Abstract
In this open-label trial, ten male patients with active Behcet’s uveitis were enrolled. Initially, two infliximab infusions (5 mg/kg) were given at weeks 0 and 2. The patients continued to receive conventional therapy on recurrence of severe uveitis (RSU) attack. The patients with further attack were regularly given infliximab infusions every 8 weeks. In cases of further RSU attacks, the infusion interval was reduced to 6 weeks. The total follow-up period was 3 years. The patients were monitored for RSU, visual acuity and adverse effects. Reduction in the doses of prednisolone was also monitored. After receiving two infliximab infusions at weeks 0 and 2, three patients remained attack-free and seven patients had another RSU attack between 8th and 47th week. These patients were regularly given infliximab at 8-week intervals. Five out of seven patients remained attack-free. In two patients who had further attack, infusion frequency was increased to 6 weeks. There was a remarkable improvement in visual acuity with no significant adverse reaction except mild respiratory tract infection (two patients), headache (one patient) and mild infusion reaction (one patient). Infliximab is a safe and effective drug for the management of Behcet’s uveitis. Selection of optimal dose and frequency of infusion required standardization for individual patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behcet’s disease (BD) is a multisystem inflammatory disorder with chronic, relapsing course. Patients with ocular involvement typically have bilateral nongranulomatous panuveitis and retinal vasculitis that involves all elements of the retinal vasculature. The course of the disease is characterized by recurrent, explosive attacks of uveitis followed by spontaneous remissions [1]. The severity and frequency of these inflammatory episodes determine the extent of permanent damage to the intraocular structures and resultant visual loss [2]. Although the course of the disease shows individual variability, male patients with a younger age at disease onset generally suffer from more severe disease and they are also at a higher risk of visual losses compared to female patients [2].
The main goals in management of patients with Behcet’s uveitis are rapid resolution of intraocular inflammation, prevention of recurrent attacks, achievement of complete remission and preservation of vision. Corticosteroids are generally used for the treatment of acute episodes of inflammation; however, their long-term use is associated with unacceptable side effects. Moreover, recurrences are common in the setting of corticosteroid monotherapy, and conventional immunosuppressive agents such as azathioprine and cyclosporine are effective for the long-term treatment of Behcet’s uveitis [3, 4]. In fact, combination of azathioprine and cyclosporine is more effective than the monotherapy with either agents. However, in some patients, disease is unresponsive to this regimen or to other cytotoxic drugs [5]. An analysis of 880 patients with Behcet’s uveitis who were treated with conventional immunosuppressive agents showed that, 16% of the eyes with a potential visual acuity of >0.1 had irreversibly lost useful vision during follow up [2]. The results of these studies clearly warrant the search of new safe and effective mode of treatment.
Basic and clinical researches suggest that proinflammatory cytokines, including tumor necrosis factor [alpha] (TNF [alpha]), have a key role in the pathogenesis of inflammation in BD [6–8]. Infliximab, a recombinant chimeric monoclonal antibody specific for human TNF [alpha], which effectively binds and stabilizes both the soluble and membranous forms of TNF [alpha], has been shown to be an effective agent in the treatment of rheumatoid arthritis, Crohn’s disease and spondylarthropathies [9–11]. Anti-TNF agents have also begun to be used as a new therapeutic approach in other inflammatory disorders, including BD, and early studies on the use of infliximab in Behcet’s uveitis have shown remarkably good results. However, the data is quite scanty and limited. In this study, we conducted an open-label prospective trial to evaluate the efficacy of infliximab in patients with severe uveitis secondary to BD.
Patients and methods
The study was conducted at Riyadh Military Hospital (Department of Medicine, Division of Rheumatology and Department of Ophthalmology). In this longitudinal open study, we enrolled ten adult patients who met the international study group criteria for a diagnosis of BD. All these patients had been receiving cyclosporine plus prednisolone except two patients who were receiving Azathioprine instead of Cyclosporine. In spite of corticosteroid and immunosuppressant therapy, these patients had history of recurrent uveitis attacks.
An initial systemic examination (Table 1) and detailed ophthalmological assessment including visual acuity, measurement of intraocular pressure, slit-lamp biomicroscopy and indirect ophthalmology of the posterior segment were followed by fundus photography. Acute deterioration of visual acuity and involvement of posterior and/or anterior chambers with or without retinitis were recorded in all the patients. Visual acuity was assessed using a Snellen chart. Anterior uveitis were scored by using a standard scoring system (on a scale of 0–4); uveitis attacks involving the posterior segment were defined as an increase in or the development of vitreous haze (0–3), the emergence of inflammatory sheathing of retinal vessels, vascular occlusion, retinal hemorrhages, retinal infiltrates, macular edema or papillitis. Both unilateral and simultaneous bilateral uveitis attacks were evaluated as a single occurrence. Chest radiography, purified protein derivative (PPD) and skin pathergy testing, routine blood chemistry and hematological examinations were also registered before the infusion. The outcome measures were intraocular inflammation, visual acuity, reduction of daily corticosteroid dose, frequency of ocular attacks post-infliximab infusion and adverse effects.
In this study, infliximab was administered at a dose of 5 mg/kg at weeks 0 and 2 for over 2 h of intravenous infusion. The existing therapy with corticosteroid and immunosuppressant was continued. The efficacy of drug therapy was monitored regularly by recording the number of uveitis attacks, visual acuity and other parameters described above. The patients who did not have complete remission following two infusions or who developed recurrent attacks were given 8 weekly infusions regimen. There was a further increase in frequency (6 weekly infusions), if 8 weekly infusion failed to prevent uveitis attacks. The primary outcome measures were remission, which was defined as the absence of uveitis attacks involving the posterior segment during the follow-up periods.
Adverse events were recorded throughout the study period. An infusion reaction was defined as an adverse event that manifested during the infusion and persisted until 2 h after the end of the infusion.
Results
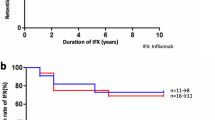
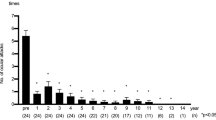
Treatment of ten Behcet's Disease Uveitis (BDU) patients with 5 mg/kg body weight on weeks 0 and 2 along with corticosteroids and immunosuppression therapy resulted in transient suppression of acute ocular inflammation. Three out of ten patients remained completely attack-free throughout the study period. Remaining seven patients suffered further uveitis attacks at weeks 8, 9, 17, 21, 30, 35 and 47 after two infliximab infusions. These patients were started on a regular 8 weekly infusion of 5 mg/kg infliximab. Only two of the seven patients receiving 8 weekly course of infliximab showed a further uveitis attack. In these patients, the frequency of infliximab infusion was increased with a 6 weekly regular infusion. With these measures, all the patients remained attack-free during the 3 years of study period.
Anti-inflammatory effect of infliximab
Table 2 shows that infliximab therapy results in a significant remission of ocular attacks. The cell count in anterior chamber was reduced to 0 in 17 out of 20 eyes (ten patients); only very mild (+1) activity was observed in three eyes. The pre- and post-infliximab infusion scarring of vitiritis also showed a highly significant improvement. On the other hand, macular edema, vasculitis and retinal vasculitis completely resolved in the patients following infliximab infusion (Table 2).
Effect of visual acuity
There was a significant improvement of the visual acuity of the patients treated with infliximab (Table 3). The improvement of vision started within the days following the first infliximab infusion. However, two patients already had irreversible retinal damage before starting infliximab infusion and showed only poor recovery (Table 3).
Adverse drug reaction
The treatment with infliximab infusion was well tolerated except mild infusion reaction (one patient), headache (one patient) and upper respiratory tract infection (two patients).
Discussion
The results of this study clearly showed a profound and transient effect of infliximab in sight-threatening uveitis. Infliximab therapy has been reported as being generally effective in anecdotal case series of BD patients with various DMARD-refractory manifestations, including mucocutaneous lesions, uveoretinitis, arthritis and gastrointestinal involvement. Tolerability was mostly satisfactory with only negligible adverse effects (Table 1). This finding is in agreement with earlier reports [12–15].
In this study, three out of ten patients remained attack-free following only two initial transfusion of infliximab at weeks 0 and 2 (Table 4). The remaining seven patients were then given 8 weekly transfusions; however, due to further recurrence, two patients needed 6 weekly transfusions to remain attack-free. Our findings are in agreement with earlier investigators who suggested a need for 6–8 weekly infusion of infliximab to achieve sustained remission of BDU [13, 16]. The present trial is the first report showing that only two initial infusion of infliximab at a dose of 5 mg/kg at weeks 0 and 2 might result in a sustained (until 3 years in this study) remission in 30% (three out of ten) of patients with severe BDU. Nakamura and Ohno [17] reported that both 5 and 10 mg/kg of infliximab are equally effective, thus suggesting that 5 mg/kg is more appropriate dose.
There was a rapid remission of macular edema and remarkable improvement in visual acuity (Table 3) suggesting the ability of infliximab to effectively suppress acute ocular inflammation in patients with BDU. A rapid therapeutic effect is essential in these patients to prevent development of fixed retinal lesions, which may cause irreversible visual impairment. The action of infliximab in resolving acute ocular inflammation is spectacular and much faster as compared to conventional immunosuppressants. In fact, the degree of inflammation may decrease by 50% during first 24 h and more than 90% within 4 days of infliximab infusion [18]. Furthermore, long-term use of nonselective immunosuppressant is associated with drug resistance as well as variety of adverse effects, which may overshadow their beneficial effects [19]. In this study, all the patients who were receiving higher doses of corticosteroids were shifted gradually to tapering dosage regimen of corticosteroids. In patients with extraocular findings, namely oral aphthous ulcer, skin lesions such as erythema nodosum and pseudofolliculitis, prior to the administration of infliximab, these manifestations disappeared during follow-up, suggesting that infliximab is also effective on these extraocular manifestations of BD. In this study, we did not observe any significant adverse effect of infliximab except mild upper respiratory tract infection in two patients, headache in one patient and mild infusion reaction in one patient. Our findings are supported by several earlier investigators who also confirmed the efficacy and safety of long-term use of infliximab [13, 16]. However, in some previous studies, the use of repeated infliximab therapy for refractory uveitis resulted in the development of antinuclear antibodies (ANAs) [20, 21]. In this study, three out of ten patients developed positive ANA without systematic lupus manifestations. The induction of autoantibodies in patients with rheumatic diseases treated with infliximab has been reported [22, 23]. In these studies, the development of ANAs and anti-dsDNA antibodies was associated with large number of infusions and higher doses of infliximab. However, the development of autoantibodies due to chronic use of infliximab was not associated with a loss of its therapeutic efficacy or enhancement of adverse effects [22, 23].
The result of this study and the published results of clinical trials of infliximab from other centers clearly confirm a prompt and often dramatic response in patients with severe BDU. An assessment of the efficacy of infliximab is also hampered by the frequent concomitant use of other drugs and by the mostly short duration of follow-up. Any possible bias on the part of investigator can only be ruled out by well designed randomized controlled trials using large number of patients. The effect of comedication interaction must also be investigated in this new therapy for refractory uveitis associated with BD.
References
International Study Group for Behçet’s Disease (1990) Criteria for diagnosis of Behcet’s disease. Lancet 335:1078–1080
Tugal-Tutkun J, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M (2004) Uveitis in Behcet’s disease: an analysis of 880 patients. Am J Ophthalmol 138:373–380
Atmaca LS, Batioglu F (1994) The efficacy of cyclosporine-A in the treatment of Behcet’s disease. Ophthalmic Surg 25:321–327
Ozdal PC, Ortac S, Taskintuna I, Firat E (2002) Long-term therapy with low dose cyclosporine A in ocular Behcet’s disease. Doc Ophthalmol 105:301–312
Kaklamani VG, Kaklamani PG (2001) Treatment of Behcet’s disease: an update. Semin Arthritis Rheum 30:299–312
Hamzaoui K, Hamza M, Ayed K (1990) Production of TNF-alpha and IL-1 in active Behcet’s disease. J Rheumatol 17:1428–1429
Santos LM, Marcos MC, Gallardo Galera JM (2001) Aqueous humor and serum tumor necrosis factor-alpha in clinical uveitis. Ophthalmic Res 33:251–255
Gul A (2001) Behcet’s disease: an update on the pathogenesis. Clin Exp Rheumatol 19(Suppl 24):S6–S12
Maini SR (2004) Infliximab treatment of rheumatoid arthritis. Rheum Dis Clin North Am 30:329–347
Rutgeerts P, van Assche G, Vermeire S (2004) Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology 126:1593–1610
Braun J, Sieper J (2004) Biological therapies in the spondyloarthritides: the current state. Rheumatology (Oxford) 43:1072–1084
Haugeberg G, Velken M, Johnsen V (2000) Successful treatment of genital ulcers with infliximab in Behcet’s disease. Ann Rheum Dis 63:744–745
Tugal-Tutkun I, Mudun A, Urgancioglu M, Kamali S, Kasapoglu E, Inanc M, Gul A (2005) Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine and corticosteroids in Behcet’s disease: an open-label trial. Arthritis Rheum 52:2478–2484
Andonopoulos AP, Meimaris N, Daoussis D et al (2003) Intra-articular anti-tumor necrosis factor alpha antibody in recalcitrant arthritis of Behcet’s disease. Clin Exp Rheumatol 21(Suppl 30):S57–S58
Kram MT, May LD, Goodman S, Molinas S (2003) Behcet’s Ileocolitis: successful treatment with tumor necrosis factor-alpha antibody (infliximab) therapy: report of a case. Dis Colon Rectum 46:118–121
Sfikakis PP, Kaklamanis PH, Elezoglou A et al (2004) Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades-Behcet’s disease. Ann Intern Med 140:404–406
Nakamura S, Ohno S (2005) Anti-tumor necrosis factor alpha antibody in the treatment of Behcet’s disease. Int Ophthalmol Clin 45:179–189
Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN (2001) Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet 358:295–296
Kaklamani VG, Kaklamanis PG (2001) Treatment of Behcet’s disease: an update. Semin Arthritis Rheum 30:299–312
Carmaschi P, Biasi D, Lombathi M et al (2006) Anti-TNF therapy in rheumatoid arthritis and autoimmunity. Rheum Int 26:209–214
Comby E, Tanaff P, Mariotte D et al (2006) Evolution of antinuclear antibodies and clinical patterns in patients with active rheumatoid arthritis with long term infliximab therapy. J Rheum 33:24–30
Louis M, Rauch J, Armstrong M, Fitzcharles MA (2003) Induction of autoantibodies during prolonged treatment with infliximab. J Rheumatol 30:2557–2562
Weill B, Kahan A (2004) Induction of autoantibodies in refractory rheumatoid arthritis treated by infliximab. Clin Exp Rheumatol 22:756–758
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Rayes, H., Al-Swailem, R., Al-Balawi, M. et al. Safety and efficacy of infliximab therapy in active behcet’s uveitis: an open-label trial. Rheumatol Int 29, 53–57 (2008). https://doi.org/10.1007/s00296-008-0606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-008-0606-8