Abstract
The objective of this study was to identify factors predictive of malignancy in patients with polymyositis (PM) and dermatomyositis (DM) in Japan. We conducted a retrospective study of PM and DM patients who were admitted to our hospital between January 1992 and September 2017. Among 134 patients, 29 (21.6%) were diagnosed with cancer in the 3 years prior to and 3 years after the initial diagnosis of PM or DM. According to multivariate analyses, male sex [odds ratio (OR) = 3.65, p = 0.03], old age (OR = 1.05, p = 0.02), and a past history of diabetes mellitus (OR = 10.4, p = 0.005) were associated with an increased risk of malignancy. The absence of interstitial lung disease (ILD) (OR = 0.25, p = 0.03) was also associated with an increased risk of malignancy. Diabetes mellitus was observed in 28.6% of PM and DM patients with malignancy, but in only 7.3% of those with malignancy. Survival was significantly lower in patients with malignancy than in those without malignancy (p < 0.001). Independent factors associated with malignancies in patients with PM or DM were male sex, old age, the absence of ILD, and, especially, a past history of diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polymyositis (PM) and dermatomyositis (DM) are rare autoimmune disorders known as inflammatory myopathies. The main clinical features include progressive weakness of the proximal muscles, which in DM is accompanied by the development of cutaneous lesions. Following the first report of malignancy related to PM and DM published in 1916 [1], several studies have examined the potential relationship between PM and DM and malignant diseases [2,3,4,5,6,7,8,9,10,11]. Of these studies, the vast majority have shown an increased incidence of malignancy associated with myositis, particularly in DM patients [2, 4, 5, 7, 8, 10, 11]. Large-scale studies have reported a three- to sixfold higher risk of malignancy in DM patients [11,12,13]. Furthermore, numerous studies have found that malignancies in PM or DM patients are associated with high mortality [14,15,16,17,18,19,20,21,22]. However, these studies typically produced inconclusive results. Moreover, only few cohorts have been previously reported in Japan. The objective of this study was to determine the relationship between malignancy and PM and DM by retrospectively reviewing the records of 134 patients treated over 25 years.
Materials and methods
Patient population
We conducted a retrospective study that examined all adult patients diagnosed with PM (n = 53) or DM (n = 81), including 14 clinically amyopathic DM patients) who were inpatients at the National Center for Global Health and Medicine Hospital, Tokyo, Japan, between January 1992 and September 2017.
Among all patients diagnosed with PM or DM, we excluded two patients with malignancies diagnosed at an unknown time, five patients with malignancies diagnosed more than 2 years before myositis diagnosis, and five with malignancies diagnosed more than 3 years after myositis diagnosis, resulting in a final cohort of 134 patients. To be considered malignancy-related, the neoplasm had to be diagnosed 3 years before or 3 years after the detection of PM or DM (Fig. 1). PM and DM were diagnosed based on the Bohan and Peter criteria.
The appearance of malignancy in relation to PM/DM diagnosis. To be considered malignancy-related, the malignancy had to have appeared between 2 years prior to or 3 years after the diagnosis of PM/DM. In 11 (38.0%) patients, the malignancy appeared before the diagnosis of PM/DM, and in 16 (55.0%), it appeared after the diagnosis of PM/DM. Two patients developed cancer at the time of diagnosis of myositis
Following this initial screening, clinical data and laboratory test results from the medical records were reviewed. Clinical data regarding gender, age at onset of symptoms, diagnosis of PM or DM, interstitial lung disease (ILD), fever, arthralgia, dysphagia, myalgia, distal muscle weakness, Raynaud’s phenomenon, heliotrope rash, Gottron’s sign, V-neck sign, cutaneous necrosis, pruritus, and any past history of hypertension or diabetes mellitus were collected. Laboratory tests at presentation were performed for the measurement of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC) count, hemoglobin (Hb), platelet (Plt) count, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin (Alb), creatinine (Cre), creatine kinase (CK), C3, and C4. Clinical features and current complications were collected in all clinical courses. Past histories of hypertension and diabetes mellitus were determined at the time of myositis diagnosis because of the possible adverse reactions caused by steroids that were administered to all patients. One patient in the group of myositis with malignancy and 16 patients in the group of myositis without malignancy had been diagnosed at another hospital, but all of them were initially administered with steroids at our hospital. Four patients (treatment for ILD in two patients, Sjogren’s syndrome in one patient, and chronic eosinophilic pneumonia in one patient) had taken steroids prior to the diagnosis of myositis, although none had diabetes mellitus. All study participants were followed up from the time of the baseline visit until loss to follow-up, death, or September 1, 2017. The mean follow-up duration for all participating patients was 6.75 years.
The myositis-specific autoantibodies were measured in 15 patients using protein-immunoprecipitation assay at the University of Kyoto and in other patients using the EIA method.
This study was approved by the Ethics Committee of the National Center for Global Health and Medicine Hospital.
Statistical analyses
Fisher’s exact test was used for categorical variables. For numerical data, the Mann–Whitney U test was used to compare the two groups. Two-sided p values < 0.05 were considered statistically significant. Univariate and multivariate analyses of malignancy predictors associated with PM or DM were performed using logistic regression analyses to identify independent risk factors. Variables with a p value < 0.05 in univariate analyses were selected for inclusion in the multivariate analysis model. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Patient survival was analyzed using a Kaplan–Meier curve and log-rank test.
Results
Demographic data of the study subjects
Of the enrolled study subjects (134 patients with PM/DM hospitalized in our department), 53 had PM and 81 had DM. Demographic and clinical data of these patients are presented in Table 1. The study population was comprised of 41 (30.6%) men, and the median age at onset of PM/DM was 57.8 [range 56.7–66.3] years. Twelve out of 53 (22.6%) patients with PM and 17 out of 81 (20.1%) DM patients had cancer.
Malignancy in association with PM/DM
Twenty-nine (21.6%) patients (11 women and 18 men) had cancer-associated PM (n = 12) or DM (n = 17). The appearance of malignancy in relation to the time of diagnosis of PM/DM is shown in Fig. 1. In particular, malignancy developed in 11 (38.0%) patients before the diagnosis of PM/DM, in 2 (6.9%) patients at the time of diagnosis of myositis, and in 16 (55.2%) patients after the diagnosis of myositis. The mean duration from the diagnosis of myositis to malignancy development in the 16 patients was 8.9 months.
The most common underlying malignancies in female patients were uterine cancer (n = 3) and breast cancer (n = 3); lung cancer (n = 5) was the most common malignancy in men (Table 2). Overall, esophageal cancer (n = 5) and lung cancer (n = 5) were the most common malignancies in our study population.
Factors associated with underlying malignancies
In univariate analyses (Table 3), male sex, older age at onset, dysphagia, and diabetes mellitus were significantly associated with underlying malignancies in patients with PM/DM (p < 0.05). Also, ILD, arthralgia, and Raynaud’s phenomenon were more rare in the cancer-associated vs myositis group without malignancy. Independent factors included male sex, older age at onset, absence of ILD, and diabetes mellitus (Table 4). In particular, the prevalence of diabetes mellitus was significantly higher in patients with malignancies (OR = 10.4; 95% CI 2.00–54.3; p = 0.005).
A patient with malignancy exhibited anti-TIF1-γ antibodies and no patients with malignancies exhibited autoantibodies against aminoacyl-transfer synthetases (ARS); however, 37 (35.2%) patients without malignancies had autoantibodies against ARS. Thirty-seven patients with anti-ARS antibodies included anti-Jo-1 (n = 19), anti-EJ (n = 4), anti-PL-7 (n = 3), anti-OJ (n = 3), anti-PL-12 (n = 2), and anti-KS (n = 1). Five other patients were proved to be anti-ARS antibody positive, but the type was not determined.
Survival analyses
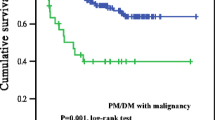
Of the 134 patients included in this study, survival outcomes were unknown for 35 patients. An additional 38 patients, including 18 with malignancies, died during the follow-up period. Of these 18 patients, five died as a result of the malignancy, two died from pneumonia not caused by ILD, one died from hemophagocytic syndrome, one died from acute exacerbation of ILD; one died from asphyxia; one died from septic shock; and one died from cerebral hemorrhage. Kaplan–Meier survival curves were used to assess survival probability for patients with and without malignancies (Fig. 2). The cumulative survival rates of the malignancy group were 68.2% (95% CI 44.6–83.4) at 1 year and 31.0% (95% CI 12.5–51.8) at 5 years; in contrast, those of the non-malignancy group were 89.6% (95% CI 80.2–94.6) at 1 year and 86.4% (95% CI 76.2–92.5) at 5 years. According to our results, the 1- and 5-year survival rates were significantly higher in the primary myositis group than in the malignancy-associated myositis group (p < 0.001).
Discussion
In this retrospective study, we sought to identify factors associated with concomitant malignancies in Japanese patients with PM/DM. Only few cohorts have been previously reported in Japan. We believe that presenting Japanese data is the strength of this study. Previous large-scale investigations into the prevalence of malignancies in PM and DM revealed incidence rates of 0–43% and 0–60% for PM and DM, respectively [23]. The prevalence of malignancies in our patient cohorts was 22.6% for PM and 20.1% for DM, well within the ranges reported by previous studies. Our study presents a slightly higher prevalence of malignancy than that reported in other cohort studies. Thus, the median age of the patients in our study is higher than that of those in the previous ones, resulting in a possibly high prevalence of malignancy.
Yang et al. [24] performed a meta-analysis and concluded that the risk of malignancy remained unchanged for several years following the diagnosis of myositis; however, it was highest during the first year after initial diagnosis. Similarly, our study also found most malignancies were detected during the first year after diagnosis, although these findings are not without exceptions, including one patient diagnosed with a malignancy 11 years after the diagnosis of myositis. The malignancy might have no relationship with myositis, but these findings suggest that routine screenings and examinations may be warranted even several years following the diagnosis of myositis.
Our study found that the most common underlying malignancy in male patients was lung cancer, whereas uterine and breast cancer were most common in women, consistent with previous findings [11, 12, 17]. According to the Cancer Registry and Statistics, Cancer Information Service, National Cancer Center, Japan, gastric cancer was the most common form of cancer among Japanese men in 2013, followed by lung and colon cancers. Breast cancer was the most common cancer among Japanese women in 2013, followed by colon and gastric cancers. The types and locations of malignancy in patients with myositis is, therefore, consistent with the expected probability of cancer in a Japanese population. Previous literature reviews have demonstrated that certain cancers, such as lung and prostate cancer in men and ovarian and breast cancer in women, as well as nasopharyngeal carcinoma in all patients, are associated with idiopathic inflammatory myopathies in patients of Southeast Asian descent [4]. The Japanese cohort described here is broadly consistent with these findings, although it is notable that only one male patient (5.0%) and one female patient (7.1%) had pharyngeal cancer.
Some factors associated with malignancy in the population of PM/DM patients in this study have been consistently implicated in previous studies, such as age, male sex, and absence of ILD [2,3,4,5, 7, 25]. The prevalence of malignant diseases increases with age among the general population; this may be the underlying reason for the higher prevalence of cancer cases among older PM/DM patients.
An important, novel finding from this study is the association between past history of diabetes mellitus and the risk of malignancy. A previous trial that analyzed whether a past history of diabetes mellitus was associated with risk of malignancy found that more patients with cancer had a past history of diabetes mellitus than those without cancer (24.2% vs. 12.6%, p = 0.103); however, the difference was not statistically significant [5]. A prospective study conducted in Japan showed that 7.3% of patients with malignancy had a past history of diabetes mellitus [26]. In comparison, we found that 28.6% of myositis patients with malignancy had a past history of diabetes mellitus. In addition, when we included patients who developed cancer more than 3 years before or more than 3 years after myositis, fewer patients (23.7%) had a past history of diabetes mellitus. These data support our hypothesis that diabetes mellitus affects not only cancer itself but also myositis-associated cancer. The associations between diabetes mellitus and cancer were attributable to the metabolic and hormonal aberrations associated with diabetes mellitus, and common biological mechanisms may be at least partially associated with insulin and insulin-like growth factors (IGFs) [26]. The most obvious difference in diabetes mellitus patients is the reduced insulin sensitivity with compensatory hyperinsulinemia and elevated levels of IGF-1 that may in turn stimulate cell proliferation in various organs. Insulin activates the IGF-1 receptor that modulates cell cycle progression. Excess insulin may also influence the development of cancer indirectly by downregulating the expression of IGF-binding protein 1, resulting in greater bioavailability of total circulating IGF-1. Moreover, the presence of diabetes mellitus may increase the availability of reactive oxygen species, resulting in increased DNA damage and a greater overall risk of cancer and other malignancies [26]. There may be additional mechanisms by which diabetes mellitus increases the malignancy risk in myositis. In terms of paraneoplastic syndrome, not only myositis but also diabetes mellitus may possibly be caused by a mechanism of autoimmunity. Further studies are needed to confirm these mechanisms.
Recent meta-analyses have shown that ILD and arthritis are associated with a relatively lower risk of malignancy in patients with myositis [6]. Several other studies, including the data presented here, have confirmed that Raynaud’s phenomenon [3], anti-ENA antibodies [2, 3, 6], and anti Jo-1 antibodies were associated with fewer malignancies in myositis patients. Anti-synthetase syndrome is defined by the presence of autoantibodies against aminoacyl-transfer synthetases (ARS), which are a common feature of diseases such as ILD, arthritis, and Raynaud’s phenomenon but appear to be protective against other malignancies. Our study found no patients with malignancies with autoantibodies against ARS. A previous study showed that patients with anti-TIF1-γ have reduced prevalence of ILD compared to anti-TIF1-γ-negative patients [27]. In old days we were not able to measure anti-TIF1-γ antibodies, so in our study, only 22 patients received the anti-TIF1-γ antibody test, and only one patient exhibited the positive of antibody with malignancy.
Over the course of our study, 18 patients with malignancies died, with five of the deaths directly attributed to the malignancy, making secondary malignancies the most common cause of death. Also, we suspect that patients with malignancy are at greater risk of pneumonia, septic shock, and cerebral hemorrhage. Therefore, having malignancy possibly may have contributed to these deaths, either directly or indirectly. Given the increased risk of cancer in these patients, it is, therefore, important to identify risk factors for cancer in patients with PM/DM.
Our study has certain limitations. First, this was a retrospective study. Second, we cannot exclude biases and confounding factors that may have influenced our model estimates. The third limitation is the relatively small sample size. In addition, our study was performed on patients treated in a single tertiary-care center; therefore, the generalizability of the results is limited.
Conclusions
Independent factors associated with malignancies in patients with PM or DM were male sex, older age, the absence of ILD and especially past history of diabetes mellitus. The prevalence of diabetes mellitus was considerably higher in PM and DM patients than in those with malignancies. Our study demonstrated a high prevalence of malignancies in PM/DM patients and was associated with decreased survival.
References
Stertz G (1916) Polymyositis. Berl Klin Wochenschr 53:489
Antiochos BB, Brown LA, Li Z, Tosteson TD, Wortmann RL, Rigby WF (2009) Malignancy is associated with dermatomyositis but not polymyositis in Northern New England, USA. J Rheumatol 36:2704–2710
András C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P et al (2008) Dermatomyositis and Polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol 35:438–444
So MW, Koo BS, Kim YG, Lee CK, Yoo B (2011) Idiopathic inflammatory myopathy associated with malignancy: a retrospective cohort of 151 Korean patients with dermatomyositis and polymyositis. J Rheumatol 38(11):2432–2435
Fang YF, Wu YJ, Kuo CF, Luo SF, Yu KH (2016) Malignancy in dermatomyositis and polymyositis: analysis of 192 patients. Clin Rheumatol 35(8):1977–1984
Wang J, Guo G, Chen G, Wu B, Lu L, Bao L (2013) Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol 169:838–847
Ponyi A, Constantin T, Garami M, András C, Tállai B, Váncsa A et al (2005) Cancer-associated myositis:clinical features and prognostic signs. Ann N Y Acad Sci 1051:64–71
Fardet L, Dupuy A, Gain M, Kettaneh A, Chérin P, Bachelez H et al (2009) Factors associated with underlying malignancy in a retrospective cohort of 121 patients with dermatomyositis. Medicine (Baltimore). 88(2):91–97
Lu X, Yang H, Shu X, Chen F, Zhang Y, Zhang S et al (2014) Factors predicting malignancy in patients with polymyositis and dermatomyositis: a systematic review and meta-analysis. PLoS One 9(4):e94128
Zantos D, Zhang Y, Felson D (1994) The overall and temporal association of cancer with polymyositis and dermatomyositis. J Rheumatol 21:1855–1859
Hill CL, Zhang Y, Sigurgiersson B, Pukkala E, Mellemkjaer L, Airio A et al (2001) Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet 357:96–100
Buchbinder R, Forbes A, Hall S, Dennett X, Giles G (2001) Incidence of malignant disease in biopsy-proven inflammatory myopathy. A population-based cohort study. Ann Intern Med 134:1087–1095
Stockton D, Doherty VR, Brewster DH (2001) Risk of cancer in patients with dermatomyositis or polymyositis, and follow-up implications: a Scottish population-based cohort study. Br J Cancer 85:41–45
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis. N Engl J Med 292:344–347
Torres C, Belmonte R, Carmona L, Gómez-Reino FJ, Galindo M, Ramos B et al (2006) Survival, mortality and causes of death in inflammatory myopathies. Autoimmunity 39:205–215
Kuo CF, See LC, Yu KH, Chou IJ, Chang HC, Chiou MJ et al (2011) Incidence, cancer risk and mortality of dermatomyositis and polymyositis in Taiwan: a nationwide population study. Br J Dermatol 165:1273–1279
Sigurgerisson B, Lindelof B, Edhag O, Allander E (1992) Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med 326:363–367
Liu WC, Ho M, Koh WP, Tan AW, Ng PP, Chua SH et al (2010) An 11-year review of dermatomyositis in Asian patients. Ann Acad Med Singap 39:843–847
Marie I, Hachulla E, Hatron PY, Hellot MF, Levesque H, Devulder B et al (2001) Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J Rheumatol 28:2230–2237
Wakata N, Kurihara T, Saito E, Kinoshita M (2002) Polymyositis and dermatomyositis associated with malignancy: a 30-year retrospective study. Int J Dermatol 41:729–734
Maoz CR, Langevitz P, Livneh A, Blumstein Z, Sadeh M, Bank I et al (1998) High incidence of malignancies in patients with dermatomyositis and polymyositis: an 11-year analysis. Semin Arthritis Rheum 27:319–324
Airio A, Kautiainen H, Hakala M (2006) Prognosis and mortality of polymyositis and dermatomyositis patients. Clin Rheumatol 25:234–239
Tiniakou Eleni, Mammen Andrew L (2017) Idiopathic Inflammatory myopathies and malignancy: a comprehensive review. Clin Rev Allerg Immunol 52:20–33
Yang Z, Lin F, Qin B, Liang Y, Zhong R (2015) Polymyositis/dermatomyositis and malignancy risk: a metaanalysis study. J Rheumatol 42(2):282–291
Sparsa A, Liozon E, Hermann F, Ly K, Lebrun V, Soria P et al (2002) Routine vs extensive malignancy search for adult dermatomyositis and polymyositis: a study of 40 patients. Arch Dermatol 138:885–890
Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S (2006) Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med 166:1871–1877
Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L (2015) Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ anti-bodies in adults with dermatomyositis. J Am Acad Dermatol 72:449–455
Acknowledgements
This work was supported in part by Grants-in-Aid for Research from the National Center for Global Health and Medicine (29-2001). The authors would like to thank Dr. Tsuneyo Mimori at the Kyoto University for the measurement of myositis-specific autoantibodies.
Author information
Authors and Affiliations
Contributions
KM designed the study, wrote the initial draft of the manuscript and analyzed date. HY contributed to analysis and interpretation of data, and assisted in the preparation of the manuscript. SY, YT and HK, members of the Division of Rheumatic Diseases, National Center for Global Health and Medicine have contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors, including Kyoko Motomura, Hiroyuki Yamashita, Saeko Yamada, Yuko Takahashi and Hiroshi Kaneko have any conflicts of interest associated with this article.
Ethical approval
This study complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee at the National Center for Global Health and Medicine (2018-5-11/NCGM-G-002515-00).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Motomura, K., Yamashita, H., Yamada, S. et al. Clinical characteristics and prognosis of polymyositis and dermatomyositis associated with malignancy: a 25-year retrospective study. Rheumatol Int 39, 1733–1739 (2019). https://doi.org/10.1007/s00296-019-04428-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04428-z