Abstract
Certain non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with an increased risk of myocardial infarction (MI), a risk linked to cyclo-oxygenase-2 inhibition. There are limited studies assessing the risk of MI associated with meloxicam, an increasingly popular drug with COX-2 inhibiting properties. A nested matched case–control study using The Health Improvement Network, a UK population-based database was conducted. NSAID users between 35 and 89 years of age with at least 1 year enrollment in the cohort were included. Incident MI cases were matched on age, sex, practice and event date with up to 4 controls. NSAID exposure was categorized as remote (between 60 days and 1 year), recent (between 1 and 60 days) or current relative to the event date. Current users were further classified as naproxen (negative control), diclofenac (positive control), meloxicam or other NSAID users. Multivariable conditional logistic regression was conducted to determine the risk of MI for each NSAID use categories compared with that of remote users. 9291 MI cases were matched with 30,676 controls. The cases had a higher prevalence of traditional cardiac risk factors, chronic kidney disease and inflammatory arthritis and cardioprotective drug utilization. The adjusted odds ratio of MI for current user compared to remote users were: meloxicam 1.38 (1.17–1.63), naproxen 1.12 (0.96–1.30) and diclofenac 1.37 (1.25–1.50). In this large population-based study, meloxicam increased the risk of MI by 38%. This study warrants cautious use of this increasingly popular drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most commonly used analgesic agents. However, since 2000, data from large clinical trials, such as VIGOR and APPROVe, began to demonstrate that rofecoxib, a selective cyclo-oxygenase-2 (COX-2) inhibitor was associated with an increased risk of myocardial infarction (MI) [1, 2]. Consequently, it was removed from the market in 2004. Since then, several NSAIDs, both selective COX-2 inhibitors and non-selective inhibitors, have been associated with an increased risk of MI [3,4,5,6,7].
The exact mechanism by which NSAIDs exert such an effect has not been completely elucidated; however, the hypothesis of an imbalance between prostacyclin and thromboxane-A2 leading to a pro-thrombotic state has gained the most prominence [8]. This has two important implications: first, given the short half-life of prostaglandins, the risk of MI is expected to occur with current NSAID use; and second, the imbalance between the prostacyclin and thromboxane-A2 is at least partly dependent on the degree of COX-2 relative to COX-1 inhibition [9, 10]. This translates into higher cardiovascular risk being associated with selective COX-2 inhibitors and non-selective drugs which preferentially inhibit COX-2 over COX-1. Meta-analyses of randomized controlled trials and observational studies support this: COX-2 inhibitors were associated with the highest cardiovascular risk and diclofenac, a non-selective inhibitor but preferentially more COX-2 inhibiting, was associated with a higher risk than other non-selective NSAIDs. Naproxen has been consistently shown to be neutral with regards to the risk of MI [11,12,13].
Clinical trials have not assessed the cardiovascular safety of other frequently used NSAIDs worldwide, such as meloxicam. Meloxicam is a derivative of the oxicam and enolic acid group and is extensively protein bound (95–99%), which facilitates once-a-day dosage [14, 15]. This, along with its favorable gastrointestinal safety profile has led to its increasing use [16,17,18,19,20]. In 2011, there were approximately 20 million prescriptions for meloxicam in the U.S [21]. However, meloxicam is significantly more COX-2 inhibiting than COX-1, raising a concern about its cardiovascular safety [14, 15]. There are only a limited number of studies with small sample sizes which have evaluated the risk of MI with meloxicam use.
Hence, we hypothesized that meloxicam use would be associated with an increased risk of myocardial infarction. We sought to address this question using a nested case–control study in a population-based database, The network health improvement (THIN).
Methods
Study sample
The health improvement network is an anonymized electronic medical records database representing approximately 10.2 million patients in the United Kingdom. It has systematically and prospectively recorded data collected by the 580 GPs on demographics, diagnoses, consultation rates, referrals, hospitalizations, laboratory test results, and prescriptions (including the dose, strength and formulation) among patients covered in the practices. The diagnoses are identified using Read-codes and prescriptions coded according to the drug dictionary, Multilex [22]. Read clinical classification system was developed as a thesaurus of medical terms capturing not only diagnoses but also history, examination, procedures, social information as well as administrative information, thus being more comprehensive than other coding systems. Quality control checks are done regularly, and this database has been validated for pharmacoepidemiologic studies and for MI as an outcome [23].
For the present study, data recorded on individuals from January 2000 to September 2013 was used. All individuals aged 35–89 years with at least 1 prescription for an NSAID during the study time were included for the analysis. Further, each study individual was required to have at least 1 year of enrollment in the database and 1 visit with the GP to be included in the study. This facilitated adequate recording of covariates. To capture incident MI cases, individuals with a history of MI were excluded.
Study design
We used a nested matched case–control study of NSAID users to determine the risk of MI with current meloxicam use compared with remote use of NSAIDs.
Identification of cases and controls
Cases of MI were identified using Read codes and date on which the Read code was recorded considered the index date. Individuals with codes for angina or merely an ECG abnormality were not considered as cases, an approach validated previously in THIN [23]. We selected up to 4 controls for each case, using the date of the case’s event (index date) to obtain the control’s data on age, sex, GP practice to which the patient belongs and the date of inclusion of control in the analysis. Matching was done without replacement.
Assessment of NSAID exposure
Amongst the NSAID users, we first determined how recent the NSAID use was for each patient relative to the index date. For each NSAID, the frequency of dosing and number of pills prescribed were used to calculate prescription completion date. While it is possible that an individual could have taken a prescription beyond the calculated end date, it would lead to misclassification of current users as recent users and hence bias the results towards null.
The prescription completion date was used to classify patients into 3 categories. Those whose prescription duration overlapped the index date were classified as “current users”, those whose prescription ended 1–60 days prior to the index date as “recent users” and those whose prescription ended more than 60 days but within 1 year prior to the index date as “remote users”. Current NSAIDs users were further categorized into four groups based on the drugs of interest: meloxicam, diclofenac (positive control), naproxen (negative control), and other NSAIDs. Thus, six mutually exclusive categories of NSAID exposure were created: the 4 current NSAID user groups, recent users (of any NSAIDs), and remote users (of any NSAIDs). If multiple overlapping prescriptions for different NSAIDs were made, the most recent prescription was used for classification into exposure categories. Given that the risk of MI is with current NSAID use, remote users of NSAID are expected to have no increased MI risk and were chosen as the reference group. Remote users were chosen oven non-users to reduce confounding. Similar approach has been used previously by Garcia et al. [24].
Confounders
Traditional cardiovascular risk factors were assessed as follow: smoking status (current, ex-smokers, non-smokers), body mass index (kg/m2; obese, overweight, normal, underweight), diabetes mellitus, hypertension and hyperlipidemia. Data regarding potential cardiovascular risk factors such as chronic kidney disease, inflammatory rheumatic disease (e.g. rheumatoid arthritis, systemic lupus erythematosus and other rheumatic disease previously demonstrated to be associated with MI risk), osteoarthritis and history of ischemic heart disease and stroke were also recorded. All disease conditions were treated as dichotomous variables. The number of visits to the GP within the year prior to the index date was recorded as a marker of healthcare utilization. Finally, use of cardioprotective agents including aspirin, β blockers, ACE inhibitors and statins was recorded as a dichotomous variable. All comorbidities were assessed prior to the index date: most proximate data within 5 years of index date was used for smoking status and BMI; drug use was assessed within 1 year prior to the index date.
Statistical analysis
Baseline characteristics were expressed as mean ± SD for continuous variables and compared using a t test, or as percentages for ordinal or dichotomous variables and compared using a Chi-square test. Multivariable conditional logistic regression was performed to estimate adjusted odds ratio (aOR) of MI for each current/recent NSAID exposure category relative to remote users of any NSAIDs adjusting for each of the covariates identified in Table 1. For the primary analysis we included all individuals, and missing data regarding BMI and smoking status were imputed by sequential regression method based on a set of covariates as predictors (IVEware for SAS version 9.2; SAS Institute, Cary, NC, USA) [25]. It has been previously suggested that risk of MI associated with NSAID use is dependent on concomitant use of aspirin [24]. Therefore, we conducted an analysis by introducing an interaction term for NSAID and aspirin use and presenting stratified analysis of individual NSAID.
We performed multiple sensitivity analyses: A. By excluding individuals with ischemic heart disease, B. Given the lack of exact knowledge of when the risk of MI conferred by NSAID use ceases, we redefined current users using 2 different time frames (i) Prescription end date within 7 days prior to index date and (ii) Prescription end date within 15 days prior to the index date and C. Complete case analysis by including individuals with only complete data and excluding those with missing data on BMI and smoking. We also performed an additional analysis using 12 mutually exclusive drug exposure categories using the combination of actual drug use (diclofenac, meloxicam, naproxen and other NSAIDs) and timing of drug use (current, recent and remote) which would provide information regarding when the risk of MI ceases.
Assuming the probability of exposure among control of 0.025 (a low estimate given 20 million annual prescriptions in US) and a correlation coefficient for exposure between matched cases and controls of 0.2, if the true odds ratio were 1.25 (a relatively low estimate for COX-2 inhibitors), we estimated the need for 5299 cases with 4 matched controls to be able to reject the null hypothesis with a power of 0.8; two-sided alpha being 0.05 [21].
We internally validated the MI definition by reviewing charts of 50 cases and 50 controls. Overall, 97% of profiles were appropriately categorized as cases or controls (48 cases and 49 controls). NSAID classification into each of the exposure categories was appropriate for all 100 subject profiles.
Results
9291 cases of MI and 30,676 matched controls were identified. The average age and gender were similar between the 2 groups. As expected, individuals with MI had a higher prevalence of traditional cardiovascular risk factors (diabetes, hypertension, hyperlipidemia, obesity and being an active smoker), chronic kidney disease (CKD) and inflammatory arthritis. They had a higher prevalence of ischemic heart disease and stroke in the past. In addition, use of cardio-active agents (β blockers, aspirin, statins and ACE inhibitors) was more common amongst the cases (Table 1).
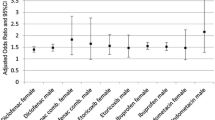
For the main analysis, we included all individuals and imputed data regarding smoking status and BMI. Multivariable logistic regression revealed that compared with remote use of any NSAIDs, the aORs of MI were 1.38 (95% CI 1.17–1.63) for current meloxicam use, 1.37 (95% 1.25–1.50) for current diclofenac use, and 1.12 (95% 0.96–1.30) for current naproxen use, respectively. Recent use of any NSAID (between 1 and 60 days prior to the event days) was also associated with an increased risk of MI (aOR 1.25, 95% CI 1.17–1.33) (Table 2).
To examine the effects of aspirin on the risk of MI, we performed an analysis by introducing an interaction for each NSAID use and aspirin use. The aOR of MI in current meloxicam users who did not use concurrent aspirin remained elevated whereas this risk was eliminated in current meloxicam users who used concurrent aspirin; remote users being the referent group in each case (Table 3). We conducted an additional analysis in patients without any prevalent ischemic heart disease to eliminate any residual confounding conferred while examining association between NSAID use and risk of MI. The results were largely unchanged; aOR for diclofenac was 1.37 (95% CI 1.25–1.50), for meloxicam was 1.40 (95% CI 1.17–1.68) and for naproxen was 1.14 (95% CI 0.96–1.34) (Table 4).
We used 2 alternative definitions of current NSAID use (prescriptions ending up to 7 and 15 days prior to the index date) for sensitivity analyses, the results did not change materially in either case (Supplementary Table S1). We also conducted sensitivity analysis by excluding individuals with any missing data (complete case analysis) which yielded 6212 cases and 19,408 controls. The distribution of baseline characteristics of this subset was similar to the underlying cohort (Supplementary Table S2). These sensitivity analyses did not affect the results (Supplementary Table S3). Finally, we conducted yet another analysis with 12 different mutually exclusive categories of NSAID exposure using different combinations actual NSAID use (diclofenac, meloxicam, naproxen and other NSAID) and timing of drug exposure as compared to index date (current, recent or remote). Similar to primary analysis, we found an increased use associated with current and recent use of diclofenac and meloxicam. There was no increased risk of MI seen with naproxen use. Remote use was not associated increased risk of MI (Supplementary Table S4).
Discussion
In this large population-based cohort, we demonstrated that the use of meloxicam was associated with approximately 38% higher odds of MI than remote use of NSAIDs. We confirmed that diclofenac use was associated with 37% increased odds of MI whereas there was no risk associated with naproxen use.
Since the VIGOR and APPROVe trials, several studies have looked at commonly used drugs like the COX-2 inhibitors (rofecoxib, celecoxib), as well as diclofenac, ibuprofen, indomethacin and naproxen [3,4,5,6,7, 24, 26]. There have been no randomized control trials and few observational studies which have assessed the risk of MI with meloxicam, partly because its use was previously uncommon, doubling only recently between 2007 and 2011 [21]. The studies of meloxicam revealed risk estimates varying from 0.88 to 1.3 often with confidence bounds bridging one. This may be a consequence of limited sample sizes in these studies.
Two studies reveal increased but not statistically significant odds of MI with meloxicam use, one conducted in THIN (2000–2005) by Garcia-Rodriguez (2008) (aOR 1.3 (0.92–1.82)) and other Finnish case–control study (aOR 1.24 (0.99–1.55)) both [24, 27]. However, both of these studies had half the sample size of the current study; 59 and 149 MI cases with meloxicam use, respectively. Two studies using the UK General Practice Research Database with small numbers of MI with meloxicam use, 25 and 137, respectively, reveal aOR of 0.97 (0.6–1.6) and 1.19 (1.01–1.41), respectively [28, 29]. All four studies used non-users of NSAIDs as a control group.
A case-crossover study conducted using National Health Insurance claims database (2005–2006) in Taiwan demonstrated that current meloxicam use had an aOR of MI 0.97 (0.73–1.3) (n = 327) [30]. This study used a 30-day window prior to the event to assess exposure as compared to concurrent use in the present study. This could have caused under-estimation of the risk. Another study in the same database showed MI risk to be similar between long-term rofecoxib and meloxicam use [31]. Given that the MI risk with rofecoxib is well established, the likely explanation would be that the risk is increased by both the drugs. Two additional studies, using the universal health insurance data from Quebec, Canada prior to 2002 revealed no increased risk with meloxicam use. However, there were only 7 cases in this database [32, 33].
Two meta-analyses assessing the risk of MI with meloxicam showed disparate results. Meta-analysis conducted by McGettigan et al. which included 4 of the above mentioned and 1 additional study presented as an abstract (Singh et al.), revealed an increased risk of MI with meloxicam use (aOR 1.2 (1.07–1.33)) [34, 35]. Our results are similar to this meta-analysis. Another meta-analysis conducted by Ashgar et al. revealed no increased risk of MI (aOR 1.13 (0.98–1.32)) [36]. However, this meta-analysis did not include the Finnish study and included additional studies with small numbers [37,38,39]. In addition, the study required meloxicam use to be at least 90 days which may introduce a prevalent user bias (long term use possible only in those who did not experience side effect during the 90 days) thereby attenuating the increased risk.
We observed that the risk of MI associated with meloxicam use was mostly in individuals not using concomitant aspirin. This is consistent is similar to that observed in a nested case–control study conducted Garcia Rodriguez et al. [24]. It has been suggested that meloxicam does not alter the anti-platelet effect of aspirin and that aspirin may counter the prothrombotic effects of meloxicam [40, 41].
The MI risk we found for diclofenac was slightly lower than that reported in older randomized controlled studies. Diclofenac use has dropped substantially since the 2008 American Heart Association position statement (with similar publicity in the UK) which identified diclofenac as a drug with increased cardiovascular risk. This may have led to diclofenac being used at lower doses than in the past and in a carefully selected patient population, thus lessening its cardiovascular risk.
Interestingly, we also observed that recent use of NSAIDs, particularly diclofenac and meloxicam, was associated with an increased risk of MI as compared with remote use. There are two potential explanations for this observation: (a) the risk of MI conferred with NSAID use may not cease immediately and (b) there may be misclassification of individuals in the recent user category who were truly current users. The latter is plausible since time of use was calculated by prescription date and number of pills given. If subjects filled the prescription late or had an excess supply, some of the recent use could have been current.
The current study has limitations some of which are inherent to electronic medical records database. First, we were unable to account for over-the-counter (OTC) use of NSAIDs such as ibuprofen, aspirin and naproxen. Even so, we expect that misclassification of current OTC users as remote users would have biased study results toward the null, underestimating the risk associated with meloxicam. Second, the prescription duration was calculated from number of pills prescribed and the frequency of dosing rather than actual use of the pills. This could have led to potential misclassification of exposure categories. Third, the sample size did not allow for dose–response evaluation. Finally, the database lacks information on family history of heart disease, quantity of alcohol use, smoking intensity, over-the-counter aspirin use and more granular data such as diabetes severity, lipid levels precluding our ability to include these variables in the analysis. Other cardiovascular outcomes, like heart failure, were beyond the scope of current study and the risk estimates may not be similar to that of MI [42].
Despite the above limitations, this study has important strengths. First, the current study design, a nested case–control approach for assessing risk of MI with meloxicam, diclofenac and naproxen users (positive and negative control) as compared to remote users, has inherent advantages. This approach offers an advantage over cohort analysis in which it would be difficult to control for drug switching and survival bias. In the current analysis individuals were considered exposed to the most proximate prescription. We used remote users rather than non-users as the control group. This approach has two advantages—(a) the results are less influenced by unmeasured confounders which are stable over time and (b) users of prescription NSAIDs are inherently different than non-users, which could introduce “healthy user effect”. While not eliminating it, use of remote users and positive and negative control group would minimize this effect [43]. Second, we calculated the E-value, based on aOR of 1.38 for meloxicam use and MI, to be approximately 2 [44]. The strength of association between unmeasured or residual confounding with exposure and outcome would have be greater than 2 beyond the model for the result of 1.38 to be untrue. This is unlikely to be the case and further, the risk estimates for diclofenac and naproxen are similar to prior studies suggesting that residual confounding has been minimized and that the results are unlikely to be biased. Third, the risk estimates were robust to the variations in study design used in various sensitivity analyses. Fourth, this is the largest study conducted using a population-based database assessing the risk of MI with meloxicam use, hence the results are likely to be broadly generalizable. Myocardial Infarction is an important clinical outcome and the results provide useful insight about the risk of MI associated with meloxicam as compared to diclofenac and naproxen, the known most toxic and least toxic NSAID, respectively. This would aid clinicians in choosing NSAID analgesics for their patients. Finally, despite the lack of data regarding some confounders, THIN database contains systematically and prospectively collected data and has been previously validated for MI as an outcome and pharmacoepidemiologic studies [23].
Conclusions
In summary, we demonstrated a 38% increased risk of MI associated with meloxicam use in a large population-based cohort. Results of the current study suggest that meloxicam should be used cautiously, especially in persons at risk of heart disease.
References
Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B et al (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 343:1520–1528. doi:10.1056/NEJM200011233432103
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K et al (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352:1092–1102. doi:10.1056/NEJMoa050493
Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL et al (2005) Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352:1081–1091. doi:10.1056/NEJMoa050330
Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P et al (2005) Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 352:1071–1080. doi:10.1056/NEJMoa050405
Cannon CP, Curtis SP, FitzGerald GA, Krum H, Kaur A, Bolognese JA et al (2006) Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 368:1771–1781
Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA et al (2007) Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation 115:1634–1642. doi:10.1161/CIRCULATIONAHA.106.181424
Nussmeier NA, Whelton AA, Brown MT, Joshi GP, Langford RM, Singla NK et al (2006) Safety and efficacy of the cyclooxygenase-2 inhibitors parecoxib and valdecoxib after noncardiac surgery. Anesthesiology 104:518–526
Fitzgerald GA (2004) Coxibs and cardiovascular disease. N Engl J Med 351:1709–1711. doi:10.1056/NEJMp048288
Olsen AM, Fosbol EL, Lindhardsen J, Folke F, Charlot M, Selmer C et al (2012) Long-term cardiovascular risk of nonsteroidal anti-inflammatory drug use according to time passed after first-time myocardial infarction: a nationwide cohort study. Circulation 126:1955–1963. doi:10.1161/CIRCULATIONAHA.112.112607
Cryer B, Feldman M (1998) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med 104:413–421
Salvo F, Antoniazzi S, Duong M, Molimard M, Bazin F, Fourrier-Reglat A et al (2014) Cardiovascular events associated with the long-term use of NSAIDs: a review of randomized controlled trials and observational studies. Expert Opin Drug Saf 13:573–585. doi:10.1517/14740338.2014.907792
Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM et al (2011) Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 342:c7086. doi:10.1136/bmj.c7086
Varas-Lorenzo C, Riera-Guardia N, Calingaert B, Castellsague J, Salvo F, Nicotra F et al (2013) Myocardial infarction and individual nonsteroidal anti-inflammatory drugs meta-analysis of observational studies. Pharmacoepidemiol Drug Saf 22:559–570. doi:10.1002/pds.3437
Noble S, Balfour JA (1996) Meloxicam. Drugs 51:424–430 (discussion 431–432)
Distel M (1998) Meloxicam clinical data on a preferential cyclooxygenase-2 inhibitor. J Clin Rheumatol 4:s32–s39
Castellsague J, Riera-Guardia N, Calingaert B, Varas-Lorenzo C, Fourrier-Reglat A, Nicotra F et al (2012) Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf 35:1127–1146. doi:10.2165/11633470-000000000-00000
Goei The HS, Lund B, Distel MR, Bluhmki E (1997) A double-blind, randomized trial to compare meloxicam 15 mg with diclofenac 100 mg in the treatment of osteoarthritis of the knee. Osteoarthr Cartil 5:283–288
Wojtulewski JA, Schattenkirchner M, Barcelo P, Le Loet X, Bevis PJ, Bluhmki E et al (1996) A 6-month double-blind trial to compare the efficacy and safety of meloxicam 7.5 mg daily and naproxen 750 mg daily in patients with rheumatoid arthritis. Br J Rheumatol 35(Suppl 1):22–28
Degner F, Sigmund R, Zeidler H (2000) Efficacy and tolerability of meloxicam in an observational, controlled cohort study in patients with rheumatic disease. Clin Ther 22:400–410
Dequeker J, Hawkey C, Kahan A, Steinbruck K, Alegre C, Baumelou E et al (1998) Improvement in gastrointestinal tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficacy Large-scale Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis. Br J Rheumatol 37:946–951
United States securities and exchange commission. http://www.sec.gov/Archives/edgar/data/1492426/000119312512129945/d272296d10k.htm http://www.sec.gov/Archives/edgar/data/1492426/000119312512129945/d272296d10k.htm:1-123
Chisholm J (1990) The read clinical classification. BMJ 300:1092
Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL (2007) Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 16:393–401. doi:10.1002/pds.1335
Garcia Rodriguez LA, Tacconelli S, Patrignani P (2008) Role of dose potency in the prediction of risk of myocardial infarction associated with nonsteroidal anti-inflammatory drugs in the general population. J Am Coll Cardiol 52:1628–1636. doi:10.1016/j.jacc.2008.08.041
Raghunathan T, Lepkowski J, Van Hoewyk J, Solenberger P (2001) A Multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 27:85–95
Bhala N, Emberson J, Merhi A, Abramson S, Arber N et al (2013) Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382:769–779. doi:10.1016/S0140-6736(13)60900-9
Helin-Salmivaara A, Virtanen A, Vesalainen R, Gronroos JM, Klaukka T, Idanpaan-Heikkila JE et al (2006) NSAID use and the risk of hospitalization for first myocardial infarction in the general population: a nationwide case–control study from Finland. Eur Heart J 27:1657–1663
Garcia Rodriguez LA, Varas-Lorenzo C, Maguire A, Gonzalez-Perez A (2004) Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation 109:3000–3006. doi:10.1161/01.CIR.0000132491.96623.04
van Staa TP, Rietbrock S, Setakis E, Leufkens HG (2008) Does the varied use of NSAIDs explain the differences in the risk of myocardial infarction? J Intern Med 264:481–492. doi:10.1111/j.1365-2796.2008.01991.x
Shau WY, Chen HC, Chen ST, Chou HW, Chang CH, Kuo CW et al (2012) Risk of new acute myocardial infarction hospitalization associated with use of oral and parenteral non-steroidal anti-inflammation drugs (NSAIDs): a case-crossover study of Taiwan’s National Health Insurance claims database and review of current evidence. BMC Cardiovasc Disord 12(1):4. doi:10.1186/1471-2261-12-4
Huang WF, Hsiao FY, Tsai YW, Wen YW, Shih YT (2006) Cardiovascular events associated with long-term use of celecoxib, rofecoxib and meloxicam in Taiwan: an observational study. Drug Saf 29:261–272
Levesque LE, Brophy JM, Zhang B (2005) The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med 142:481–489
Brophy JM, Levesque LE, Zhang B (2007) The coronary risk of cyclo-oxygenase-2 inhibitors in patients with a previous myocardial infarction. Heart 93:189–194
McGettigan P, Henry D (2011) Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 8:e1001098. doi:10.1371/journal.pmed.1001098
Singh G, Mithal A, Triadafilopoulos G (2005) Both COX-2 specific inhibitors and non-specific NSAIDS increase the risk of acute myocardial infarction in patients with arthritis: selectivity is with the patient, not the drug class (abstract). Ann Rheum Dis 64:85
Asghar W, Jamali F (2015) The effect of COX-2-selective meloxicam on the myocardial, vascular and renal risks: a systematic review. Inflammopharmacology 23:1–16. doi:10.1007/s10787-014-0225-9
Hosie J, Distel M, Bluhmki E (1996) Meloxicam in osteoarthritis: a 6-month, double-blind comparison with diclofenac sodium. Br J Rheumatol 35(Suppl 1):39–43
Jick SS (2000) The risk of gastrointestinal bleed, myocardial infarction, and newly diagnosed hypertension in users of meloxicam, diclofenac, naproxen, and piroxicam. Pharmacotherapy 20:741–744
Mangoni AA, Woodman RJ, Gaganis P, Gilbert AL, Knights KM (2010) Use of non-steroidal anti-inflammatory drugs and risk of incident myocardial infarction and heart failure, and all-cause mortality in the Australian veteran community. Br J Clin Pharmacol 69:689–700. doi:10.1111/j.1365-2125.2010.03627.x
Scheiman JM, Hindley CE (2010) Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther 32:667–677. doi:10.1016/j.clinthera.2010.04.009
Van Ryn J, Kink-Eiband M, Kuritsch I, Feifel U, Hanft G, Wallenstein G et al (2004) Meloxicam does not affect the antiplatelet effect of aspirin in healthy male and female volunteers. J Clin Pharmacol 44:777–784. doi:10.1177/0091270004266623
Arfe A, Scotti L, Varas-Lorenzo C, Nicotra F, Zambon A, Kollhorst B et al (2016) Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: nested case–control study. BMJ 354:i4857. doi:10.1136/bmj.i4857
Shrank WH, Patrick AR, Brookhart MA (2011) Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 26:546–550. doi:10.1007/s11606-010-1609-1
VanderWeele TJ, Ding P (2017) Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 167:268–274. doi:10.7326/M16-2607
Funding
The present study was funded by NIH AR47785.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Hyon Choi reports receiving research consultancy fee from Takeda.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Boston University Institutional Review Board and Scientific Review Committee with The Health Improvement Network database have reviewed the protocol and approved the study. De-identified publicly available data from THIN was used for purposes of the current study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dalal, D., Dubreuil, M., Peloquin, C. et al. Meloxicam and risk of myocardial infarction: a population-based nested case–control study. Rheumatol Int 37, 2071–2078 (2017). https://doi.org/10.1007/s00296-017-3835-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3835-x