Abstract
Two-component systems (TCSs) are diverse cell signaling pathways that play a significant role in coping with a wide range of environmental cues in both prokaryotic and eukaryotic organisms. These transduction circuitries are primarily governed by histidine kinases (HKs), which act as sensing proteins of a broad variety of stressors. To date, nineteen HK groups have been previously described in the fungal kingdom. However, the structure and distribution of these prominent sensing proteins were hitherto investigated in a limited number of fungal species. In this study, we took advantage of recent genomic resources in fungi to refine the fungal HK classification by deciphering the structural diversity and phylogenetic distribution of HKs across a large number of fungal clades. To this end, we browsed the genome of 91 species representative of different fungal clades, which yielded 726 predicted HK sequences. A domain organization analysis, coupled with a robust phylogenomic approach, led to an improved categorization of fungal HKs. While most of the compiled sequences were categorized into previously described fungal HK groups, some new groups were also defined. Overall, this study provides an improved overview of the structure, distribution, and evolution of HKs in the fungal kingdom.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histidine kinases (HK) have been described in prokaryotes, as well as serine/threonine kinases (S/TKs) and tyrosine kinases, and exhibit diverse cellular activities (Mascher et al. 2006). They have also been identified in amoebae, plants, fungi, viruses, and bacteriophages, where they assist in coping with external stresses (Osakabe et al. 2013; Hargreaves et al. 2014; Hérivaux et al. 2016; Schaap 2016; Galperin et al. 2018; Kabbara et al. 2019; Hoang et al. 2021).
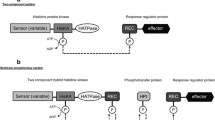
In prokaryotes, these proteins which function at the head of so-called “two-component phosphorelay systems” (TCSs) came into prominence in 1986 (Ninfa and Magasanik 1986; Nixon et al. 1986; Winans et al. 1986). The canonical structure of these proteins is composed of several domains. The N-terminal sequence, designated the “sensing domain”, represents the initial region of high variability that determines the signal perceived by the HK. The transmitter domain, located in the middle of the protein, is composed of the cognate dimerization/histidine phosphorylation (DHp) and histidine kinase-like ATPase catalytic (HATPase_c) subdomains. The H-box and X-box, which typically contain a phosphorylatable histidine, are components of the DHp domain (Jacob-Dubuisson et al. 2018). There are four types of DHp domains found to date in prokaryotic HK sensors. HisKA makes up ∼ 80% of DHp while HisKA_3, HisKA_2, or HWE-HK make up the remaining part.
Most of the TCSs are characterized by a two-step phosphorelay between a sensor HK and a downstream response regulator (RR) (Fig. 1). The active site, which catalyzes phosphorylation and dephosphorylation reactions of RRs (referred to as the receiver domain, REC), contains five conserved residues: a pair of acidic residues that bind a divalent cation (usually Mg2+), the Asp phosphorylation site, a Ser/Thr, and a Lys. The metal ion, the Ser/Thr, and the Lys coordinate the three oxygen atoms of the phosphoryl group (Bourret 2010; Gao et al. 2019). It is noteworthy that bacterial transcriptional regulators with DNA-binding domains make up more than two-thirds of all response regulators (Galperin et al. 2018). Eukaryotic TCSs usually entail sophisticated multi-step phosphorelays (Fig. 1). This system is adopted by fungi as key signal transduction mediators that sense a variety of environmental and intracellular cues, as well as host factors (Bahn 2008; Hérivaux et al. 2016). Unlike prokaryotic HKs, the majority of eukaryotic HKs possess an additional C-terminal REC domain. Therefore, eukaryotic HKs are typically designated as hybrid HKs (HHKs) (Papon and Stock 2019).
Domain organization and TCSs signaling. In most of the prokaryotic TCS systems, the HK is first autophosphorylated upon receipt of a signal and thus acts as a primary sensor. The phosphoryl group is then transferred to a RR. About two-thirds of bacterial RRs are transcriptional regulators for an adapted response. In eukaryotes, TCSs are usually composed of additional modules, including the histidine-containing phosphotransfer (Hpt) domain, and the signaling routes rely on a multi-step phosphorelay path between three families of proteins (HHK, Hpt, and RR). In most eukaryotic TCSs, the RR either acts directly as a transcription factor or regulates downstream responses
HKs are a class of proteins that exhibit considerable topological diversity across multiple domains. This sophisticated architecture has resulted in a wide range of functional properties. A number of domains previously described in bacterial and plant HKs were also identified in the N-terminal sensing regions of fungal proteins. These include the GAF domain (cGMP-specific phosphodiesterases-Adenylyl cyclases-FhlA) (Aravind and Ponting 1997), the PAS domain (Period circadian protein-Aryl hydrocarbon receptor nuclear translocator protein-Single-minded protein) (Stuffle et al. 2021; Xing et al. 2023), the HAMP domain (Histidine kinases-Adenylate cyclases-Methyl accepting proteins and Phosphatases) (Parkinson 2010), the S/TKrd domain (Serine/Threonine kinase-related domain), and the CHASE domain (Cyclase/Histidine kinase-Associated Sensing Extracellular) (Mougel & Zhulin 2001; Anantharaman and Aravind 2001; Hérivaux et al. 2017). The PAS domain is present in a number of signaling proteins, including HKs, S/TKs, and voltage-gated ion channels. It has been reported that PAS-containing HKs play a pivotal role in diverse aspects of fungal development, particularly in phytopathogenic fungi, in response to a wide range of environmental cues (Jacob et al. 2014; Shin et al. 2019). It is speculated that GAF and S_TKc (serine/threonine protein kinases) are thought to serve as signaling connectors in the regulation of HK enzymatic activity (Yamada-Okabe et al. 1999). In the fungal pathogen Candida albicans, these two functional domains have been found in the N-terminal region of CHK1 and have been recently shown to be involved in the invasiveness of hyphae in mucosal tissues (Liao et al. 2021; Feng et al. 2022).
Among fungal genera, the number of members and domain architectures in the group of HK-encoding genes is quite diverse, especially in the N-terminus sensing domain (Hérivaux et al. 2016). The expansion of genomic resources has recently enabled the identification of previously unknown HK family members in yeasts and molds, creating the opportunity to propose a complex taxonomy of the HKs identified in the Basidiomycota, the Ascomycota, and the Early Diverging Fungi (EDF). The increased use of advanced genetic techniques has provided new insights into the significance of several groups of HKs in prominent fungal diseases (Li et al. 2010). Eleven classes of fungal HKs were first proposed in the Ascomycota (Catlett et al. 2003). Over the years, a greater diversity of HKs has been identified in several fungal clades, notably in the Basidiomycota (Lavín et al. 2010, 2013, 2014) and more recently in the EDF (Hérivaux et al. 2017). According to Defosse and colleagues, fungi encompass a vast array of sensing proteins, which have been divided into sixteen groups, of which, groups III and X appear to be of particular importance for morphogenesis, stress adaptation, and virulence (Defosse et al. 2015). Other significant groups include ethylene receptors, CHASE-HK, AHK1/fungal group VI, and phytochromes (Papon and Stock 2019). The most recent classification in the Eukaryotes resulted in nineteen classes of fungal HKs, one of which (group XVII), had not been previously described (Kabbara et al., 2019). To date, HisKA is the only type of DHp domain that has been identified in the fungal kingdom.
Nevertheless, all these phylogenetic studies were based on the analysis of HKs predicted proteins in limited sets of fungal species or clades. Recently, the explosion of whole-genome sequencing in fungal species has provided an unprecedented opportunity for the identification of novel fungal HK structures, notably with the 1000 Fungal Genomes Project (http://1000.fungalgenomes.org). In this study, we sought to update our knowledge on the structural diversity and distribution of fungal HKs. Given that humans are devoid of such signaling systems, an understanding of the fungal kingdom repertoire of HKs could provide insights into new HK groups that may represent new avenues for antifungal therapy (Fihn and Carlson 2021).
Materials and methods
Analysis and annotation of HK sequences
To identify the HK protein sequences in fungal species, the HisKA (PFAM00512) and HATPase_c (PFAM02518) from the Consensus Protein Families Database were matched (BLASTP) against the NCBI and JGI genome sequence databases. A multiple alignment was performed using the Clustal Omega algorithm to eliminate redundant protein sequences. The domain structure was determined using the SMART algorithm (http://smart.embl-heidelberg.de/). Only putative HK sequences that contained at least two of the following three domains were retained: HisKA, HATPase_c, and REC (PFAM00072). The protein sequences of HKs were annotated using the first letter of the genus, followed by the first three letters of the species, HK, and the number of the sequence (e.g., WmelHK1 for Wallemia mellicola Histidine Kinase 1). To identify uncharacterized HKs, the putative HK sequences of each species were used as queries for TBLASTN searches against the fungal genome. All the HK sequences analyzed in this study are compiled in Supplementary Material File S1. Finally, the conserved H-box signature and the specific organization of the structured domains at the N-terminus were used to classify HK sequences into their respective groups based on previous classifications (Catlett et al. 2003; Lavín et al. 2010; Defosse et al. 2015; Kabbara et al. 2019).
Phylogenetic analysis
Multiple alignments were performed using ClustalOmega (v1.2.3) (Sievers et al. 2011). The resulting alignment was subsequently trimmed using trimAl (v1.4.rev15 build[2013-12-17]) (Capella-Gutiérrez et al. 2009). The complete phylogenetic tree was generated using IQ-tree (version 2.0.3) (Minh et al. 2020). The ModelFinder algorithm (Kalyaanamoorthy et al. 2017), included in IQ-tree, was used to identify the best-fit substitution model. A maximum likelihood (ML) tree was generated by IQ-tree using the LG + G4 substitution model. Ultra-fast bootstrapping (UFBoot) was employed for 1,000 iterations in the bootstrapping process. The consensus tree was visualized using the Interactive Tree of Life v5 (iToL, https://itol.embl.de) (Letunic and Bork 2021).
Results and discussion
In order to decipher the phylogenetic relationships among fungal HKs, we browsed the genomes of ninety-one species, which represent a diverse array of fungal clades (Fig. 2). As a result, 726 predicted protein sequences were compiled (Fig. 2). All the HK sequences analyzed in this study are compiled in Supplementary Material File S1. The phylogenetic tree analysis firstly highlighted that most of the deduced HK sequences could be classified into the previously described groups (Fig. 3), but interestingly, 38 predicted protein sequences could not be assigned to a defined group and were, therefore, considered as “unclassified”.
List of fungal species in which the presence of predicted HK sequences was investigated for this study. This dataset is provided as an Excel file Supplementary Material File S1
Phylogeny estimation of HK predicted protein sequences in fungi. All the HK sequences analyzed in this study are compiled in Supplementary Material File S1
Previously described HK groups
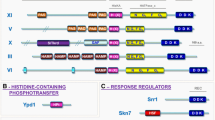
Group I
A total of 54 members of the fungal group I HKs were exclusively but broadly found in the Pezizomycotina (filamentous Ascomycota) (Fig. 3). While these predicted proteins commonly possess a single GAF-related domain within their N-terminus sensing region, these HKs remain highly divergent in the primary sequence, as previously described (Fig. 4). As previously suggested, this expansion may result from the dynamic evolution of a single-gene family in pluricellular ascomycetous species (Catlett et al. 2003). These proteins were primarily identified only in various clades of the Ascomycota. Here, predicted members are identified in representative genomes from species with highly diversified lifestyles and niches, including lichenized species, endophytes, epiphytes, ectomycorrhizal fungi, dimorphic human pathogens, human opportunist molds, nematode-trapping fungi, and plant pathogenic fungi. To date, few functional characterizations have been conducted for this fungal HK group. In the opportunistic mold Aspergillus fumigatus, which harbors five predicted members of group I fungal HKs, only one (phk6) was found to be involved in the regulation of the “fluffy” developmental program (Chapeland-Leclerc et al. 2015). Interestingly, the two major fungal pathogens of wheat, Zymoseptoria tritici and Fusarium verticillioides, also both contain five representatives, suggesting that group I HKs play an important role in environmental and pathogenic molds. In this respect, strains lacking the Mohik3 gene, which encodes a group I HK from the rice blast fungus Magnaporthe oryzae, showed reduced pathogenicity (Jacob et al. 2014).
Categorization of HKs in previously described groups of fungal sensors. This dataset and all the HK sequences analyzed in this study are compiled in Supplementary Material File S1. The conserved H-box signature and the specific organization of the structured domains at the N-terminus were used to classify HK sequences into their respective groups based on previous classifications (Catlett et al. 2003; Lavín et al. 2010; Defosse et al. 2015; Kabbara et al. 2019). For keys, refer to the caption in Fig. 1
Group II
Five fungal group II HKs were exclusively found in the Dothideomycetes, the Sordariomycetes, and the Orbiliomycetes (mostly nematode-trapping fungi). This finding is consistent with the results of previous studies (Catlett et al. 2003; Defosse et al. 2015; Kabbara et al. 2019) (Figs. 3 and 4). These proteins remain poorly investigated, and no function has yet been assigned. We must note that three predicted proteins in the peach leaf curl agent Taphrina deformans (TdefHK1-2-8) stand in sister branches of the fungal group II HKs. However, the divergence in the H-box sequence signature precludes a formal categorization of these sensors in the fungal group II HKs.
Group III
It is now well-documented that group III fungal HKs are widely distributed in the Ascomycota clades, in addition to the Basidiomycota and some EDF subphyla such as the Mucoromycota and the Zoopagomycota (Fig. 3). This is evidenced by the categorization of ninety-nine predicted proteins from our dataset into this fungal HK group (Fig. 4). Moreover, this group has undergone a significant expansion in the Entomophtoromycotina and the Mucoromycotina, as exemplified by Basidiobolus meristosporus, which has seven representatives, and by Lichtheimia corymbifera, which has five members. The most striking structural feature among group III HKs is a N-terminus cluster of a variable number of HAMP domains. These domains play a role in stress adaptation and regulate the downstream high osmolarity glycerol (HOG) pathway (Defosse et al. 2015; Yaakoub et al. 2022). Overall, group III HKs appear to be prominent mediators of morphogenesis, stress tolerance, antifungal susceptibility, and virulence in a broad panel of human-, plant-, and insect-pathogenic fungi (reviewed in Defosse et al. 2015). In molds, these sensors have pleiotropic functions including regulation of hyphal development and asexual reproduction, tolerance to osmotic stress and antifungals (Hagiwara et al. 2013; Chapeland-Leclerc et al. 2015; Defosse et al. 2015; Calcáneo-Hernández et al. 2023; Ren et al. 2024). In addition, this group of sensing proteins plays a crucial role in yeasts, including adaptation to oxidative stress, regulation of morphogenesis, and virulence in C. albicans and Cryptococcus neoformans (Yamada-Okabe et al. 1999; Bahn et al. 2006). Finally, in dimorphic fungi, group III HKs are also involved in a broad range of important physiological processes, including morphogenesis, drug susceptibility, and virulence (Nemecek et al. 2006; Boyce et al. 2011; Navarro et al. 2021).
Groups IV and V
The N-terminal regions of group IV and V HKs both contain the ligand-binding PAS/PAC (PAS-associated, C-terminal) sensor domains, yet the mechanistic involvement of these domains in fungi remains unclear (Fig. 4). The Eurotiomycetes and the Sordariomycetes were the only fungal clades in which the group IV fungal HKs have been identified thus far (9 predicted sequences were identified in our dataset). A unique study concerning this group of HKs dealt with A. fumigatus virulence (Clemons et al. 2002). Besides, the fungal group V HKs were found to be widely distributed in the Ascomycota molds (54 predicted proteins). In the plant pathogenic mold M. oryzae, these proteins are involved in a number of processes, including vegetative growth, conidiation, stress adaptation, and virulence (Jacob et al. 2014). In addition, PhkB in A. fumigatus was demonstrated to be involved in fungal development (Chapeland-Leclerc et al. 2015).
Group VI (primary fungal osmosensors)
The transmembrane osmosensors (group VI) were broadly represented in all Ascomycota clades, with the exception of Basidiomycota and EDF, which exhibited a paucity of this group (42 members in the dataset) (Fig. 4). This group includes the well-studied and unique HK Sln1 in Saccharomyces cerevisiae. Group VI HKs are known to belong to the TCS, which constitutes the main upstream branch that shuttles osmotic cues to the HOG_MAPK pathway in yeast (Hohmann 2002; Saito and Posas 2012; Salas-Delgado et al. 2017). In this regard, the phosphorylation of HOG1 (MAPK) is entirely dependent on Sln1 (Yaakoub et al. 2022). In addition to its primary role as an osmosensor, S. cerevisiae Sln1 has been shown to facilitate the transduction of numerous other stress signals, including oxidative stress, heat, acid, and ethanol (Yaakoub et al. 2022). In A. fumigatus, the loss of the gene encoding Sln1 (also referred to as TcsB) resulted in increased sensitivity to high temperature, sorbitol, and cell wall agents (Ji et al. 2012; Silva et al. 2020). Importantly, MoSln1 was demonstrated to mediate salt stress response, differentiation, and virulence in the phytopathogenic fungus M. oryzae (Jacob et al. 2014; Ryder et al. 2019).
Group VII
Fungal HKs from group VII (27 members in the dataset) were exclusively but broadly found in the Pezizomycotina (pluricellular Ascomycota) (Fig. 4). To date, the function of these proteins remains unclear in fungi, as group VII HKs gene mutant strains have been shown to exhibit no altered phenotype compared to wild-type strains (Chapeland-Leclerc et al. 2015). The predicted members are detected in representative genomes from species with highly diversified ways of life and niches that include lichenized species, endophytes, epiphytes, ectomycorrhizal fungi, dimorphic human pathogens, human opportunist molds, nematode-trapping fungi, and plant pathogenic fungi.
Group VIII (fungal phytochromes)
Group VIII HKs were represented in various fungal species (54 members in the dataset), encompassing Basidiomycota, Pezizomycotina (filamentous Ascomycota), and EDF. These fungi inhabit a vast array of environmental niches and hosts, including nematodes, insects, plants, and humans. It is predicted that species within this group can adapt to a variety of lifestyles, including saprobic, endophytic, opportunistic, parasitic, ectomycorrhizal, or lichen symbiotic (Figs. 2 and 4). Above all, group VIII HKs were primarily characterized as light-perceiving receptors known as phytochromes (Fph), which have dual functions depending on their cellular localization, and they mainly control red light perception in fungi (Blumenstein et al. 2005; Purschwitz et al. 2008, 2009; Bayram et al. 2010). Photoresponse has been extensively investigated across several fungal clades. The mutation of fphA in Alternaria alternata revealed that photoregulation affects germination, sporulation, and secondary metabolism (Igbalajobi et al. 2019). Moreover, phy1 mutants of Ustilago maydis were unable to form basidiocarps under red light illumination, suggesting that the protein plays a role in the perception of red light (Sánchez-Arreguin et al. 2020). A recent study in Aspergillus nidulans demonstrated that the light receptors mediating the red- and blue-light sensing interact with the HOG pathway. This was revealed by the expression of the stress-activated kinase (sakA) gene, which was essential for the induction of expression of approximately 100 genes by red light in the absence of phytochrome (Yu et al. 2021). It is noteworthy that, in response to light, the phytochrome FphA physically interacts with the histidine-containing phosphotransfer protein YpdA resulting in the phosphorylation of SakA and its translocation into nuclei (Yu et al. 2016).
Group IX
Fungal HKs from group IX were identified in the Ascomycota and the Basidiomycota, including a considerable number of species (46 members in the dataset). Many of these species are plant or insect pathogenic fungi (Figs. 2 and 4). To date, the function of group IX HKs remains unknown, as the deletion of MoHik2 in M. oryzae did not affect the development of this fungus (Jacob et al. 2014; Defosse et al. 2015).
Group X (S/TK domain-containing fungal HKs)
The fungal group X and its sub-groups (X-A, X-B, X-C) represent a polyphyletic series of large proteins with a typical N-terminus consisting of a GAF domain and a S/TK domain (Aravind and Ponting 1997) (Figs. 3 and 4). These proteins, similar to group III HKs, regulate essential physiological processes in pathogenic fungi, including morphogenesis, virulence, and oxidant adaptation (Defosse et al. 2015; Hérivaux et al. 2016). Group X-A HKs (13 members in the dataset) were predominantly identified in saprobic or ectomycorrhizal fungi belonging to the Ascomycota, the Basidiomycota, and the Umbelopsidales (Mucoromycota). Group X-B (31 members in the dataset) includes HKs predicted proteins of dimorphic or filamentous fungi in the Ascomycota (Pezizomycotina); and, interestingly, a predicted protein related to this group was found in Rozella allomycis (Cryptomycota), an obligate parasite of the Blastocladiomycota (Hérivaux et al. 2017). Fungal HKs from group X-C (37 members in the dataset) were identified in saprobic or opportunistic pathogens belonging to the Ascomycota, the Basidiomycota, and the Mucoromycota. Among the predicted members, this HK group is predominantly identified in plant pathogens and a limited number of human opportunistic species. In A. fumigatus, PhkA has been shown to be involved in the regulation of conidiation and the resistance to oxidative stresses (Chapeland-Leclerc et al. 2015). In C. albicans, the disruption of the cahk1 gene (equivalent to CaHK2 in our dataset study) altered hyphal formation and virulence (Yamada-Okabe et al. 1999). In contrast to C. albicans, the growth was not affected in Mohik6 mutant strains of M. oryzae but hyphae were demonstrated to be more resistant to lytic enzymes (Jacob et al. 2014).
Group XI
Group XI fungal HKs, previously described as highly divergent (Catlett et al. 2003), were widely represented in the Ascomycota (54 members in the dataset) including lichenized species, and animal or plant pathogens (Figs. 2 and 4). Among these HKs, MoHik8p was reported to be crucial for conidial development and hence pathogenicity-related morphogenesis in the plant pathogenic mold M. oryzae (Jacob et al. 2014).
Group XII (dual HKs)
Group XII (Dual HKs) are large HKs characterized by tandem duplication of two complete sets of hybrid kinase machinery: two HK transmitter modules (T) and two RR receiver domains (R) arranged in a TRTR configuration in a single polypeptide (Lavín et al. 2014) (Fig. 4). These sensors were initially believed to be restricted to the Basidiomycota. This had to be revised since related predicted proteins were recently identified in EDF (Hérivaux et al. 2017). Here, our phylogenetic analysis suggested that fungal dual HKs can be divided into three main subgroups (XII-A, XII-B, XII-C) (Fig. 3). Our compilation showed the presence of 9 members related to the XII-A subgroup in filamentous ectomycorrhizal species and basidiomycetous yeasts of the Basidiomycota (Fig. 4). Interestingly, we identified, for the first time, related sequences in the early diverging branches of the Ascomycota (Protomyces lactucae-debilis, Taphrinales). This could indicate that dual HKs have emerged in a common ancestor of dikarya but have not been retained during the evolution of Pezizomycotina (filamentous Ascomycota). The group XII-B included predicted proteins (16 members in the dataset) only found in the Mucoromycota, including 7 members in the endosymbiont of cyanobacteria Geosiphon pyriformis (Fig. 4). Interestingly, a new subgroup XII-C was exclusively found in the yeast Rhodotorula graminis and the filamentous fungus Puccinia graminis that belong to the Pucciniomycotina (Fig. 4). Although the group XII HK Tco2 from C. neoformans contributes to the regulation of the HOG pathway, the topology of the signaling pathways mediated by group XII HKs is still relatively unknown and has not been investigated yet (Bahn et al. 2006).
Group XIII
Group XIII fungal HKs were historically identified in C. neoformans (CneoHK5 = Tco5) (Bahn et al. 2006). Here, we identified 14 members in species belonging to Basidiomycota (Pucciniomycotina, Agaricales, Boletales, Dacrymycetes, and Tremellomycetes) and early diverging Ascomycota (Taphrinales) (Fig. 4). To date, the function of group XIII fungal HKs remains unknown, as the deletion of Tco5 in C. neoformans did not affect any phenotype of this fungus (Bahn et al. 2006).
Group XIV
The predicted groups XIV-A (10 members in the dataset) and XIV-B HKs (14 members in the dataset) were limited to the Basidiomycota, which includes saprobic species or those living in association with plants (pathogenic or ectomycorrhizal) (Fig. 4). Interestingly, a mutant strain of the sugarcane smut fungus Sporisorium scitamineum, which lacks a putative group XIV HK, exhibited enhanced mating and virulence capabilities (Cai et al. 2021).
Group XV and XVI (MS-HKI and MS-HKII)
The group XV HKs (also referred to as MS-HKI, Defosse et al. 2015) were exclusively identified in the EDF (11 members in the dataset), specifically in the Glomeromycotina, Mortierellomycotina, and Cryptomycota (Fig. 4). Besides, group XVI HKs (MS-HKII) were restricted to filamentous saprobic species belonging to the Mucorales and Mortierellales (11 members in the dataset) (Fig. 4). However, the function of these HKs remains unassigned.
Group XVII
The recently described group XVII of fungal HKs (Kabbara et al. 2019, 6 members in the dataset) was sporadically found in Orbiliomycetes, Dacrymycetes, Chaetothyriales, Lecanorales, and Puccinomycotina (Fig. 4) but no specific function has yet been allocated to these HKs.
Group XVII (ethylene receptors)
In the EDF, the newly identified fungal HK group homologous to the plant ethylene receptor family (Hérivaux et al. 2017) appears to have arisen from a polyphyletic origin, as these sensors were clustered into two distinct sub-groups, XVIII-A (3 members in the dataset) and XVIII-B (12 members in the dataset). These receptors are primarily identified in saprobic, aquatic, or parasitic fungal species (Fig. 4). Importantly, a candidate-based approach has recently demonstrated that group XVIII-A HKs in Rhizophagus irregularis are bona fide ethylene receptors in arbuscular mycorrhizal fungi that inhabit the root cortical cells of most plants (Mongès et al. 2023).
Group XIX (CHASE domain-containing HKs)
Finally, the CHASE domain-containing HKs (referred to as group XIX) tended to cluster together, suggesting a monophyletic origin (9 members in the dataset) (Fig. 4). This sensor group, which was previously believed to act as cytokinin receptors, has recently been demonstrated not to be involved in sensing these phytohormones in R. irregularis (Mongès et al. 2023). The function of this group of sensors remains therefore fully unknown.
New HK groups
In light of the increasing number of whole-genome sequences of fungal species available in publicly accessible repositories, we have updated the categorization of HKs in fungi. Nonetheless, the Pneumocystidiomycetes and the Microsporidia lineages were excluded from the present analysis due to the absence of HK-encoding genes resulting from substantial genome reduction (Defosse et al. 2015; Hérivaux et al. 2016). The present compilation provides evidence for the emergence of new structures in divergent species within the fungal phylogeny. This has led to multigene families that have arisen through duplication, as well as the formation of novel structures that have not been previously described. In this regard, we have identified six novel groups of fungal HKs (XX-XXV) (Fig. 5).
For instance, group XX and XXIV HKs (4 and 11 members, respectively) were exclusively identified in the EDF. These HKs possessed a sensing region comprising multiple predicted hydrophobic helices, which may suggest that they are predominantly membrane-bound sensors and that they may sense external stimuli. However, we never detected any significant homologies with other N-termini regions of prokaryotic or eukaryotic HKs, precluding any hypothetical function for XX and XXIV HKs.
The group XXI HKs were also found to be restricted to the EDF (12 members). These HKs were identified in Catenaria anguillulae, a nematode pathogen in the Blastocladiomycota, but were mainly found in the Entomophthoromycotina (Fig. 5). Besides, the group XXII HKs (8 members) were exclusively detected and expanded in the aquatic fungus Gonapodya prolifera belonging to the Chytridiomycota (Fig. 5). Sensing regions of both groups XXI and XXII HKs contain the ligand-binding PAS domains, however, once again, the lack of homologies with other previously characterized HK sequences did not allow any functional prediction for these new groups of fungal sensors.
Fungal HKs from group XXIII were only observed in 4 species of the dataset including the club-like ascomycetes Geoglossum umbratile, the truffle-like ascomycetes Leucangium carthusianum, in the yellow morel Morchella americana, and in pine root endophytic EDF Umbelopsis ramanniana. This patchy distribution in the fungal phylogeny is intriguing insofar these group XXIII HKs are present in few but distant species with distinct ecologies (saprobic, ectomycorrhizal, and endophytic) (Fig. 5). Interestingly, we detected significant homologies between the N-terminus region of group XXIII fungal HKs and some bacterial HKs displaying a MEthanogen/methylotroph DcmR Sensory (MEDS) domain. As a consequence, it could be possible that group XXIII fungal HKs could act as hydrocarbon derivatives as in bacteria (Anantharaman and Aravind 2005) but this remains to be functionally demonstrated.
Finally, group XXV HKs were identified exclusively in the filamentous saprobic species of Mortierellomycotina. These proteins exhibit a GAF domain within their N-terminus. However, we never detected any significant homologies with other prokaryotic or eukaryotic HKs (Fig. 5).
Conclusion
This study extends previous research on the distribution and classification of fungal HKs (Catlett et al. 2003; Lavín et al. 2010; Defosse et al. 2015; Hérivaux et al. 2017; Kabbara et al. 2019) by carrying out a robust phylogenetic analysis on a vast array of predicted sequences. This analysis has led to a more refined categorization of these important sensing proteins. Moreover, we consolidated the monophyletic features of previously described groups, as well as the polyphyletic origins of other groups (e.g., X, XII, and XIV). It was also of interest to shed light on fungal members belonging to new HK groups but for which the function remains fully unknown (e.g., XX, XXI, XXII, XXIII, XXIV, and XXV). This analysis revealed that a number of HK groups were phylum-specific. For example, groups VI and XI were exclusively found in the Ascomycota, while groups I and VII were exclusively found in the Pezizomycotina. Of note, some HK groups (e.g., III) have certainly emerged early in the evolution of fungi and have been maintained in nearly all fungal phyla. Although this study led to the identification of hypothetical associations between some HK groups and specific fungal lifestyles, further functional analysis will be necessary to determine the relevance of these observations. Thus, in the near future, it would be important to intensify research in order to gain further insight into the function of certain HK groups for which these aspects have never been addressed. The emergence of novel technologies, such as CRISPR/Cas9 systems and fluorescent protein fusion strategies, will likely facilitate the acquisition of new data concerning the function and the subcellular localization/dynamics of the respective HK groups (Cairns et al. 2016). Designing such novel approaches is essential to advance our understanding of the involvement of fungal HKs in regulating many aspects of host-pathogen interactions and in facilitating the adaptation of fungi to specific niches.
Data availability
No datasets were generated or analysed during the current study.
References
Anantharaman V, Aravind L (2001) The CHASE domain: a predicted ligand-binding module in plant cytokinin receptors and other eukaryotic and bacterial receptors. Trends Biochem Sci 26(10):579–582. https://doi.org/10.1016/s0968-0004(01)01968-5
Anantharaman V, Aravind L (2005) MEDS and PocR are novel domains with a predicted role in sensing simple hydrocarbon derivatives in prokaryotic signal transduction systems. Bioinformatics 21(12):2805–2811. https://doi.org/10.1093/bioinformatics/bti418
Aravind L, Ponting CP (1997) The GAF domain: an evolutionary link between diverse phototransducing proteins. Trends Biochem Sci 22(12):458–459. https://doi.org/10.1016/s0968-0004(97)01148-1
Bahn YS (2008) Master and commander in fungal pathogens: the two-component system and the HOG signaling pathway. Eukaryot Cell 7(12):2017–2036. https://doi.org/10.1128/EC.00323-08
Bahn YS, Kojima K, Cox GM, Heitman J (2006) A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol Biol Cell 17:3122–3135. https://doi.org/10.1091/mbc.E06-02-0113
Bayram O, Braus GH, Fischer R, Rodriguez-Romero J (2010) Spotlight on aspergillus nidulans photosensory systems. Fungal Genet Biol 47(11):900–908. https://doi.org/10.1016/j.fgb.2010.05.008
Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R (2005) The aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15:1833–1838. https://doi.org/10.1016/j.cub.2005.08.061
Bourret RB (2010) Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol 13(2):142–149. https://doi.org/10.1016/j.mib.2010.01.015
Boyce KJ, Schreider L, Kirszenblat L, Andrianopoulos A (2011) The two-component histidine kinases DrkA and SlnA are required for in vivo growth in the human pathogen Penicillium Marneffei. Mol Microbiol 82:1164–1184. https://doi.org/10.1111/j.1365-2958.2011.07878.x
Cai E, Sun S, Deng Y, Huang P, Sun X, Wang Y, Chang C, Jiang Z (2021) Histidine kinase Sln1 and cAMP/PKA signaling pathways antagonistically regulate sporisorium scitamineum mating and virulence via transcription factor Prf1. J Fungi 7(8):610. https://doi.org/10.3390/jof7080610
Cairns TC, Studholme DJ, Talbot NJ, Haynes K (2016) New and improved techniques for the study of pathogenic fungi. Trends Microbiol 24(1):35–50. https://doi.org/10.1016/j.tim.2015.09.008
Calcáneo-Hernández G, Landeros-Jaime F, Cervantes-Chávez JA, Mendoza-Mendoza A, Esquivel-Naranjo EU (2023) Osmotic stress responses, Cell Wall Integrity, and Conidiation are regulated by a histidine kinase sensor in Trichoderma Atroviride. J Fungi (Basel) 9(9):939. https://doi.org/10.3390/jof9090939
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25(15):1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Catlett NL, Yoder OC, Turgeon BG (2003) Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot Cell 2(6):1151–1161. https://doi.org/10.1128/EC.2.6.1151-1161.2003
Chapeland-Leclerc F, Dilmaghani A, Ez-Zaki L et al (2015) Systematic gene deletion and functional characterization of histidine kinase phosphorelay receptors (HKRs) in the human pathogenic fungus aspergillus fumigatus. Fungal Genet Biol 84:1–11. https://doi.org/10.1016/j.fgb.2015.09.005
Clemons KV, Miller TK, Selitrennikoff CP, Stevens DA (2002) fos-1, a putative histidine kinase as a virulence factor for systemic aspergillosis. Med Mycol 40(3):259–262. https://doi.org/10.1080/mmy.40.3.259.262
Defosse TA, Sharma A, Mondal AK et al (2015) Hybrid histidine kinases in pathogenic fungi. Mol Microbiol 95(6):914–924. https://doi.org/10.1111/mmi.12911
Feng Y, Bian S, Pang Z, Wen Y, Calderone R, Li D, Shi D (2022) Deletion of non-histidine domains of histidine kinase CHK1 diminishes the infectivity of Candida albicans in an oral mucosal model. Front Microbiol 13:855651. https://doi.org/10.3389/fmicb.2022.855651
Fihn CA, Carlson EE (2021) Targeting a highly conserved domain in bacterial histidine kinases to generate inhibitors with broad spectrum activity. Curr Opin Microbiol 61:107–114. https://doi.org/10.1016/j.mib.2021.03.007
Galperin MY, Makarova KS, Wolf YI, Koonin EV (2018) Phyletic distribution and Lineage-Specific Domain Architectures of Archaeal Two-Component Signal Transduction Systems. J Bacteriol 200(7):e00681–e00617. https://doi.org/10.1128/JB.00681-17
Gao R, Bouillet S, Stock AM (2019) Structural basis of Response Regulator function. Annu Rev Microbiol 8:73:175–197. https://doi.org/10.1146/annurev-micro-020518-115931
Hagiwara D, Takahashi-Nakaguchi A, Toyotome T, Yoshimi A, Abe K, Kamei K et al (2013) NikA/TcsC histidine kinase is involved in Conidiation, Hyphal morphology, and responses to osmotic stress and Antifungal Chemicals in aspergillus fumigatus. PLoS ONE 8(12):e80881. https://doi.org/10.1371/journal.pone.0080881
Hargreaves KR, Kropinski AM, Clokie MR (2014) What does the talking? Quorum sensing signalling genes discovered in a bacteriophage genome. PLoS ONE 9(1):e85131. https://doi.org/10.1371/journal.pone.0085131
Hérivaux A, So YS, Gastebois A et al (2016) Major Sensing Proteins in pathogenic Fungi: the hybrid histidine kinase family. PLoS Pathog 12(7):e1005683. https://doi.org/10.1371/journal.ppat.1005683
Hérivaux A, Dugé de Bernonville T, Roux C, Clastre M, Courdavault V, Gastebois A, Bouchara JP, James TY, Latgé JP, Martin F, Papon N (2017) The identification of phytohormone receptor homologs in early diverging Fungi suggests a role for plant sensing in Land colonization by Fungi. mBio 8(1):e01739–e01716. https://doi.org/10.1128/mBio.01739-16
Hoang XLT, Prerostova S, Thu NBA, Thao NP, Vankova R, Tran LP (2021) Histidine kinases: diverse functions in Plant Development and responses to environmental conditions. Annu Rev Plant Biol 72:297–323. https://doi.org/10.1146/annurev-arplant-080720-093057
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66(2):300–372. https://doi.org/10.1128/MMBR.66.2.300-372.2002
Igbalajobi O, Yu Z, Fischer R (2019) Red- and Blue-Light sensing in the Plant Pathogen Alternaria alternata depends on phytochrome and the White-Collar protein LreA. mBio 10(2):e00371–e00319. https://doi.org/10.1128/mBio.00371-19
Jacob S, Foster AJ, Yemelin A, Thines E (2014) Histidine kinases mediate differentiation, stress response, and pathogenicity in Magnaporthe oryzae. Microbiologyopen 3(5):668–687. https://doi.org/10.1002/mbo3.197
Jacob-Dubuisson F, Mechaly A, Betton JM, Antoine R (2018) Structural insights into the signalling mechanisms of two-component systems. Nat Rev Microbiol 16(10):585–593. https://doi.org/10.1038/s41579-018-0055-7
Ji Y, Yang F, Ma D, Zhang J, Wan Z, Liu W, Li R (2012) HOG-MAPK signaling regulates the adaptive responses of aspergillus fumigatus to thermal stress and other related stress. Mycopathologia 174(4):273–228. https://doi.org/10.1007/s11046-012-9557-4
Kabbara S, Hérivaux A, Dugé de Bernonville T, Courdavault V, Clastre M, Gastebois A, Osman M, Hamze M, Cock JM, Schaap P, Papon N (2019) Diversity and Evolution of Sensor Histidine Kinases in Eukaryotes. Genome Biol Evol 1;11(1):86–108. https://doi.org/10.1093/gbe/evy213
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14(6):587–589. https://doi.org/10.1038/nmeth.4285
Lavín JL, Ramírez L, Ussery DW, Pisabarro AG, Oguiza JA (2010) Genomic analysis of two-component signal transduction proteins in basidiomycetes. J Mol Microbiol Biotechnol 18(2):63–73. https://doi.org/10.1159/000277654
Lavín JL, García-Yoldi A, Ramírez L, Pisabarro AG, Oguiza JA (2013) Two-component signal transduction in Agaricus Bisporus: a comparative genomic analysis with other basidiomycetes through the web-based tool BASID2CS. Fungal Genet Biol 55:77–84. https://doi.org/10.1016/j.fgb.2012.09.012
Lavín JL, Sarasola-Puente V, Ramírez L, Pisabarro AG, Oguiza JA (2014) Dual-histidine kinases in basidiomycete fungi. C R Biol 337(2):111–116. https://doi.org/10.1016/j.crvi.2013.12.007
Letunic I, Bork P (2021) Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49(W1):W293–W296. https://doi.org/10.1093/nar/gkab301
Li D, Agrellos OA, Calderone R (2010) Histidine kinases keep fungi safe and vigorous. Curr Opin Microbiol 13(4):424–430. https://doi.org/10.1016/j.mib.2010.04.007
Liao B, Ye X, Chen X, Zhou Y, Cheng L, Zhou X, Ren B (2021) The two-component signal transduction system and its regulation in Candida albicans. Virulence 12(1):1884–1899. https://doi.org/10.1080/21505594.2021.1949883
Mascher T, Helmann JD, Unden G (2006) Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev 70(4):910–938. https://doi.org/10.1128/MMBR.00020-06
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol 37(5):1530–1534. https://doi.org/10.1093/molbev/msaa015. Erratum in: Mol Biol Evol. 2020;37(8):2461
Mongès A, Yaakoub H, Bidon B et al (2023) Are histidine kinases of Arbuscular Mycorrhizal Fungi involved in the response to Ethylene and Cytokinins? Mol Plant Microbe Interact 36(10):656–665. https://doi.org/10.1094/MPMI-05-23-0056-R
Mougel C, Zhulin IB (2001) CHASE: an extracellular sensing domain common to transmembrane receptors from prokaryotes, lower eukaryotes and plants. Trends Biochem Sci 26(10):582–584. https://doi.org/10.1016/s0968-0004(01)01969-7
Navarro MV, de Barros YN, Segura WD, Chaves AFA, Jannuzzi GP, Ferreira KS, Xander P, Batista WL (2021) The role of Dimorphism regulating histidine kinase (Drk1) in the pathogenic Fungus paracoccidioides brasiliensis Cell Wall. J Fungi (Basel) 7(12):1014. https://doi.org/10.3390/jof7121014
Nemecek JC, Wüthrich M, Klein BS (2006) Global Control of Dimorphism and Virulence in Fungi. Science 312:583–588. https://doi.org/10.1126/science.1124105
Ninfa AJ, Magasanik B (1986) Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A 83(16):5909–5913. https://doi.org/10.1073/pnas.83.16.5909
Nixon BT, Ronson CW, Ausubel FM (1986) Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A 83(20):7850–7854. https://doi.org/10.1073/pnas.83.20.7850
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2013) Sensing the environment: key roles of membrane-localized kinases in plant perception and response to abiotic stress. J Exp Bot 64(2):445–458. https://doi.org/10.1093/jxb/ers354
Papon N, Stock AM (2019) Two-Component systems. Curr Biol 29(15):R724–r725. https://doi.org/10.1016/j.cub.2019.06.010
Parkinson JS (2010) Signaling mechanisms of HAMP domains in chemoreceptors and sensor kinases. Annu Rev Microbiol 64:101–122. https://doi.org/10.1146/annurev.micro.112408.134215
Purschwitz J, Müller S, Kastner C, Schöser M, Haas H, Espeso EA et al (2008) Functional and physical interaction of blue and red-light sensors in aspergillus nidulans. Curr Biol 18:255–259. https://doi.org/10.1016/j.cub.2008.01.061
Purschwitz J, Müller S, Fischer R (2009) Mapping the interaction sites of aspergillus nidulans phytochrome FphA with the global regulator VeA and the white collar protein LreB. Mol Genet Genomics 281:35–42. https://doi.org/10.1007/s00438-008-0390-x
Ren W, Han W, Huan T et al (2024) A new point mutation (D1158N) in histidine kinase Bos1 confers high-level resistance to fludioxonil in field gray mold disease. Pestic Biochem Physiol 198:105750. https://doi.org/10.1016/j.pestbp.2023.105750
Ryder LS, Dagdas YF, Kershaw MJ, Venkataraman C, Madzvamuse A, Yan X, Cruz-Mireles N, Soanes DM, Oses-Ruiz M, Styles V, Sklenar J, Menke FLH, Talbot NJ (2019) A sensor kinase controls turgor-driven plant infection by the rice blast fungus. Nature 574(7778):423–427. https://doi.org/10.1038/s41586-019-1637-x
Saito H, Posas F (2012) Response to hyperosmotic stress. Genetics 192(2):289–318. https://doi.org/10.1534/genetics.112.140863
Salas-Delgado G, Ongay-Larios L, Kawasaki-Watanabe L, Lopez-Villasenor I, Coria R (2017) The yeasts phosphorelay systems: a comparative view. World J Microbiol Biotechnol 33(6):111. https://doi.org/10.1007/s11274-017-2272-z
Sánchez-Arreguin JA, Cabrera-Ponce JL, León-Ramírez CG, Camargo-Escalante MO, Ruiz-Herrera J (2020) Analysis of the photoreceptors involved in the light-depending basidiocarp formation in Ustilago maydis. Arch Microbiol 202(1):93–103. https://doi.org/10.1007/s00203-019-01725-w
Schaap P (2016) Evolution of developmental signalling in Dictyostelid social amoebas. Curr Opin Genet Dev 39:29–34. https://doi.org/10.1016/j.gde.2016.05.014
Shin JH, Gumilang A, Kim MJ, Han JH, Kim KS (2019) A PAS-Containing histidine kinase is required for Conidiation, Appressorium formation, and Disease Development in the Rice Blast Fungus, Magnaporthe oryzae. Mycobiology 47(4):473–482. https://doi.org/10.1080/12298093.2019.1689037
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Silva LP, Frawley D, Assis LJ, Tierney C, Fleming AB, Bayram O, Goldman GH (2020) Putative membrane receptors contribute to activation and efficient signaling of mitogenactivated protein kinase cascades during adaptation of aspergillus fumigatus to different stressors and carbon sources. mSphere 5(5):e00818–e00820. https://doi.org/10.1128/mSphere.00818-20
Stuffle EC, Johnson MS, Watts KJ (2021) PAS domains in bacterial signal transduction. Curr Opin Microbiol 61:8–15. https://doi.org/10.1016/j.mib.2021.01.004
Winans SC, Ebert PR, Stachel SE, Gordon MP, Nester EW (1986) A gene essential for agrobacterium virulence is homologous to a family of positive regulatory loci. Proc Natl Acad Sci USA 83(21):8278–8282
Xing J, Gumerov VM, Zhulin IB (2023) Origin and functional diversification of PAS domain, a ubiquitous intracellular sensor. Sci Adv 9(35):eadi4517. https://doi.org/10.1126/sciadv.adi4517
Yaakoub H, Sanchez NS, Ongay-Larios L, Courdavault V, Calenda A, Bouchara JP, Coria R, Papon N (2022) The high osmolarity glycerol (HOG) pathway in fungi. Crit Rev Microbiol 48(6):657–695. https://doi.org/10.1080/1040841X.2021.2011834
Yamada-Okabe T, Mio T, Ono N, Kashima Y, Matsui M, Arisawa M, Yamada-Okabe H (1999) Roles of three histidine kinase genes in hyphal development and virulence of the pathogenic fungus Candida albicans. J Bacteriol 181:7243–7247. https://doi.org/10.1128/jb.181.23.7243-7247.1999
Yu Z, Armant O, Fischer R (2016) Fungi use the SakA (HogA) pathway for phytochrome-dependent light signalling. Nat Microbiol 1:16019. https://doi.org/10.1038/nmicrobiol.2016.19
Yu Z, Streng C, Seibeld RF, Igbalajobi OA, Leister K, Ingelfinger J, Fischer R (2021) Genome-wide analyses of light-regulated genes in aspergillus nidulans reveal a complex interplay between different photoreceptors and novel photoreceptor functions. PLoS Genet 17(10):e1009845. https://doi.org/10.1371/journal.pgen.1009845
Acknowledgements
We acknowledge the GenOuest bioinformatics core facility (https://www.genouest.org) for providing the computing infrastructure.
Author information
Authors and Affiliations
Contributions
S.M. and N.P. conceptualized and designed the study. S.M., A.H., M.W., and N.P. analyzed data. S.M., H.Y., V.C., and N.P. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mina, S., Hérivaux, A., Yaakoub, H. et al. Structure and distribution of sensor histidine kinases in the fungal kingdom. Curr Genet 70, 17 (2024). https://doi.org/10.1007/s00294-024-01301-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00294-024-01301-w