Abstract
Hydrogels based on polyacrylamide (PAM) and sodium alginate (AG) were fabricated via gamma irradiation. The structure–property behavior of PAM/AG hydrogels was characterized by FTIR spectroscopy, and water absorption measurements. The PAM/AG hydrogels were applied as drug delivery taking chlortetracycline and ketoprofen as drug models. In addition, the water diffusion and drug release kinetics were investigated by applying the Fick's law, in which the mechanism for water diffusion and drug release was suggested. The results clarified the pH-sensitivity of the PAM/AG blend hydrogels towards the drug-release medium in the case of both drugs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are three-dimensional network structures, which are capable of swelling and absorbing large amounts of water or biological fluids allowing a wide range of medical applications [1,2,3]. Drug-delivery hydrogels synthesized by chemical initiation or gamma radiation have been extensively reported. A novel pH-sensitive and macroporous NaAlg-based hydrogels were prepared, in the presence of N-methylolacrylamide (NMAAm) and acrylic acid, using N,N-methylene-bis-acrylamide (MBA) as a cross-linker, 2,2-dimethoxy-2-phenylacetophenone as a photoinitiator via ultraviolet irradiation [4]. 5-fluorouracil (5-FU) as a model drug was loaded on hydrogel, the drug encapsulation efficiency of products reached to 85.2% and the cumulative release rate of drug was 78.4 and 37% in the intestinal and gastric fluid, respectively. Chemical cross-linking of monomers methacrylic acid (MAA) and itaconic acid (IA) through ethylene glycol dimethacrylate (EGDMA) was carried out for synthesizing robust hydrogel system for oral administration and tuned for targeted release of 5-fluorouracil (5-FU) and leucovorin calcium (LV) at colonic site [5]. PVP hydrogel was synthesized using gamma radiation technique [6]. In the first step, PVP, agar and PEG concentrations were modified as a function of dose rate. The sample containing 3% of PEG was selected to test its potential as antileishmanial drug carrier. Polyethylene glycol (PEG)-and polyethylene glycol–polycaprolactone (PEG–PCL)—based hydrogels were synthesized with various compositions of prepolymers by using ROP and click chemistry methods [7]. The kinetics of diclofenac sodium release from hydrogels showed similar behavior so that the hydrogels with the highest PCL concentration, the release mechanism fully changed from non-Fickian to Fickian diffusion. Novel colon targeting xanthan gum/polyvinylpyrrolidone-co-poly acrylic acid hydrogels for controlled delivery of 5-fluorouracil at colon-specific site by combining the properties of natural and synthetic polymers was prepared [8]. Drug release kinetics revealed the controlled release pattern of 5-fluorouracil in developed polymeric network. Cross-linked XG/PVP-copoly (AA) hydrogels can be used as promising candidate for controlled delivery of 5-FU for prolonged treatment period at colon-specific site. Chitosan-based hydrogels with poly(ethylene glycol)/polycaprolactone for the controlled release of drugs/growth was studied [9]. A casting/solvent evaporation method was used to obtain films of alginate and gelatin, crosslinked with Ca2+, taking ciprofloxacin hydrochloride as model drug [10]. The results showed a decrease of the ciprofloxacin release with increasing the ratios of gelatin. Ph-sensitive composite hydrogel based on chitosan-g-poly (acrylic acid)/ attapulgite/sodium alginate (CTS-g-PAA/APT/SA) was fabricated as drug delivery crosslinked by Ca2+ [11]. It was found that the rates of accumulative release of diclofenac sodium (DS) from the composite hydrogel beads were 3.76% in pH 2.1 solution and 100% in pH 6.8 solutions within 24 h, respectively. Interpenetrating network (IPN) tablets of diltiazem-HCl (DTZ) based on polyacrylamide-grafted-sodium alginate (PAam-g-SAL) copolymer and sodium alginate (SAL) to be applied as the drug delivery was investigated [12]. Hydrogels based on alginate-g-poly(itaconic acid) (NaAlg-g-PIA) microspheres as drug delivery matrices of Nifedipine crosslinked by glutaraldehyde (GA) in hydrochloric acid catalyst was prepared [13]. The results indicated that Nifedipine released from grafted microspheres was faster in the pH 7.4 buffer solution than that at pH 1.2 solutions. In addition, it was found that by increasing time, drug amount, GA and NaAlg-g-PIA concentrations, the release of nifedipine from microspheres was decreased. The release characteristics of diclofenac sodium (DS) from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads were reported [14]. The highest DS release obtained was 92% for 1/1 PVA-g-PAAm/NaAlg ratio beads and that the release results showed that DS release from the beads through the external medium is much higher at high pH (6.8 and 7.4) than that at low pH (1.2). Hybrid alginate hydrogel with shells of porous CaCO3 micro-particles formed by templating water-in-oil emulsion then in situ gelation was used for Brilliant blue (BB) as a drug model [15]. The formation of the shells of CaCO3 microparticles slowed down the BB released from the colloid some. A drug delivery system for preparing microcapsule by liposome in alginate was used for bovine serum albumin (BSA) as a model drug [16]. The results showed that Ca2+ and Ba2+ made hydrogels compose easier than Al3+ and particle size was uniform, circular. However, the microcapsule prepared by Al3+ was flat circular and easily adhere to each other. CaCl2 as a crosslinking agent was employed to prepare biopolymer microspheres of sodium alginate and starch [17]. The prepared microspheres were loaded with an insecticide; chlorpyrifos, and FTIR and SEM techniques were used to characterize both the native (unloaded) and loaded microspheres. Fe3+ crosslinked alginate-carboxymethyl cellulose in several volume rates was prepared [18]. The vitro release test was used to monitor the controlled release of albumin from hydrogel beads under simulated gastrointestinal conditions over 24 h. The release of protein was protected and controlled by The Fe3+ crosslinked AC beads, showing that such beads introduce a promising protein therapeutic carrier for the oral delivery.
The radiation synthesis of hydrogels in aqueous solution based on natural and synthetic water-soluble polymers has been a special interest and widely used as drug delivery systems. In this contest, pH sensitive hydrogels based on acrylamides and their swelling and diffusion characteristics with drug delivery behavior taking 5-fluorouracil as a model drug [14].
In previous studies, we were interested with the radiation synthesis of drug-delivery responsive hydrogels from natural polymers. In this regard, the temperature and pH responsive behavior of carboxymethyl cellulose/acrylic acid hydrogels prepared by electron beam irradiation was reported [19]. The properties of swelling and drug release of acrylamide/carboxymethyl cellulose networks composed by gamma irradiation were studied [20]. Gamma irradiated concentrated aqueous solutions of chitosan/sodium alginate blends were characterized and their drug uptake-release characters were investigated [21]. The radiation synthesis of pH-sensitive hydrogels from carboxymethyl cellulose/poly(ethylene oxide) blends as drug delivery systems was studied [22]. The physico-chemical and drug release properties of poly(vinyl alcohol)/gum arabic/TiO2 nanocomposite hydrogels formed by gamma radiation [23] and radiation synthesis and drug delivery properties of interpenetrating networks (IPNs) based on poly(vinyl alcohol)/ methylcellulose blend hydrogels [24] were investigated. Recently, the biological applications of nanocomposite hydrogels prepared by gamma-radiation copolymerization of acrylic acid (AAc) onto plasticized starch (PLST)/montmorillonite clay (MMT)/chitosan (CS) blends was reported [25].
As seen above, most of the drug delivery hydrogels prepared by gamma irradiation or ionizing radiation was based on using acrylamide, sodium alginate and cross-linked PVP as a single component and network of xanthan gum and poly(vinyl pyrrolidone) prepared by a chemical method. All those hydrogels were investigated taking 5-fluorouracil as a drug model. In the present study, gamma radiation was used for the preparation of a drug delivery hydrogels from polyacrylamide (PAM) and sodium alginate (AG) blends taking new drugs such as Tetracycline and Ketoprofen as drug models. The importance of choosing PAM and AG is due the presence of many hydrophilic groups capable of forming covalent bonds with those on the drugs. The study of the pH-sensitivity of swelling and applying PAM/AG as pH-responsive hydrogels as drug delivery systems is an important objective.
Experimental
Materials
A laboratory grade chemicals sodium alginate (AG) used in this work was obtained from Aldrich Chemical Co. (Milwaukee, WI). A laboratory grade of polyacrylamide (PAM) was purchased from Merck, Germany. Calcium chloride, pure grade chemicals was used as a crosslinking agent for sodium alginate. Tetracycline, antibiotic drug, and Ketoprofen were purchased from Sigma Chemical Co., USA. All chemicals used such as, citrate, phosphate buffer were of analytical reagent grade and were purchased from El-Nasr Co. for Chemical Industries, Egypt, and used as received.
Preparation of PAM/AG blend hydrogels
Films of PAM/AG blends were first prepared by solution casting. In this procedure, aqueous solutions with different concentrations of AG were prepared using CaCl2 (1 wt%) as a crosslinking agent in distilled water. PAM was dissolved in distilled water. The solutions of PAM and AG were then mixed with continuous stirring to obtain homogenous solutions to obtain PAM/AG compositions of 80/20, 50/50 and 20/80 wt%. The blend solutions were put into Petri dishes, and made free of air by purging nitrogen for 5 min at least and sealed. The films were then exposed to gamma irradiation at a dose rate of 6.92 kGy/h. The hydrogel films were first washed with excess distilled water to remove unreacted materials, extracted with distilled water using a Soxhlet system for 6 h to remove homopolymers and finally dried in a vacuum oven at 80 °C to constant weight to get films with thickness of ~ 0.5 mm.
The gamma irradiation procedure was carried out in a cobalt-60 gamma cell (made in Russia) instilled at the National Center for Radiation Research and Technology, Egyptian Atomic Authority, Cairo, Egypt.
Characterization
FT-IR spectroscopic analysis
The IR spectra were performed on a FT-IR spectrometer model Mattson 100, Unicam, and were recorded over the range 500–4000 cm−1.
Swelling characters
A dry weight of the Pam/AG hydrogel (Wo) was immersed in distilled water for different periods up to 24 h at room temperature and at pH of 7.4 (10 mM NaH2PO4–Na2HPO4 buffered solution). At each period, the sample was removed and blotted on filter paper to remove the excess water on the surface and weighed (Ws). The swelling ratio was calculated according to Eq. 1:
The pH-sensitivity of swelling was determined by the same procedure but in buffer solutions with various pH values (2.1, 5, and 8) at room temperature for 12 h to reach equilibrium swelling. The different solutions of NaH2PO4, Na2HPO4, NaCl and NaOH were combined to form buffer solutions with various pH values. All experiments were done in triplicates.
Tensile mechanical properties
The tensile mechanical properties were tested in the form of stripes (1 cm in width and 3 cm in length). The tensile strength and elongation at break point were determined at a crosshead speed of 10 mm/min on a testing machine, Mecmesin, multi Test 25-I (United Kingdom).
Scanning electron microscopy (SEM)
The surface morphology of the PAM/AG hydrogels was examined by scanning electron microscopy (SEM). The SEM micrographs were taken on a JSM-5400 instrument (Joel, Japan). A sputter coater was used to pre-coat conductive gold onto the fracture surfaces at 30 kV.
Preparation of the hydrogels (PAM/AG) loaded with the drug (tetracycline and ketoprofen)
In order to determine the amount of tetracycline and ketoprofen drugs uptake or release, a standard calibration curves representing the absorbance of different concentrations of tetracycline and ketoprofen drugs were first constructed. From this relation, a concentration of unknown sample can be determined. A dry weight PAM/AG hydrogel (Wo) was immersed into different concentrations of drug solutions at room temperature for 12 h until complete sorption (W1). UV spectrophotometer model UV2 series made by Unicam was used at the specific wavelength of drugs. The drug uptake (%) was calculated using the Eq. 2:
Drug release characters of PAM/AG blend hydrogels
Release profiles of the drugs tetracycline and ketoprofen were carried out by placing drug-loaded blend hydrogels into 20 ml of buffer solutions at pH 2.1, 5 and 8 and allowed to complete release. The release experiment was performed three times for every sample and the average value was used to plot the release profiles.
Results and discussion
Synthesis of PAM/AG copolymer hydrogels via gamma radiation
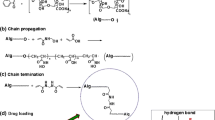
Sodium alginate is a natural non-toxic polysaccharide available in the brown algae. It is a water soluble salt of alginic acid. Sodium alginate has a tendency to undergo crosslinking in the presence of multivalent cations, such as calcium ions in aqueous media [26]. On the other hand, the crosslinking of vinyl polymers in solutions by gamma radiation was established [27]. The crosslinking of PAM in aqueous solution by gamma radiation may be briefly summarized as follows:
-
(1)
Gamma radiation is absorbed by the polymer PAM and the solvent H2O, the radicals –(–C·H–C·H–CONH2–)n, HO· and H· are formed. The radicals resulted from the radiolysis of water transfer to PAM polymer and thus increase the concentration of PAM radicals and increase the rate of crosslinking.
-
(2)
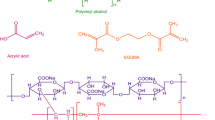
Two PAM polymer radicals with n and m repeat units combine to form a crosslinked point. It is expected that the formed PAM/AG blend hydrogel represents an interpenetrating polymer network (IPN). These kinds of network structure contain an entangled combination of two crosslinked polymers not bonded to each other as shown in Fig. 1. However, the possible formation of hydrogen bonding between PAM and AG still exist.
FT-IR Spectroscopic analysis
Figures 2 and 3 show The FT-IR spectra of unirradiated PAM and AG polymers and PAM/AG blend hydrogels at different rates before and after gamma irradiation at different doses, respectively. The IR spectrum of unirradiated AG showed a broad peak at 3355 cm−1 due to -OH group, two peaks at 1576 and 1412 cm−1 due the –COO− group, and one sharp peak at 1036 cm−1, due to the C–O group. The characteristic band of AG can be seen at 819 cm−1. The IR spectrum of unirradiated PAM showed bands at 3408 cm−1 and 3116 cm−1, assigned to the stretching vibration of N–H, 1676 cm−1 (C–O stretching) and 1567 cm−1 (N–H bending). Also bands at 2940 cm−1 (CH stretching) and 1515–1370 cm−1 (various CH bending) can be observed. The IR spectra of the unirradiated PAM/AG blends are characterized by the absorption bands typical of the pure components. However, the AG characteristic band appeared at 819 cm−1 was not observed in all the IR spectra of PAM/AG blends. For the unirradiated PAM/AG blends at different ratios, the absorption band seen at 1576 cm−1 is due to the asymmetric stretching vibration of –COO− groups coupled with the PAM band at 1676 cm−1 were shifted to 1562 cm−1. These findings suggest the existence of new hydrogen bonds formed between –COO− groups of alginate and -CONH2 groups of PAM. The PAM bands at 3408–3116 cm−1 are due to the stretching vibration of -NH2 groups are coupled with –OH band of AG at 3506 cm−1 indicating the formation of hydrogen bonds between –OH groups of AG and -NH2 groups of PAM molecules [28, 29].
In the case of blend hydrogels prepared by gamma irradiation, the absorption bands typical of the pure components were also observed. In this regard, the AG characteristic band, appeared at 819 cm−1, was absent in all the spectra of PAM/AG blend hydrogels. It is clear that all the characteristic bands for pure components exist in the different PAM/AG blend hydrogels. In general, it is believed that the peak near 1129 cm−1 originated from glycosidic linkages in polysaccharides is due to asymmetric α-(1–4) stretching mode of the glycosidic [30, 31]. It is clear that, the AG band shifted from 1129 to 1095 cm−1 after blending with PAM for the blend 50/50%. Upon exposure of the blends to gamma irradiation at 20 to 50 kGy, this band was shifted to 1071 cm−1 in case of the PAM/AG (50/50) blend composition. It was proposed that based on the proposal that the 1124 cm−1 band is assigned to C–O stretching vibrations, which may have appeared due to the C–OH group at the C6 position [32].
Swelling characters
The swelling in buffer solutions of various pH values and at different temperature for PAM/AG blend hydrogels of different ratios, prepared at the dose of 20 kGy of gamma irradiation is illustrated in Fig. 4. Few points may be indicated:
-
(1)
The swelling was suddenly increased after to 5 h and then reached the equilibrium stat up to 12 h, regardless of hydrogel composition and pH value however, the swelling ratio was found to increase with the increase of the pH value up to 8.
-
(2)
The swelling ratio increases with increasing temperature from 25 to 50 °C.
-
(3)
At all, values of pH and temperature, the swelling ratio increases with increasing the AG component.
-
(4)
It is evident that the PAM/AG hydrogels displayed pH-sensitive swelling behavior. This sensitivity resulted from the existence of the hydrophilic carboxylic acid and amide groups along the hydrogels chains. At higher pH values than the pKa, the carboxylic acid groups became ionized, leading to swollen networks due to the electrostatic repulsion between charged groups [33]. The low swelling ratio within the pH values 2–5, is due to electrostatic interaction. On increasing the pH to 8, the hydrogen bonds broke as the carboxylic acid groups ionized. Meanwhile electrostatic repulsion caused the network to expand; therefore, the swelling ratio reached a relatively larger value.
-
(5)
The increase of swelling ratio with increasing the AG ratio has a direct relation with the gel fraction of PAM/AG blend hydrogels, in which it was found that the crosslinking degree (gel content) was decreased by increasing the ratio of AG. Thus, as the gel content decreases, thee hydrogel structure become incompact and hence facilitate the diffusion of water molecules.
-
(6)
The increase of temperature would increase the mobility of chains and hence facilitate the diffusion of water molecules from the surroundings.
The mechanism of diffusion into hydrophlic polymeric systems has been paid great attention due to the important biomedical, pharmaceutical and environmental applications [34]. The nature of water diffusion into PAM/AG hydrogels was determined by applying Fick's law according to the following equation [35]:
where Wt is the amounts of water absorbed by the hydrogel at time t and We is the amounts of water absorbed by the hydrogel at equilibrium, k represents a constant characteristic of the networks structure and n is the exponent determining the mode of water diffusion. When ln F is plotted against ln t, it gives a straight line from which the constant k is determined by the intercept and the slope gives the number n. In this concern, a value of n = 0.5 implies a Fickian diffusion mechanism, in which the sorption is diffusion controlled, while a value of 0.5 < n < 1 implies an anomalous non-Fickian type diffusion and adds to the water-sorption process.
Figure 5 shows the plots of ln F against ln t for the swelling in buffer solutions of different pH values and different temperatures for PAM/AG blend hydrogels of different ratios, prepared at the dose of 20 kGy of gamma irradiation. Table 1 presents the kinetic parameters calculated for the PAM/AG hydrogels of different compositions. The data indicated that all the hydrogels represented a non-Fickian type of diffusion. Thus, it may conclude that the water diffusion into the hydrogel networks is not controlled but it depends on water sorption process, which in turn depends on the structure and pathways of water through the networks. This result indicates that the relatively high crosslinking density restricts the water pathways diffusion onto the hydrogels.
Tensile mechanical properties
The tensile strength and elongation at break parameters are indicative for the handling properties and mechanical performance of the hydrogel films. The tensile strength and elongation at beak of PAM/AG blend hydrogels of different rates are shown in Fig. 6. From the data in Fig. 6, few points may be indicated:
-
(1)
The tensile strength and elongation at break of either unirradiated or gamma irradiated PAM/AG blend hydrogels was found to decrease with increasing the ratio of AG polymer is due to the brittle nature of the natural AG.
-
(2)
It is obvious that the tensile strength of PAM/AG blend hydrogels increased with increasing irradiation dose and decreased with the increase of AG ratio, regardless of blend composition. This finding is due the crosslinking occurred to the PAM component. These findings are true for the PAM/AG blend hydrogels up to the 75/25%. This behavior could be attributed to the occurrence of degradation to the AG component.
Scanning electron microscopy (SEM)
Figure 7 shows the SEM micrographs of pure PAM and AG polymers and PAM/AG (50/50%) blend hydrogels, before and after gamma irradiation to a dose of 20 kGy. The SEM micrograph of the fracture surface of unirradiated AG polymer is smooth while the fracture surface of unirradiated PAM polymer showed a number of small particles dispersed along the micrograph. The fracture surface of the unirradiated PAM/AG (50/50%) is full of cavities, small particles and small halls spread all over the surface. Gamma irradiation improved greatly the surface. It became smoother, in which the cavities and small particles were almost disappeared and were replaced by very small particles spread homogeneously all over the surface. These findings indicate the occurrence of crosslinking.
Drug uptake-release characters of PAM/AG hydrogels
The crosslinking affected directly the swelling of PAM/AG and reducing the permeability of different solutes. Consequently, the release of loaded drugs onto PAM/AG matrices will eventually allowing these systems to be used in drug-controlled release [36]. The drug release mechanisms and the resulting release patterns are determined by the physicochemical nature of the matrix. Several processes, i.e. drug dissolution and diffusion, swelling and erosion of the matrix or a combination of two or more of these processes are related to the overall control of drug release [37, 38].
Uptake and release of tetracycline drug
The chemical structure of tetracycline drug (shown below) contains many active groups capable of forming covalent and hydrogen bonding with the chains of PAM/AG blends hydrogels. The uptake of tetracycline by PAM/AG (%) hydrogels at different ratios was at room temperature and pH 7 to the equilibrium state is shown in Fig. 8. It can be seen that the uptake (%) was increased with increasing drug concentration and with increasing AG ratio. It seems that the bonding of drug with AG through hydrogen bonding was favorable than with PAM chains.

The tetracycline release profiles at room temperature and in buffer solutions of different pH values and the equilibrium drug release at various pH values from PAM/AG blend hydrogels at different compositions, prepared by gamma irradiation to a dose of 20 kGy. It is clear that the release process of tetracycline depends largely on the pH of the medium. The highest release was reached at pH 8.0 while the lowest release was at pH 2.0, depending on hydrogel composition. The release however was found to increase with increasing the ratio of AG in the hydrogel composition. It seems that the release is greatly related to the swelling in water of non-loaded PAM/AG hydrogel films at different pH values as shown in Fig. 4. At lower pH value, the amount of COO− on alginate is almost equal to the amount of NH 3+ on PAM, and hence the macromolecular chains in the hydrogel film matrix attract each other causing a shrink and therefore, the lowest value of water swelling of blank matrix film at pH 2.0. At higher pH, the equilibrium ratio between the amount of COO− and NH3+ was broken and the macromolecules chains of the film matrix take each other apart, which increased the swelling of water and thus decrease the drug release (Fig. 9).
Release of Ketoprofen drug
Ketoprofen is a non-steroidal anti-inflammatory drug having a 4.94 pKa and its chemical structure name is shown below. It is clear that the chemical structure contains –COOH and C=O groups. Thus, it is expected that when the drug is loaded onto the PAM/AG hydrogels networks, it would form hydrogen bonding with the available groups along the PAM/AG hydrogels networks. The Ketoprofen release profiles at room temperature and in buffer solutions at different pH values are shown in Fig. 10. The drug release at equilibrium from PAM/AG blend hydrogels at different pH values for the same hydrogels is shown in Fig. 10. It is evident that the ketoprofen releases gradually and then reaches the equilibrium release state after ~ 3 h depending on the medium pH. However, the release (%) of ketoprofen at The equilibrium state of ketoprofen from the PAM/AG hydrogel of compositions 80/20 and 50/50% at pH 8 was found to be 85.5 and 40.8%, respectively.

Sensitivity and drug release kinetics
Sensitivity of drug release
A useful parameter in the in the field of drug delivery applications is to measure the sensitivity of drug delivery character of PMA/AG copolymer hydrogel by plotting the initial part of the release profiles of the drugs tetracycline and ketoprofen against time on a linear regression plots, as shown in Fig. 11. The calculated slopes (rate of release) are listed in Table 2. The data show that the ketoprofen release rate, in different buffer solutions of various pH values, from PAM/AG hydrogels is ~ 3–5 times higher than the release rate of tetracycline under the same conditions. This finding may be referred to the relatively weak hydrogen bonding of ketoprofen through the carboxylic groups in PAM/AG hydrogels and the relatively smaller the size of ketoprofen.
Drug release kinetics
The nature of drug release was studied by applying Fick's law. The application of this law on the present system is by plotting ln F versus ln t for drugs release profiles of tetracycline and ketoprofen at room temperature and in buffer solutions of various pH values from PAM/AG blend hydrogels at different ratios, prepared by gamma irradiation to a dose of 20 kGy in illustrated in Fig. 12. The calculated data parameters for "n" and "k" are presented in Table 2. Based on the data on Table 2, few conclusions may be outlined:
-
(1)
The "n" values for the drug delivery of Tetracycline from PAM/AG blend hydrogels was calculated to be ~ 0.5 indicating a Fickian release mechanism, where the drug delivery diffusion is controlled, regardless of the pH value or blend hydrogel composition.
-
(2)
The "n" values for the drug delivery of Ketoprofen from PAM/AG blend hydrogels were calculated to be 0.5 < n < 1 indicating anomalous non-Fickian type release [39]. It may be deduced that the drug release of Ketoprofen from the hydrogel networks depends on the structure and pathways of water through the networks rather being controlled.
-
(3)
The lower accumulated release values rate at pH 2 may be explained as follows: Since the blend hydrogel chains would combine via hydrogen bonding and electrostatic interaction at acidic condition, it is hard to relax, and only a part of Tetracycline or Ketoprofen drugs could enter into the buffer solution, which causing a lower release. Thus, the release behaviors of Tetracycline and ketoprofen from the PAM/AG hydrogels would be higher in alkaline medium rather than in acidic medium.
Conclusions
In this study, films of a new pH-sensitive blend hydrogel based on polyacrylamide (PAM) and sodium alginate (AG) were successfully prepared by gamma radiation. The results indicated that the pH-sensitivity of PAM/AG hydrogels was greatly affected the swelling in water and the drug release characters. The uptake and release of Tetracycline drug increased with the increase in AG ratio in the blend mixture. At pH range 2.1–5, the drug accumulated release ratio from the hydrogel films is slower than that at pH 7.4–8, and decreased with increasing PAM ratio. These affairs hold true in the case of ketoprofen drug. At pH 7.4–8, the drug release mechanism of the hydrogel films was swelling-controlled. The results indicated that introducing AG in polymeric network might present simple and unique ways to prepare new controlled drug delivery systems.
Availability of data and materials
The authors declare that the data and materials are available.
References
Xu X, Liu Y, Fu W, Yao M, Ding Z, Xuan J, Li D, Wang S, Xia Y, Cao M (2020) Poly(N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers (Basel) 12:1–22
Feng S, Wang S, Lv Y, He L, Li Q, Zhang T (2019) Dual pH- and thermal-responsive nanocomposite hydrogels for controllable delivery of hydrophobic drug baicalein. Polym Int 68(2019):494–502
McCarthy PC, Zhang Y, Abebe F (2021) Recent applications of dual-stimuli responsive chitosan hydrogel nanocomposites as drug delivery tools. Molecules 26:1–15
Wu C, Cong Li C, Zhang X, Cheng C, Wang J (2019) An alginate-based hydrogel composite obtained by UV radiation and its release of 5-fluorouracil. Polym Bull 76:1167–1182
Abdullah O, Minhas MU, Ahmad M, Ahmad S, Ahmad A (2019) Synthesis of hydrogels for combinatorial delivery of 5-fluorouracil and leucovorin calcium in colon cancer: optimization, in vitro characterization and its toxicological evaluation. Polym Bull 76:3017–3037
Ammar NEB, Essid R, Saied T, Şen M, Elkahoui S, Hamzaoui AH (2020) Synthesis and characterization of radiation cross-linked PVP hydrogels and investigation of its potential as an antileishmanial drug carrier. Polym Bull 77:1343–1357
Saidi M, Dabbaghi A, Rahmani S (2020) Swelling and drug delivery kinetics of click-synthesized hydrogels based on various combinations of PEG and star-shaped PCL: influence of network parameters on swelling and release behavior. Polym Bull 77:3989–4010
Anwar M, Pervaiz F, Shoukat H, Noreen S, Shabbir K, Majeed A, Ijaz S (2021) Formulation and evaluation of interpenetrating network of xanthan gum and poly(vinyl pyrrolidone) as a hydrophilic matrix for controlled drug delivery system. Polym Bull 78:59–80
Wen Y, Li F, Li C, Yin Y, Li J (2017) High mechanical strength chitosan-based hydrogels cross-linked with poly(ethylene glycol)/polycaprolactone micelles for the controlled release of drugs/growth factors. J Mater Chem B 5:961–971
Dai C, Wang B, Zhao H, Li B (2006) Alginate/gelatin blend films and their properties for drug controlled release. J Membr Sci 280:37–44
Wang Q, Zhang J, Wang A (2009) Preparation and characterization of a novel pH-sensitive chitosan-g-poly (acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohyd Polym 78:731–737
Mandal S, Basu SK, Sa B (2010) Ca2+ ion crosslinked interpenetrating network matrix tablets of polyacrylamide-grafted-sodium alginate and sodium alginate for sustained release of diltiazem hydrochloride. Carbohyd Polym 82:867–873
Isiklan N, İnal M, Kurşun F, Ercan G (2011) pH responsive itaconic acid grafted alginate microspheres for the controlled release of nifedipine. Carbohyd Polym 84:933–943
Şanli O, Ay N, Isiklan N (2007) Release characteristics of diclofenac sodium from poly(vinyl alcohol)/sodium alginate and poly(vinyl alcohol)- grafted-poly(acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm 65:204–214
Liu H, Wang C, Gao Q, Liu X, Tong Z (2008) Fabrication of novel core-shell hybrid alginate hydrogel beads. Int J Pharm 351:104–112
Dai C, Wang B, Zhao H, Li B (2005) Factors affecting protein release from microcapsule prepared by liposome in alginate. Colloids Surf, B 42:253–258
Roy A, Bajpai J, Bajpai AK (2009) Dynamics of controlled release of chlorpyrifos from swelling and eroding biopolymeric microspheres of calcium alginate and starch. Carbohyd Polym 76:222–231
Kim MS, Park S P, Gu BK, Kim, C (2012) Ionically crosslinked alginate-carboxymethyl cellulose beads for the delivery of protein therapeutics. Applied Surface Science. In Press, Accepted Manuscript, Available online 20 January (2012)
El-Naggar AM, Abd Alla SG, Said HM (2006) Temperature and pH responsive behaviour of CMC/AAc hydrogels prepared by electron beam irradiation. Mater Chem Phys 95:158–172
Nizam El-Din HM, Abd Alla SG, El-Naggar AM (2010) Swelling and drug release properties of acrylamide/carboxymethyl cellulose networks formed by gamma irradiation. Radiat Phys Chem 79:725–730
Nizam El-Din El-Naggar (2011) Characterization of gamma irradiated concentrated aqueous solutions of chitosan/sodium alginate blends and their drug uptake-release characters. J Appl Polym Sci 122:2383–2390
Nizam El-Din HM, El-Naggar AM, Abu-El Fadle FI (2013) Radiation synthesis of pH-sensitive hydrogels from carboxymethyl cellulose/poly(ethylene oxide) blends as drug delivery systems. Int J Polym Mater Polym Biomater 62:1–8
Nizam El-Din HM, Khafaga MR, El-Naggar AM (2015) Physico-chemical and drug release properties of poly(vinyl alcohol)/gum Arabic/TiO2 nanocomposite hydrogels formed by gamma radiation. J Macromol Sci Part A Pure Appl Chem 52:821–829
El-Naggar AM, Senna MM, Mostafa TA, Reham H (2017) Radiation synthesis and drug delivery properties of interpenetrating networks (IPNs) based on poly(vinyl alcohol)/ methylcellulose blend hydrogels. Int J Biol Macromol 102:1045–1051
Nizam El-Din HM, Ibraheim DM (2021) Biological applications of nanocomposite hydrogels prepared by gamma-radiation copolymerization of acrylic acid (AAc) onto plasticized starch (PLST)/montmorillonite clay (MMT)/chitosan (CS) blends. Int J Biol Macromol 192(2021):151–160
Yotsuyanagi T, Yoshioka I, Segi N, Ikeda K (1991) Acid-induced and calcium-induced gelation of alginic acid: bead formation and pH dependent swelling. Chem Pharm Bull 39:1072–1074
Chapiro A (1962) Radiation chemistry of polymeric systems; interscience: New York, 22–81
Xiao C, Lu Y, Liu H, Zhang L (2000) Preparation and physical propertied of blends films from sodium alginate and polyacrylamide solutions. J Macromol Sci, Pure Appl Chem 37:1663
Shiaw-Guang HuD, Jium-Nan Chou K (1996) Kinetics of water swelling and development of porous structure in ionic poly(acrylonitrile-acrylamide-acrylic acid) hydrogels. J Polym 37:1019
Kacurakova M, Capek P, Sasinkova V, Wellner N, Ebringerova A (2000) FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohyd Polym 43:259–203
Nikonenko NA, Buslov DK, Sushko NI, Zhbankov RG (2000) Spectroscopic manifestation of stretching vibrations of glycosidic linkage in polysaccharides. J Mol Struct 752:20–24
Sekkal M, Dincq V, Legrand P, Huvenne JP (1995) Investigation of the glycosidic linkages in several oligosaccharides using FT-IR and FT Raman spectroscopies. J Macromol Struct 349:349–352
Bose PK, Polavarapu PL (2000) Evidence for covalent binding between copper ions and cyclodextrin cavity: a vibrational circular dichroism study. Carbohyd Res 323:63–72
Van Krevelen DW (1990) Properties of Polymers, 3rd edn. Elsevier, Amsterdam, p 21
Peppas NA, Gurny R, Doelker E, Buri D (1989) Modelling of drug diffusion through swellable polymeric. J Membr Sci 7:241
Dong Z, Wang Q, Du Y (2006) Alginate/gelatin blend films and their properties for drug controlled release. J Membr Sci 280:37–44
Goskonda SR, Hilman GA, Upadrashta SM (1994) Controlled release pellets by extrusion-spheronization. Int J Pharm 111:89–97
Kojima M, Nakagami H (2002) Development of controlled release matrix pellets by annealing with micronized water-insoluble or enteric polymers. J Control Release 82:335–343
Chen J, Rong L, Lin H, Xiao R, Wu H (2009) Radiation synthesis of pH-sensitive hydrogels from β-cyclodextrin-grafted PEG and acrylic acid for drug delivery. Mater Chem Phys 65:159–169
Funding
The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript. The authors gratefully acknowledge the financial support of the National Center for Radiation Research and Technology, Egyptian Atomic Energy Authority, Cairo Egypt.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation: FIAEF, Data collection and analysis: HMNED. The first draft of the manuscript: AWMEN, and HMNED. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent for publication
The authors declare no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nizam El-Din, H.M., El-Naggar, A.W.M. & Abu-El Fadle, F.I. Characterization and drug release kinetics of polyacrylamide/sodium alginate blend hydrogels synthesized by gamma irradiation. Polym. Bull. 81, 6149–6171 (2024). https://doi.org/10.1007/s00289-023-04991-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-04991-3