Abstract
Bacterial cells are critically dependent on their ability to divide. The process of division is carried out by a large and highly dynamic molecular machine, known as the divisome. An understanding of the divisomes’ architecture is highly sought after, as it is essential for understanding molecular mechanisms and potentially designing antibiotic molecules that curb bacterial growth. Our current view, which is mainly based on high-resolution imaging of Escherichia coli, is that it is a patchy ring or toroid structure. However, recent super-resolution imaging has shown that the toroid structure contains at least three concentric rings, each containing a different set of proteins. Thus, the emerging picture is that the divisome has different functional modules that are spatially separated in concentric rings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In the model Gram-negative bacterium Escherichia coli, the divisome is assembled from more than 30 different proteins (Adams and Errington 2009; Egan and Vollmer 2013; Haeusser and Margolin 2016; Lutkenhaus et al. 2012; Meier and Goley 2014). A third of these proteins (FtsZ, FtsA, ZipA, FtsK, FtsQ, FtsL, FtsB, FtsW, FtsI, and FtsN) are generally counted as the core of the divisome, as they are essential for division, and as a consequence, for cell viability (Haeusser and Margolin 2016). The essential division proteins are either cytoplasmic or inner membrane proteins, and their assembly at the division site has been well studied (see Goehring and Beckwith 2005). It is initiated by the arrival of thousands of copies of FtsZ, FtsA, and ZipA, which form a dynamic ring structure that is commonly referred to as the proto-ring (Erickson et al. 2010; Rico et al. 2013). This ring then acts as an assembly platform for the remaining division proteins, and most likely, also provides some of the force required to constrict the cell envelope (Ortiz et al. 2016). In a second step of assembly, FtsK, FtsQ, FtsL, FtsB, FtsW, FtsI, and FtsN are recruited (Aarsman et al. 2005). These proteins are involved in chromosome segregation and remodelling of the peptidoglycan layer. The latter is important for constriction of the cell envelope and scission of the daughter cells.
Structural information on the architecture of the divisome has been a long-standing goal of the cell division community. Unfortunately, the size, complexity, membrane location, and highly dynamic nature of the divisome make it extremely challenging to purify as a whole, and, therefore, to study by the traditional structural approaches, such as X-ray crystallography or cryo-electron microscopy. Our current understanding of the divisome structure is based on super-resolution imaging of fluorescently labelled FtsZ, FtsA, and ZipA, which indicates a patchy ring or toroid structure (Coltharp et al. 2016; Fu et al. 2010; Holden et al. 2014; Jacq et al. 2015; Rowlett and Margolin 2014, 2015; Strauss et al. 2012). It has been largely assumed that most other divisome proteins are in the toroid, because protein–protein interaction studies have indicated a high level of interconnectivity between divisome proteins (reviewed in Egan and Vollmer 2013). However, new super-resolution fluorescence microscopy data have provided some thought provoking insight: is the toroid composed of more than a single ring?
The first indication that the toroid structure may be more complex than a single ring came from studies done by Galli and Gerdes, who co-expressed FtsZ-GFP together with the non-essential divisome protein ZapB-mCherry in E. coli. Their subsequent high-resolution 3D reconstruction images showed that the signal from ZapB was inside of the signal from FtsZ, indicating that ZapB formed a concentric ring inside the ring containing FtsZ (Galli and Gerdes 2010). This conclusion was supported by two-colour PhotoActivated Localization Microscopy (PALM) imaging of E. coli cells co-expressing FtsZ-PAmCherry and ZapB-Dronpa (Buss et al. 2015). The latter study also showed that MatP, another non-essential divisome protein, was inside of ZapB, thus, indicating that there might be two rings (or layers) inside of the ring containing FtsZ.
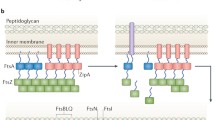
Additional support for the concept that the divisome could be more that a single ring was obtained when we co-expressed pairs of the core divisome proteins in E. coli, with either a GFP or mCherry tag, then used dual-colour super-resolution Structured Illumination Microscopy (SIM) to image these proteins at the division site (Söderström et al. 2016). The resulting images crisply showed that the fluorescence signal from FtsZ, FtsA, and ZipA always overlapped, consistent with their assembly in a single ring (see also Rowlett and Margolin 2014). However, the fluorescence signals from FtsQ, FtsL, FtsI, and FtsN were approximately 100 nm wider (Fig. 1a). The interpretation of these 2D images is that the enzymes involved in peptidoglycan biosynthesis form a concentric ring that is located outside of the ring containing FtsZ.
The E. coli divisome can be visualized as concentric rings. a Time-lapse SIM images of the division septum in a cell co-expressing FtsZ-mCherry (red) and GFP-FtsN (green). The images clearly show that the signal from FtsZ-mCherry is inside that of GFP-FtsN throughout constriction. ‘T’ indicates the time in minutes lapsed since the first image. Scale bar 0.2 μm and dotted lines roughly represent the cell outline. Beneath each image are line scans through a cross section of the divisome (drawn as dashed line at T = 0). Image reprinted with permission from (Söderström et al. 2016). b Schematic representation of concentric divisome rings observed in E. coli. Left panel pairwise imaging of fluorescently labelled divisome proteins, carried out in three different studies (Buss et al. 2015; Galli and Gerdes 2010; Söderström et al. 2016), has indicated that some pairs of divisome proteins are spatially separated at the septum. Right panel the pairwise images represent concentric rings containing different sets of proteins. Note that only divisome proteins that have been assigned to a particular ring are shown
Taken together, the available data suggest that the structure of the divisome is a toroid made up of concentric rings (Fig. 1b). Intriguingly each ring layer appears to have a specific complement of proteins, indicating that it has a specific functional role; the inner layer(s) appears to have a regulatory role, since it contains ZapB and MatP. The middle proto-ring is the assembly scaffold; this ring may also provide some of the force necessary for constriction, as it contains FtsZ, FtsA, and ZipA. And finally, there is the outer ring, containing FtsQ, FtsL, FtsI, and FtsN, which are involved in peptidoglycan synthesis and ingrowth.
The reason(s) why the modular organisation of the divisome has eluded detection for so long can be attributed to two major factors: (1) it requires dual-colour labelling of divisome proteins, and (2) super-resolution imaging to unambiguously resolve them. With this knowledge in hand, it should now be possible to refine the existing low-resolution map, and, importantly, to validate key findings using approaches that probe the native divisome, such as Immuno-Fluorescence Microscopy (IFM). Outstanding questions that spring to mind are related to whether other divisome proteins, such as those in the periplasm and outer membrane, are assembled into the known rings? Or whether they form separate rings that are yet to be observed? Can the various rings be reconstituted in vitro? And how does the composition of each ring change during constriction? Regarding the latter point, it is already known that the proto-ring is a highly dynamic entity with components like FtsZ and ZipA checking in and out every 10 s (Stricker et al. 2002), and FtsZ disassembling before the cytoplasm has been sealed (Söderström et al. 2014). It is, therefore, plausible that the internal structure and composition of the divisome might change as envelope constriction proceeds.
References
Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Disteche M, den Blaauwen T (2005) Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol 55:1631–1645
Adams DW, Errington J (2009) Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol 7:642–653
Buss J, Coltharp C, Shtengel G, Yang X, Hess H, Xiao J (2015) A multi-layered protein network stabilizes the Escherichia coli FtsZ-ring and modulates constriction dynamics. PLoS Genet 11:e1005128
Coltharp C, Buss J, Plumer TM, Xiao J (2016) Defining the rate-limiting processes of bacterial cytokinesis. Proc Natl Acad Sci USA 113:E1044–1053
Egan AJ, Vollmer W (2013) The physiology of bacterial cell division. Ann N Y Acad Sci 1277:8–28
Erickson HP, Anderson DE, Osawa M (2010) FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev 74:504–528
Fu G, Huang T, Buss J, Coltharp C, Hensel Z, Xiao J (2010) In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM). PLoS One 5:e12682
Galli E, Gerdes K (2010) Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol Microbiol 76:1514–1526
Goehring NW, Beckwith J (2005) Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr Biol 15:R514–526
Haeusser DP, Margolin W (2016) Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol 14:305–319
Holden SJ, Pengo T, Meibom KL, Fernandez Fernandez C, Collier J, Manley S (2014) High throughput 3D super-resolution microscopy reveals Caulobacter crescentus in vivo Z-ring organization. Proc Natl Acad Sci USA 111:4566–4571
Jacq M, Adam V, Bourgeois D, Moriscot C, Di Guilmi AM, Vernet T, Morlot C (2015) Remodeling of the Z-ring nanostructure during the Streptococcus pneumoniae cell cycle revealed by photoactivated localization microscopy. MBio 6. doi:10.1128/mBio.01108-15
Lutkenhaus J, Pichoff S, Du S (2012) Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton 69:778–790
Meier EL, Goley ED (2014) Form and function of the bacterial cytokinetic ring. Curr Opin Cell Biol 26:19–27
Ortiz C, Natale P, Cueto L, Vicente M (2016) The keepers of the ring: regulators of FtsZ assembly. FEMS Microbiol Rev 40:57–67
Rico AI, Krupka M, Vicente M (2013) In the beginning, Escherichia coli assembled the proto-ring: an initial phase of division. J Biol Chem 288:20830–20836
Rowlett VW, Margolin W (2014) 3D-SIM super-resolution of FtsZ and its membrane tethers in Escherichia coli cells. Biophys J 107:L17–20
Rowlett VW, Margolin W (2015) The bacterial divisome: ready for its close-up. Philos Transac Royal Soc London Ser B, Biol Sci 370. doi:10.1098/rstb.2015.0028
Söderström B, Skoog K, Blom H, Weiss DS, von Heijne G, Daley DO (2014) Disassembly of the divisome in Escherichia coli: evidence that FtsZ dissociates before compartmentalization. Mol Microbiol 92:1–9
Söderström B, Mirzadeh K, Toddo S, von Heijne G, Skoglund U, Daley DO (2016) Coordinated disassembly of the divisome complex in Escherichia coli. Mol Microbiol. doi:10.1111/mmi.13400
Strauss MP, Liew AT, Turnbull L, Whitchurch CB, Monahan LG, Harry EJ (2012) 3D-SIM super resolution microscopy reveals a bead-like arrangement for FtsZ and the division machinery: implications for triggering cytokinesis. PLoS Biol 10:e1001389
Stricker J, Maddox P, Salmon ED, Erickson HP (2002) Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc Natl Acad Sci USA 99:3171–3175
Acknowledgments
Work in the SCB unit is supported by the Okinawa Institute of Science and Technology (OIST) Graduate University. BS acknowledges support form Stiftelsen Olle Engkvist Byggmästare and Signhild Engkvists Stiftelse. DOD acknowledges support from Carl Trygger Stiftelsen and the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Söderström, B., Daley, D.O. The bacterial divisome: more than a ring?. Curr Genet 63, 161–164 (2017). https://doi.org/10.1007/s00294-016-0630-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0630-2