Abstract
Rheumatoid arthritis is an autoimmune disorder which involves inflammation of the synovial tissue, leading to synovial proliferation, bone erosion and ultimately joint disability. It is a complex disorder, and the proper etiology is still unknown. Both environmental and genetic factors are responsible for the development of Rheumatoid arthritis. Clinically, the disease is generally diagnosed by the presence of auto-antibodies like Rheumatoid factor. But these are not specifically associated with Rheumatoid arthritis. These are also present in patients with other autoimmune disorders and also in healthy persons. Citrullinated epitopes are shown to be more specific for Rheumatoid arthritis. Citrullination normally occurs in cells undergoing apoptosis, and hence, citrullinated proteins are cleared from body and not encountered by immune system. However, in Rheumatoid arthritis patients, these are not cleared. Anti-citrullinated protein antibodies are detectable in patients at risk of Rheumatoid arthritis long before the onset of the disease. The concentration of which normally increases as the disease progress. Hence, these are important for diagnosis of Rheumatoid arthritis. This review is focused on the importance of anti-citrullinated protein antibodies in disease pathogenesis and its importance in the diagnosis of Rheumatoid arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systematic, inflammatory joints disease, occurs as a chronic inflammatory disorder that is characterized by cartilage destruction [4, 24]. It is related to other autoimmune disease families like insulin-dependent diabetes mellitus (IDDM) and thyroid diseases [45]. Worldwide, its prevalence is about 0.5–1 % while 0.55–0.75 % of the Pakistani population is affected by RA. Women are three times more susceptible than men with sex ratio of 2–4. Although people at any age are vulnerable to the disease, it is more frequent at the age of 40–50 years. Rheumatoid arthritis is not a life-threatening disease directly, but a patient’s life quality is severely affected by it [39].
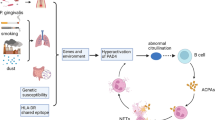
Exact etiology of RA is not so far known, but its development depends upon interaction between genetics elements in individual and non-genetic factors. Considerable amount of data have suggested that autoimmunity markers and genetic factors and are very good indicators [20]. Genetic factors like MHC-II (major histocompatibility complex II) and non-MHC alleles, processes related to autoimmunity and environmental factors are very crucial in RA pathogenesis [14]. Pathogenesis of RA involves in a number of cellular responses. Autoimmune responses mediated T cell and B cells are important in inflammatory cascade initiation. Then T cells, B cells, macrophages and neutrophils migrate into synovial tissues, where they produce immune mediator that break down the extracellular matrix, in particular that of cartilage [30]. As a result synovial hypertrophy and angiogenesis occurs this leads into osteoclasts activation and then bone erosion. T cells and synovial fibroblast also generate inflammatory cytokines that differentiate monocyte-macrophage lineage cells to mature osteoclasts that leads to bone resorption and erosion [44]. So, diagnosis at early stage and instant, valuable therapy is important in order to avoid joint destruction, functional disability and horrific outcome of infection [2, 35].
The availability of sophisticated and efficient therapies [40] and early intervention is important in preventing irreversible damage of joints [7, 23]. It is utmost crucial to identify RA in the early stage. Although the classification criteria for RA described by American College of Rheumatology (1987) are often used as diagnostic tool for RA in clinical practice, they are not well suited for early diagnosis for RA [5, 36, 51]. The above-mentioned criteria rely mainly on the appearance of RA clinical symptoms but most of the time clinical symptoms do not appear in early stages. So, there is a need to develop a sensitive and specific serological marker, which is present in the early stages. So that rheumatologists would be able to use the expensive and toxic drugs to only those patients who are at high risks [19]. Therefore, a marker should be able to identify the erosive and non-erosive aggression of the infection. The anti-CCP antibodies fortunately meet the criteria for an efficient and useful marker for early RA.
In RA, auto-antibodies are present in patient’s serum. These are often not that much specific because these antibodies may also be present in patients with other diseases. Rheumatoid factor (RF) is one of the examples of such antibodies. It is a well-known highly non-specific autoantibody which is intended for to the Fc part of IgG molecules (Waaler 2007). It is normally present in 80 % of RA patients, but in many other diseases, it can be detected. It can also be detected in healthy people, especially in old age people (10–30 %) [31], thus lowering its specificity. Other RA-associated antibodies include ANA (antibodies to nuclear antigens), anti-RA33, anti-GPI (glucose-6-phosphate isomerase), anti-calpastatin, ANCA (anti-neutrophil cytoplasmic antibodies), anti-fibronectin and anti-collagen type II. Such type of auto-antibodies can also be detected in many other autoimmune diseases like SLE (systemic lupus erythematosus) and MCTD (mixed connective tissue disease) and in many normal individuals.
In the past decade, it has been shown that the autoantibodies which are highly specific for RA are aimed at citrulline-containing epitopes. Citrulline is a non-standard amino acid. It is named as because it is not incorporated into proteins during protein production, however, during posttranslational modification of arginine residues, it is incorporate which is carried out by peptidylarginine deiminase (PAD) [41]. The process of citrullination usually takes place in those cells that are going under the process of apoptosis. To our knowledge, the presence of citrullinated antigens in inflamed synovial tissue do not always indicate the presence of ACCP antibodies in serum and synovial fluid, whereas the exact structure of HLA molecules facilitates the induction of autoantibodies directed to citrullinated proteins [15]. History of ACPA started when anti-perinuclear antibodies (APF) and anti-keratin antibodies (AKA) were described [29, 56]. These antibodies can be detected with high specificity in about half RA patients. As a new diagnostic tool for RA, first-generation CCP test (anti-CCP1) has 68 % sensitivity with 97–98 % specificity [37]. For the improvement of CCP test sensitivity, several citrulline-containing peptides libraries were screened with RA serum pool and this led second-generation CCP test development (CCP2). CCP2 test is more sensitive as compared to RF (80 %), with superior specificity (98 %) [51].
Role in pathogenesis of RA
Discovery of ACPA provide us a new way to investigate those factors that are involve in RA. Anti-citrullinated protein antibodies are extremely specific for RA [52]. Before the onset of clinical symptoms, these auto-antibodies can be detected with increasing titers as patients approach disease onset [8, 28, 34]. Anti-citrullinated protein antibodies have been shown to be able to initiate and enhance arthritis in murine models of arthritis [22, 47], and they are able to activate both FcR-positive cells and the complement system, arguing that they could play a role in disease pathogenesis [9, 38, 46].
Several human leukocyte antigen (HLA) alleles, particularly those encoding the shared epitope (SE), are known to be associated with RA susceptibility, especially with ACPA-positive RA [13, 49]. These data indicate that antigen presentation and T cell involvement are important in the induction of ACPA. With T cell help, antigen-exposed B cells can undergo class-switching and avidity maturation. This occurs in germinal centers, where B cells compete for a limited source of antigens on follicular dendritic cells under antigen-specific control of follicular helper T cells [12, 33]. It is known that ACPA-producing B cells undergo isotype switching since ACPA of all isotypes can be detected in sera of RA patients [54]. Relatively little is known about the avidity maturation of ACPA before and during disease manifestation. Recently, we have shown that the avidity of the ACPA response is relatively low as compared to antibody responses against recall antigens in patients with established RA [42]. Recently, several studies have shown that during the pre-disease stage of RA, the ACPA response recognizes more epitopes [48], uses more isotypes and increases in levels [6]. In conclusion, the avidity of ACPA is in general low, but when analyzing individual patients, marked differences in the ACPA avidity can be observed. The avidity maturation of ACPA takes place before disease onset and then stabilizes.
There are a lot of studies that demonstrate the ACPA presence as a prognostic tool for severity of disease, RA development, as well as radiographic erosions in synovitis of recent onset [14, 18, 26, 27]. ACPA higher levels have been found in both, individuals who developed RA or who did not develop it. Several studies marked the predictive value ACPA presence [1, 11, 17]. Though, it is unclear until now, whether ACPA high levels predict poorer outcome or not [10, 25, 43]. In a study of 104 RA patients, ACPA higher baseline levels were linked with erosive disease after 2 years [53]. Ninety-nine RA patients in another study reported a slightly significant correlation between radiographic progression and baseline serum ACPA levels (Meyer et al. 2006). Another study of early 238 RA individuals showed a higher radiographic progression rate of high-positive ACPA versus low-positive ACPA patient groups after 10 years [43]. Two studies marked the levels of ACPA levels in individuals having longstanding RA. One study found a weak association in almost 180 patients between radiographic progression rate and ACPA levels. The other study was cross-sectional, consisting 241 RA patients. In this study, mean of disease duration was about for 8.6 years. In patients, mean of ACPA levels were similar with or without erosions [10, 25]. Several single nucleotide polymorphisms are linked with this disease like PTPN22, and it is specifically correlated with RA-ACPA positive [21, 32]. Whereas DRB1*03 is correlated with RA-ACPA negative disease, but not all of studies have confirmed this correlation [16, 55]. So such differences indicate that there are different disease entities of ACPA-positive and ACPA-negative RA.
Role as a new diagnostic biomarker
The diagnosis and treatment of RA has made tremendous progress in the last few years. Experts are even suggesting that a paradigm shift has occurred in the field of rheumatology. The development of effective diagnostic sero-markers and corresponding test systems, particularly ELISA, APF and AKA test assays, represents enormous progress in RA diagnostics but their problematic and lengthy immunofluorescence test format never let them to become typical tests for diagnosis. The widely used Anti-CCP2 assays that have high diagnostic sensitivity and specificity are widely used nowadays. It also shows significant prognostic and predictive worth in RA. It is a more specific test than RF because other types of arthritis and immunological disorders can be distinguished on the basis of this, and the signs and symptoms of which are mostly similar to RA [1, 11].
Anti-CCP2 test is more appropriate for diagnosis of early RA because these antibodies are present at early stage of the disease, and probably, this is due to this reason that in some advanced stage RA patients, the anti-CCP2 test shows negative results [28, 34]. But this is hard to say that a person showing positive result of anti-CCP2 test will develop RA in coming years or months, and this can also be just simply a false positive test. However, this test is in particular of great advantage to the clinician as these antibodies can be used as early markers for the onset of RA. In some studies, it was reported that a positivity of anti-CCP2 indicates the stage of undifferentiated arthritis, which will progress to RA within 3 years [3, 50]. In conclusion, anti-CCP2 assay has a lot more importance in the diagnosis of RA, and on the basis of these advantages, it has been included in the 2010 classification criteria for RA.
Conclusion
There is no proper assay available for the diagnosis of RA. Current assays like RF are not much specific for RA and are also detected in patients with other autoimmune disorders and in healthy individuals. So there is a need for the development of an assay which may be more specific for RA and which may also help in early diagnosis of RA before the onset of the diseased condition. ACPAs, which are citrullinated antibodies, are more specific for RA. This is second-generation CCP test (CCP2), which has more sensitivity as compared to RF and CCP1 (80 %) and more specificity than ever test available for RA (98 %). The presence of ACPA is prognostic for the disease severity. As shown in different studies, it is concluded that the level of ACPA is low before the onset of the disease and as the disease progress, ACPA level raises. However, ACPA is also detected in persons who are at risk of RA, before the disease onset.
References
Agrawal S, Misra R, Aggarwal A (2007) Autoantibodies in rheumatoid arthritis: association with severity of disease in established RA. Clin Rheumatol 26:201–204
Alamanos Y, Drosos AA (2005) Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 4(3):130–136
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G (2010) Rheumatoid arthritis classification criteria: an American college of rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 69(9):1580–1588
Andreas K, Lübke C, Häupl T, Dehne T, Morawietz L, Ringe J, Kaps C, Sittinger M (2008) Key regulatory molecules of cartilage destruction in rheumatoid arthritis: an in vitro study. Arthritis Res Ther 10(1):R9
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1998) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Bos WH, Wolbink GJ, Boers M, Tijhuis GJ, de Vries N, van der Horst-Bruinsma IE, Tak PP, van de Stadt RJ, van der Laken CJ, Dijkmans BA, van Schaardenburg D (2010) Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 69(3):490–494
Bukhari MA, Wiles NJ, Lunt M, Harrison BJ, Scott DG, Symmons DP et al (2003) Influence of diseasemodifying therapy on radiographic outcome in inflammatory polyarthritis at five years: results from a large observational inception study. Arthritis Rheum 48:46–53
Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW (2009) Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol 36(4):706–711
Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, Serre G (2008) Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum 58(3):678–688
De Rycke L, Peene I, Hoffman IE, Kruithof E, Union A, Meheus L, Lebeer K, Wyns B, Vincent C, Mielants H, Boullart L, Serre G, Veys EM, De Keyser F (2004) Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis 63:1587–1593
Del Val Del Amo N, Ibanez BR, Fito MC, Gutierrez PR, Loza CE (2006) Anti-cyclic citrullinated peptide antibody in rheumatoid arthritis: relation with disease aggressiveness. Clin Exp Rheumatol 24:281–286
Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ (2010) PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 11(6):535–542
Gregersen PK, Silver J, Winchester RJ (1987) The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30(11):1205–1213
Van der Helm-van AHM, Mil KNV, Breedveld FC, Toes RE, Huizinga TW (2005) Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res 7(5):R949–R958
Huizinga TW, Amos CI, van der Helm-van MAH, Chen W, van Gaalen FA, Jawaheer D, Schreuder GM, Wener M, Breedveld FC, Ahmad N, Lum RF, de Vries RR, Gregersen PK, Toes RE, Criswell LA (2005) Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum 52(11):3433–3438
Irigoyen P, Lee AT, Wener MH, Li W, Kern M, Batliwalla F, Lum RF, Massarotti E, Weisman M, Bombardier C, Remmers EF, Kastner DL, Seldin MF, Criswell LA, Gregersen PK (2005) Regulation of anti–cyclic citrullinated peptide antibodies in rheumatoid arthritis: contrasting effects of HLA–DR3. Arthritis Rheum 52(12):3813–3818
Karlson EW, Chibnik LB, Cui J, Plenge RM, Glass RJ, Maher NE, Parker A, Roubenoff R, Izmailova E, Coblyn JS, Weinblatt ME, Shadick NA (2008) Associations between human leukocyte antigen, PTPN22, CTLA4 genotypes and rheumatoid arthritis phenotypes of autoantibody status, age at diagnosis and erosions in a large cohort study. Ann Rheum Dis 67:358–363
Kastbom A, Strandberg G, Lindroos A, Skogh T (2004) Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 63:1085–1089
Kirwan JR, Quilty B (1997) Prognostic criteria in rheumatoid arthritis: can we predict which patients will require specific anti-rheumatoid treatment? Clin Exp Rheumatol 15:S15–S25
Klareskog L, Widhe M, Hermansson M, Ronnelid J (2008) Antibodies to citrullinated proteins in arthritis: pathology and promise. Curr Opin Rheumatol 20(3):300–305
Kokkonen H, Johansson M, Innala L, Jidell E, Rantapaa-Dahlqvist S (2007) The PTPN22 1858C/T polymorphism is associated with anti-cyclic citrullinated peptide antibody-positive early rheumatoid arthritis in northern Sweden. Arthritis Res Ther 9:R56
Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, Michael Holers VM (2006) Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest 116(4):961–973
Landewe RB (2003) The benefits of early treatment in rheumatoid arthritis: confounding by indication, and the issue of timing. Arthritis Rheum 48:1–5
Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F (1998) Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 41(5):778–799
Lee DM, Phillips R, Hagan EM, Chibnik LB, Costenbader KHMM, Schur PH (2009) Quantifying anti-cyclic citrullinated peptide titres: clinical utility and association with tobacco exposure in patients with rheumatoid arthritis. Ann Rheum Dis 68:201–208
Machold KP, Stamm TA, Nell VP, Pflugbeil S, Aletaha D, Steiner G, Uffmann M, Smolen JS (2007) Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology 46:342–349
Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A, Nicaise-Roland P, Sibilia J, Combe B (2003) Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 62:120–126
Nielen MM, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MH, Habibuw MR, Vandenbroucke JP, Dijkmans BA (2004) Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 50(2):380–386
Nienhuis RL, Mandema E (1964) A new serum factor in patients with rheumatoid arthritis; the antiperinuclear factor. Ann Rheum Dis 23:302–305
Okamoto H, Kobayashi A (2011) Tyrosine kinases in rheumatoid arthritis. J Inflamm 8:21
Palosuo T, Tilvis R, Strandberg T, Aho K (2003) Filaggrin related antibodies among the aged. Ann Rheum Dis 62(3):261–263
Plenge RM, Padyukov L, Remmers EF, Purcell S, Lee AT, Karlson EW, Wolfe F, Kastner DL, Alfredsson L, Altshuler D, Gregersen PK, Klareskog L, Rioux JD (2005) Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am J Hum Genet 77:1044–1060
Randall KL, Lambe T, Johnson A, Treanor B, Kucharska E, Domaschenz H, Whittle B, Tze LE, Enders A, Tanya L, Crockford TL, Bouriez-Jones T, Alston D, Cyster JG, Lenardo MJ, Mackay F, Deenick EK, Tangye SG, Chan TD, Camidge T, Brink R, Vinuesa CG, Batista FD, Cornall RJ, Goodnow CC (2009) Dock8 mutations cripple B cell immunological synapses, germinal centers and long-lived antibody production. Nat Immunol 10(12):1283–1291
Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, Sundin U, van Venrooij WJ (2003) Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 48(10):2741–2749
Raza K, Buckley CE, Salmon M, Buckley CD (2006) Treating very very early rheumatoid arthritis. Best Pract Res Clin Rheumatol 20(5):849–863
Saraux A, Berthelot JM, Chales G, Le Henaff C, Thorel JB, Hoang S et al (2001) Ability of the American college of rheumatology 1987 criteria to predict rheumatoid arthritis in patients with early arthritis and classification of these patients two years later. Arthritis Rheum 44:2485–2491
Schellekens GA, Visser H, de Jong BA, van den Hoogen FH, Hazes JM, Breedveld FC, van Venrooij WJ (2000) The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum 43(1):155–163
Schuerwegh AJ, Ioan-Facsinay A, Dorjee AL, Roos J, Bajema IM, van der Voort EIH, Huizinga TWJ, Toes REM (2010) Evidence for a functional role of IgE anticitrullinated protein antibodies in rheumatoid arthritis. Proc Natl Acad Sci 107(6):2586–2591
Scott DL, Coulton BL, Symmons DPM, Popert AJ (1987) Long-term outcome of treating rheumatoid arthritis: results after 20 years. The Lancet 329(8542):1108–1111
Smolen JS, Steiner G (2003) Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov 2:473–488
Steiner G, Smolen J (2002) Autoantibodies in rheumatoid arthritis and their clinical significance. Arthritis Res 4(Suppl 2):S1–S5
Suwannalai P, Scherer HU, van der Woude D, Ioan-Facsinay A, Jol-van der Zijde CM, van Tol MJ, Drijfhout JW, Huizinga TW, Toes RE, Trouw LA (2011) Anti-citrullinated protein antibodies have a low avidity compared with antibodies against recall antigens. Ann Rheum Dis 70(2):373–379
Syversen SW, Gaarder PI, Goll GL, Odegard S, Haavardsholm EA, Mowinckel P, van Der HD, Landewe R, Kvien TK (2008) High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 67:212–217
Taneja V, Krco CJ, Behrens MD, Luthra HS, Griffiths MM, David CS (2007) B cells are important as antigen presenting cells for induction of MHC-restricted arthritis in transgenic mice. Mol Immunol 44(11):2988–2996
Torfs CP, King MC, Huey B, Malmgren J, Grumet FC (1986) Genetic interrelationship between insulin-dependent diabetes mellitus, the autoimmune thyroid diseases, and rheumatoid arthritis. Am J Hum Genet 38(2):170–187
Trouw LA, Haisma EM, Levarht EW, van der Woude D, Ioan-Facsinay A, Daha MR, Huizinga TW, Toes RE (2009) Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum 60(7):1923–1931
Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, Serre G, Burkhardt H, Thunnissen MM, Holmdahl R (2009) Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med 206(2):449–462
van de Stadt LA, van der Horst AR, de Koning MH, Bos WH, Wolbink GJ, van de Stadt RJ, Pruijn GJ, Dijkmans BA, van Schaardenburg D, Hamann D (2011) The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann Rheum Dis 70(1):128–133
van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR (2006) The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum 54(4):1117–1121
van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, de Jong BA, Breedveld FC, Verweij CL, Toes RE, Huizinga TW (2004) Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: a prospective cohort study. Arthritis Rheum 50(3):709–715
Van Venrooij WJ, Hazes JM, Visser H (2002) Anticitrullinated protein/peptide antibody and its role in the diagnosis and prognosis of early rheumatoid arthritis. Neth J Med 60:383–388
van Venrooij WJ, van Beers JJ, Pruijn GJ (2008) Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci 1143:268–285
Vencovsky J, Machacek S, Sedova L, Kafkova J, Gatterova J, Pesakova V, Ruzickova S (2003) Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 62:427–430
Verpoort KN, Jol-van der Zijde CM, Papendrecht-van der VEA, Ioan-Facsinay A, Drijfhout JW, van Tol MJ, Breedveld FC, Huizinga TW, Toes RE (2006) Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum 54(12):3799–3808
Verpoort KN, van Gaalen FA, van der Helm-van Mil AH, Schreuder GM, Breedveld FC, Huizinga TW, de Vries RR, Toes RE (2005) Association of HLA–DR3 with anti–cyclic citrullinated peptide antibody–negative rheumatoid arthritis. Arthritis Rheum 52:3058–3062
Young BJ, Leslie RD, Clark CJ, Hamblin TJ (1979) Anti-keratin antibodies in rheumatoid arthritis. Br Med J 2(6182):97–99
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moeez, S., John, P. & Bhatti, A. Anti-citrullinated protein antibodies: role in pathogenesis of RA and potential as a diagnostic tool. Rheumatol Int 33, 1669–1673 (2013). https://doi.org/10.1007/s00296-012-2635-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2635-6