients [18, 19]. As a self-administered instrument, the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) Scale was applied. Its total range was from zero to 52 indicating the worst possible fatigue as zero and no fatigue as 52 [20, 21]. The Work Productivity and Activity Impairment (WPAI) questionnaires were applied to assess the impact of PsA on work productivity or other daily activities [22, 23]. Overall work productivity and impairment of regular activities due to poor health and symptom scores [presenteeism, absenteeism, work productivity loss (WPL) and WPAI 4)] were calculated [22, 24].
Statistical analysis
The sample size was calculated as at least 79 using G Power 3.1.9 with assumptions of α = 0.05 and power = 80%. Interclass correlation analysis was performed by Mangold Reliability Calculator (v. 1.5). Jamovi (v. 1.0.7.0) was used for all other statistical analyses. Normality was tested by the Shapiro–Wilk test and Q–Q plot. The quantitative data were stated as the average ± standard deviation or median [interquartile ranges (IQR) as 25th–75th percentile], while the qualitative data were stated as frequency (%). Internal consistency, which reflects the homogeneity of the scale, was determined by calculating Cronbach’s α coefficient. An intra-class correlation coefficient (ICC) was used for test–retest reliability. For that purpose, a total of 23 patients have a second assessment of the PSAID questionnaire 3 days later. A Cronbach’s α and ICC score of greater than 0.7 was accepted as satisfactory. Item reliability statistics were performed to evaluate the reliability of the subscales of the PSAID by excluding one item consecutively in each analysis. Validity shows how much the scale can measure the intended item to be measured. Construct validity was tested using the “convergent validity” method. For this purpose, the PSAID scores were compared with other scales. Pearson or Spearman’s correlation coefficients (r) were used for validity analyses. A value of p < 0.05 was accepted as statistically significant.
Results
There were 80 patients with a mean age of 50.2 ± 9.9 years. Female to male ratio was 3:1. The median duration of the disease was 8 years (IQR 3.0–13.3). Demographic and clinical features are given in Table 1. Although there was no nail pathology in 16 patients (20%), pitting, onycholysis and subungual hyperkeratosis were the nail problems detected in 53 (66.3%), 17 (21.3%) and 13 patients (16.3%), respectively. Axial disease and erosive arthritis were detected in 44 (55%) and 32 patients (40%), respectively.
The median score for PGA was 50 (47.5–70.0). The median value of VAS was obtained as 50 (IQR 40.0–80.0). Other characteristics are detailed in Table 1.
The median cDAPSA and PASI scores were 18.8 (IQR 15.1–27.0) and 1.55 (IQR 0.0–5.62), respectively. Based on the scoring of cDAPSA, there were 44 (55%) MDA, 19 (23.8%) HDA and 17 (21.2%) LDA patients. There was no patient with REM.
Cronbach's α value of the PSAID scale and ICC were 0.799 and 0.984, respectively. The calculation of the final PSAID score revealed the median value of 4.7 (IQR 3.14–6.45). The scores for the PSAID subscales are given in Table 2. Pain, fatigue, ability to work, functional capacity and feeling of discomfort were the five highest-scoring subscales in relation to greater disease impact. Item reliability statistics showed that there was satisfactory internal consistency for each subscale of the PSAID. PSAID-depression was shown to be the least reliable subscale for this analysis. Inter-class correlation analysis also revealed that there were significant correlations between the subscales (Table 3).
Although the PSAID scores in female and unemployed patients were significantly higher than that of male and employed patients (p = 0.004 and p = 0.048, respectively), there was no significant association between the final PSAID score and other demographic and clinical features (Table 4). The duration of the disease, the duration of morning stiffness, VAS, tender joint score, LEI, cDAPSA, and level of ESR were significantly correlated with the final.
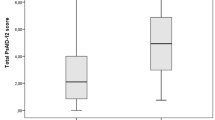
PSAID score (p < 0.05 for all) (Table 5). As the duration of the disease decreased, significantly higher PSAID scores were detected (p = 0.03). The higher tender joint score, longer morning stiffness time and higher LEI were significantly correlated with higher PSAID scores (p < 0.05 for all). In patients with higher cDAPSA scores, significantly higher PSAID scores were detected (p < 0.001). Subgrouping of cDAPSA as LDA, MDA, and HDA was significantly correlated with the PSAID final scores (p < 0.001). As the disease severity increased from low (LDA) to medium (MDA) and high (HDA), the mean PSAID scores significantly increased from 3.03 ± 2.26 to 4.86 ± 1.63 and 6.06 ± 1.74, respectively. Subgroup analysis (pairwise comparisons) revealed that there were significant differences between all subgroups (p < 0.05 for all).
The scores of all scales are given in Table 6. There were acceptable correlations between the PSAID and all scales except DQLI and COPE (p < 0.001) (Table 6). The strongest correlations were detected between the PSAID and pain subscale of Nottingham Health Profile (r = 0.732), Physician’s global assessment (r = 0.686), WPAI-WPL (r = 0.647) and WPAI4 (r = 0.646).
Discussion
The association of the PSAID with clinical outcomes and other PROMs has been shown in this study in which a comprehensive validity and reliability analysis was performed in a Turkish population. Besides the presence of the internal consistency of the PSAID, there were also significant correlations between other commonly used scales and the PSAID. We have also demonstrated that PSAID is significantly correlated with the disease activity in this study population. All these findings supported that the PSAID is a feasible, valid and reliable questionnaire for the assessment of PsA in this Turkish population.
The use of the PSAID for assessment of PsA symptoms has been evaluated by a recent systematic literature review [25]. In this paper, the authors reported that the PSAID questionnaire can be recommended to evaluate symptoms of PsA due to its sufficient content validity. This recommendation can be regarded as evidence supporting the use of the PSAID in PsA patients.
In a previous study by Kalyoncu et al. [8] that was also performed in Turkey, use of the PSAID has been studied to assess the values of PSAID in patients with clinically diagnosed PsA patients with anti-tumor necrosis factor treatment [1, 3, 4, 6, 7]. In Gossec’s study [2] in which the PSAID was elaborated and validated at first, but all patients who did not formally fulfill the CASPAR criteria were regarded as the weakness of the study. They also suggested further validation studies in subgroups. Therefore, we included only the patients who were classified in accordance with the CASPAR criteria.
Correlation of the PSAID with DAPSA and cDAPSA as measures of disease activity has been shown by Di Carlo et al. [4] as contrary to Johnson’s study [5]. The PSAID has been shown to be a useful instrument for identifying disease activity remission following DMARD treatment [26]. Although cut-off values of the PSAID have not been determined regarding the different disease activity levels, they tried to find cut-off values for each group as REM, LDA, MDA, and HDA. But, we did not use such approach in our study. But, we showed that there were significant associations between the PSAID scores of LDA, MDA and HDA. Due to the absence of patients with REM, we could not analyze the association of REM with other disease activity groups. Besides cDAPSA, we also showed that there were significant correlations of disease activity measures including VAS, tender joint score, and LEI to the PSAID total score. Although it is known that the PSAID is not a disease activity index, we may recommend the PSAID as a “patient-reported disease activity index” in accordance with Di Carlo et al. [4] due to this close relation between PSAID and cDAPSA and other disease activity measures.
In previous studies, higher scores of PROMs in female patients have been reported. In Holland’s study [3], there were higher PSAID scores in female patients in accordance with our study. They also reported that the correlation between PROMs and clinical outcomes was stronger in male patients. Although it has been thought that the differences in clinical features may cause some changes in the PSAID scores, the difference have remained significant after adjustment for disease activity [3]. Similar results have been also reported by other researchers [4, 9]. Thus, female sex should be taken into consideration during the evaluation of the results of the PSAID and other PROMS.
Correlation of the PSAID and other known PROMS have been studied by many researchers. In these studies, variable levels of correlation of the PSAID to other PROMs were demonstrated. In Holland’s study (3), the strongest correlation was shown to be between the EuroQoL-5D index and PSAID. In Tälli’s and Johnson’s studies [5, 7], it has been shown that there was a good correlation between the PSAID and PGA as in the original study of Gossec et al. [2]. High sensitivity and specificity of the PsAID with the HAQ was shown in PsA patients only with a group of MDA [5]. Leung et al. [27] showed the validity of functional capacity using one subscale of the PSAID in PsA patients. However, in most of the studies, there was a poor correlation between DLQI and the PSAID as in the present study [3, 5, 7]. It has been speculated that the presence of similar domains may cause higher correlations between different PROMs. There were also weak correlations between the PsAID, the PASI, and DLQI [5]. Therefore, disease impact of some major clinical outcomes including work, sleep, fatigue, and skin related issues may not be captured by clinical scores necessitating detailed studies. Besides, heterogeneity of the study groups regarding demographic characteristics, severity of disease activity and associated comorbidities cause difficulties to reach to widely acknowledged result. Therefore, controlling these confounding factors should be kept in mind for future studies.
The scores of the PSAID in different studies have been questioned [5]. In some studies, the mean score was shown to be an average of 3 to 4 which was lower than that of other studies [1, 3, 5, 6, 8, 28]. In Kalyoncu’s study [8], the baseline PSAID score was calculated as 6.6. In the present study, the mean scores were 5.13 and 3.63 in female and male patients, respectively. Besides the authors did not discuss the impact of lower or higher scores in different study groups, correlation of the PSAID scores with other PROMs or follow-up scores in the same groups for treatment or disease process should be the main focus for the studies.
Non-random selection of the patients in two different centers might cause selection bias. However, the prospective design of the study that was performed in two specialized centers and analyses of the PSAID with a large number of PROMs were the main strengths.
In conclusion, we demonstrated that the PSAID is a reliable and valid questionnaire that can be used in patients with PsA. It can be used to tailor individualized treatment choices due to the presence of significant correlations with the disease activity of PsA.
References
Walsh JA, Wan MT, Willinger C, Husni ME, Scher JU, Reddy SM et al (2019) Measuring outcomes in psoriatic arthritis: comparing Routine Assessment of Patient Index Data (RAPID3) and Psoriatic Arthritis Impact of Disease (PSAID). J Rheumatol. https://doi.org/10.3899/jrheum.190219
Gossec L, de Wit M, Kiltz U, Braun J, Kalyoncu U, Scrivo R et al (2013) A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 73:1012–1019. https://doi.org/10.1136/annrheumdis-2014-205207
Holland R, Tillett W, Korendowych E, Cavill C, Waldron N, Brooke M et al (2018) Validation of the Psoriatic Arthritis Impact of Disease (PsAID) Questionnaire and its potential as a single-item outcome measure in clinical practice. Ann Rheum Dis 77:343–347. https://doi.org/10.1136/annrheumdis-2017-211996
Di Carlo M, Becciolini A, Lato V, Crotti C, Favalli EG, Salaffi F (2017) The 12-item Psoriatic Arthritis Impact of Disease Questionnaire: construct validity, reliability, and interpretability in a clinical setting. J Rheumatol 44:279–285. https://doi.org/10.3899/jrheum.160924
Johnson K, Ye JY, Chandran V, Gladman DD (2019) A novel role for the psoriatic arthritis impact of disease (PsAID) questionnaire. Semin Arthritis Rheum 49:241–245. https://doi.org/10.1016/j.semarthrit.2019.04.001
Salaffi F, Di Carlo M, Carotti M, Farah S, Gutierrez M (2016) The psoriatic arthritis impact of disease 12-item questionnaire: equivalence, reliability, validity, and feasibility of the touch-screen administration versus the paper-and-pencil version. Ther Clin Risk Manag 12:631–642. https://doi.org/10.2147/TCRM.S101619
Tälli S, Etcheto A, Fautrel B, Balanescu A, Braun J, Cañete JD et al (2016) Patient global assessment in psoriatic arthritis—what does it mean? An analysis of 223 patients from the Psoriatic arthritis impact of disease (PsAID) study. Jt Bone Spine 83:335–340. https://doi.org/10.1016/j.jbspin.2015.06.018
Kalyoncu U, Kiraz S, Bilgen SA, Karadag O, Akdogan A, Kilic L et al (2019) Change in PsAID-12 scores in patients continuing or discontinuing anti-TNF treatments in psoriatic arthritis: results from the HUR-BIO biologic registry. Clin Rheumatol 38:1187–1192. https://doi.org/10.1007/s10067-019-04426-3
Orbai AM, Perin J, Gorlier C, Coates LC, Kiltz U, Leung YY et al (2019) Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with gender in 458 patients from 14 countries. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.24090
Mease PJ (2011) Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res (Hoboken) 63(Suppl 11):S64–85. https://doi.org/10.1002/acr.20577
Mease PJ (2011) Psoriatic arthritis: update on pathophysiology, assessment and management. Ann Rheum Dis 70(Suppl 1):i77–84. https://doi.org/10.1136/ard.2010.140582
Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS (2010) Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 69:1441–1447
Küçükdeveci AA, Sahin H, Ataman S, Griffiths B, Tennant A (2004) Issues in cross-cultural validity: example from the adaptation, reliability, and validity testing of a Turkish version of the Stanford Health Assessment Questionnaire. Arthritis Rheum 51:14–19
Oztürkcan S, Ermertcan AT, Eser E, Sahin MT (2006) Cross validation of the Turkish version of dermatology life quality index. Int J Dermatol 45:1300–1307
Aydemir O (1997) Validity and reliability of Turkish version of Hospital Anxiety and Depression Scale. Turk Psikiyatri Derg 8:280–287
Kücükdeveci AA, McKenna SP, Kutlay S, Gürsel Y, Whalley D, Arasil T (2000) The development and psychometric assessment of the Turkish version of the Nottingham Health Profile. Int J Rehabil Res 23:31–38
Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH (2011) Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res (Hoboken) 63(Suppl 11):S383–412. https://doi.org/10.1002/acr.20541
Tuncay T, Musabak I, Gok DE, Kutlu M (2008) The relationship between anxiety, coping strategies and characteristics of patients with diabetes. Health Qual Life Outcomes 13(6):79. https://doi.org/10.1186/1477-7525-6-79
Howells L, Chisholm A, Cotterill S, Chinoy H, Warren RB, Bundy C (2018) Impact of disease severity, illness beliefs, and coping strategies on outcomes in psoriatic arthritis. Arthritis Care Res (Hoboken) 70:295–302. https://doi.org/10.1002/acr.23330
Montan I, Löwe B, Cella D, Mehnert A, Hinz A (2018) General population norms for the functional assessment of chronic illness therapy (FACIT)-Fatigue Scale. Value Health 21:1313–1321. https://doi.org/10.1016/j.jval.2018.03.013
Akin S, Kas Guner C (2019) Investigation of the relationship among fatigue, self-efficacy and quality of life during chemotherapy in patients with breast, lung or gastrointestinal cancer. Eur J Cancer Care (Engl) 28:e12898. https://doi.org/10.1111/ecc.12898
Reilly MC, Zbrozek AS, Dukes EM (1993) The validity and reproducibility of a work productivity and activity impairment instrument. Pharmaeconomics 4:353–365
Mumcu G, Lehimci F, Fidan Ö, Gük H, Alpar U, Ünal AU et al (2017) The assessment of work productivity and activity impairment in Behçet's disease. Turk J Med Sci 47:535–541. https://doi.org/10.3906/sag-1603-161
de Hooge M, Ramonda R, Lorenzin M, Frallonardo P, Punzi L, Ortolan A et al (2016) Work productivity is associated with disease activity and functional ability in Italian patients with early axial spondyloarthritis: an observational study from the SPACE cohort. Arthritis Res Ther 18:265
Perez-Chada LM, Balak D, Cohen JM, Ogdie A, Merola JF, Gottlieb AB (2020) Measurement properties of instruments assessing psoriatic symptoms for psoriasis clinical trials: a systematic literature review. Expert Rev Clin Immunol 8:1–17. https://doi.org/10.1080/1744666X.2020.1724090
Pardo E, Charca L, Alonso S, Alperi M, Arboleya L, Queiro R (2020) Disease Activity in Psoriatic Arthritis Index and Psoriatic Arthritis Impact of Disease Questionnaire: correlation and sensitivity to change in a real clinical setting. Clin Exp Rheumatol (PMID: 31969229)
Leung YY, Orbai AM, de Wit M, Balanescu A, Dernis E, Soubrier M et al (2020) Comparing the patient reported physical function outcome measures in a real-life international cohort of patients with psoriatic arthritis. Arthritis Care Res (Hoboken). https://doi.org/10.1002/acr.24139
Queiro R, Cañete JD, Montilla C, Abad M, Montoro M, Gómez S (2017) Minimal disease activity and impact of disease in psoriatic arthritis: a Spanish cross-sectional multicenter study. Arthritis Res Ther 19:72. https://doi.org/10.1186/s13075-017-1277-1
Funding
No funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Güler, T., Bora Karslı, P., Ataman, Ş. et al. Psoriatic arthritis impact of disease questionnaire: validity, reliability and its clinical potential. Rheumatol Int 40, 959–967 (2020). https://doi.org/10.1007/s00296-020-04575-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04575-8