Abstract
A series of novel aromatic polyamides containing both sulfone linkages and cardo groups were synthesized via a heterogeneous palladium-catalyzed carbonylation and condensation reaction of aromatic diiodides bearing ether sulfone linkages, carbon monoxide, and aromatic diamines with cardo groups. Polycondensation reaction proceeded smoothly under 1 atm of CO at 120 °C in N,N-dimethylacetamide (DMAc) by using a bidentate phosphine ligand-modified magnetic nanoparticles-anchored palladium complex [2P-Fe3O4@SiO2-PdCl2] as a recyclable catalyst with 1,8-diazabicycle[5,4,0]-7-undecene (DBU) as a base, furnishing cardo poly(ether sulfone amide)s with inherent viscosities between 0.70 and 0.77 dL/g. The resulting polyamides could be readily dissolved in polar aprotic organic solvents and even dissolved in less polar pyridine and tetrahydrofuran at room temperature and could be easily converted into flexible, transparent, and tough films via casting from their solutions in DMAc. These polymers exhibited excellent thermal stability with the glass transition temperatures between 241 and 283 °C and the temperatures at 5% weight loss ranging from 438 to 475 °C in an atmosphere of nitrogen. The polyamide films displayed good mechanical behavior with tensile strengths of 78.8–84.4 MPa, tensile moduli of 2.08–2.57 GPa, and elongations at breakage of 10.2–12.5%, and optically high transparency with cut-off wavelengths in the range of 338–368 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyamides, such as poly(p-phenyleneterephthalamide) (PPTA) and poly(m-phenyleneisophthalamide) (MPIA), exhibit many desirable characteristics including excellent mechanical properties, high thermal stability, and good chemical resistance, along with low flammability [1,2,3,4,5,6,7,8,9]. Despite the outstanding combined properties, most of aromatic polyamides suffer from some drawbacks such as infusibility and limited solubility in most organic solvents because of the strong interchain interaction caused by the highly rigid and regular polymer backbone and the existence of intermolecular hydrogen bonding, which result in their poor processability and restrict their wide-spread applications. Thus, considerable efforts have been devoted to the design of the chemical structure of the rigid polymer backbone to obtain aromatic polyamides that are easily processable by traditional techniques [10,11,12,13,14].
On the other hand, aromatic poly(ether sulfone)s, such as those derived from 4,4′-dichlorodiphenyl sulfone and bisphenols like 2,2-bis(4-hydroxyphenyl)propane, biphenyl-4,4′-diol, and 4,4′-dihydroxydiphenyl sulfone, have already been developed into commercial high-performance thermoplastic materials because of their good mechanical properties, high thermooxidative stability, as well as excellent hydrolytic stability [15,16,17]. These polymers are generally amorphous and transparent materials having comparatively high glass transition temperatures and have been extensively employed as thermoplastic matrices in fiber-reinforced composites using Kevlar, carbon, and glass fibers as reinforcement [18]. It is well known that the introduction of sulfone linkages into polymer main chain could lead to an enhanced solubility, an increased Tg value, and excellent thermooxidative stability. In order to modify the properties of aromatic polyamides, aromatic poly(ether sulfone amide)s have been synthesized by low temperature solution polycondensation reactions of 4,4′-(4,4′-sulfonylbis(4,1-phenylene)bis(oxy))dianiline with diaroyl chlorides [19], 4,4′-(4,4′-sulfonylbis(4,1-phenylene)bis(oxy))dibenzoyl chloride with aromatic diamines [20], and aromatic diamines containing sulfone and amide units with diaroyl chlorides [21]. The resulting polyamides were characterized by outstanding thermooxidative stability and solubility, good mechanical behavior, and higher glass transition temperatures.
The carbonylation and condensation reactions of aromatic dihalides, carbon monoxide, and aromatic diamines under the catalysis of palladium have been developed into an alternative approach for the synthesis of aromatic polyamides due to some attractive advantages such as the elimination of corrosive and moisture-sensitive aromatic diacid chlorides, the use of CO as a inexpensive and easily available C1 source, and the easy availability of various bishalogenated arene monomers [22,23,24,25,26,27,28,29,30]. However, all the carbonylative polymerizations were carried out using homogeneous PdCl2(PPh3)2 (typically 6 mol%) as the catalyst and an excess of PPh3 as the ligand. Homogeneous catalysis suffers from the difficult separation of the palladium catalyst from the desired product and the inability to recycle the expensive catalyst. Moreover, the polycondensation catalyzed by a homogeneous palladium complex might result in a high level of residual palladium in the desired polymer because of palladium leaching. The process for removal of palladium impurity from the resulting polymer is rather tedious since the residual palladium species was firmly embedded in the curled polymer chain, thereby limiting application of this methodology in large-scale preparation of highly purified polymers. Therefore, development of highly active and recyclable heterogeneous palladium catalysts for the carbonylative polycondensation without palladium leaching remains a challenging task and is highly desirable.
In recent years, the application of magnetic nanoparticles-bound palladium complexes in organic transformations has received much attention since the palladium complexes anchored onto magnetic nanoparticles can be facilely separated from the product and recovered simply by placing a magnet near the reaction vessel without centrifugation and/or filtration, which minimizes loss of the palladium catalyst and greatly improves its recyclability [31,32,33,34,35]. Recently, we have described the preparation of a bidentate phosphine ligand-modified magnetic nanoparticles-anchored palladium complex [2P-Fe3O4@SiO2-PdCl2] and its application to heterogeneous carbonylation and condensation reaction of aromatic diiodides, carbon monoxide, and aromatic diamines towards new aromatic polyamides [36,37,38]. To further expand the application of this heterogeneous palladium catalyst and examine the combined effects of sulfone linkages and cardo groups on the properties of aromatic polyamides, herein we report the synthesis of new aromatic polyamides containing both sulfone linkages and cardo groups through a heterogeneous carbonylation and condensation reaction of aromatic diiodides bearing ether sulfone linkages, carbon monoxide, and aromatic diamines with cardo groups by using 2P-Fe3O4@SiO2-PdCl2 as a recyclable palladium catalyst. The new polyamides obtained were characterized by FT-IR, 1H-NMR, WAXD, DSC, TGA, UV–vis, etc. Primary characterization results indicated that they might serve as new candidates for solution processable high-performance engineering plastic and optoelectronic materials.
Experimental
Materials
The 2P-Fe3O4@SiO2-PdCl2 complex was prepared via our previously described route [36]. N,N-Dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), N-methyl-2- pyrrolidone(NMP), 1,3-dimethyl-2-imidazolidone (DMI), dimethyl sulfoxide (DMSO), and hexamethylphosphoramide (HMPA) were purified by distillation under a reduced pressure and stored over 4 Å molecular sieve. Diphenylphosphine, 3-aminopropyltriethoxysilane, and 1,8-diazabicyclo[5,4,0]-7-undecene (DBU) were purified by distillation under a reduced pressure. 4,4′-Di(3-iodophenoxy)diphenyl sulfone (1a) [38], 4,4′-di(4-iodophenylsulfonyl)diphenyl ether (1b) [38], 9,9-bis[4-(4-aminophenoxy)phenyl]fluorene (2a) [39], 9,9-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]fluorene (2b) [40], 9,9-bis-[4-(4-aminophenoxy)phenyl]xanthene (2c) [41], and 9,9-bis[4-(4-amino-2-trifluoromethylphenoxy)phenyl]xanthene (2d) [42] were prepared by referring to literature methods. All other starting materials were of analytical grade and were employed as received from different commercial sources.
Characterization
FT-IR spectra of the polymers in KBr pellets were obtained with a Horiba FT-720 FTIR spectrometer. 1H NMR (400 MHz) spectra were recorded on a Bruker Avance 400 (400 MHz) spectrometer in DMSO-d6 as solvent with Me4Si as the internal reference. Elemental analyses were conducted on a PerkinElmer model 2400 CHN element analyzer. Inherent viscosities (ηinh = (ln ηr)/c) were measured on a Cannon–Fenske viscometer at a concentration of 0.5 g/dL in DMAc at 30 °C, in which the polyamides were pretreated by drying in oven at 120 °C for 2 h to remove the adsorbed moisture. Molecular weights were measured on a gel permeation chromatograph (GPC) with polystyrene calibration using JASCO HPLC equipped with Shodex KD-80 M column at 40 °C in DMF. Differential scanning calorimetry (DSC) analyses were conducted on a Mettler Toledo DSC 821e instrument under nitrogen protection at a heating rate of 10 °C/min, and the Tg values were read at the middle of the transition in the heat capacity in the second scan. The samples were pre-heated at 150 °C for 1 h before DSC measurements. Thermogravimetric analysis (TGA) was carried out with a Netzsch Sta 449c thermal analyzer system under nitrogen protection at a heating rate of 20 °C/min. The measurements were taken after an initial 250 °C/10 min drying step. The stress–strain behavior of the polyamide films were studied on an Instron model 1130 universal tester with 60 × 5 mm specimens at a drawing rate of 5 mm/min, and an average of at least five individual determinations was reported. Wide angle X-ray diffraction (WAXD) patterns were recorded at room temperature on a Rigaku D/MAX-IIA X-ray diffractometer with nickel-filtered CuKα radiation (40 kV and 20 mA). Ultraviolet–visible spectra of the polyamide films were obtained on a V-550 UV–vis spectrophotometer. The refractive indices of the polymer films were determined by a prism-coupler method on Sairon SPA-3000 model at 623.8 nm. The palladium content of the catalyst was measured with a Jarrell-Ash 1100 ICP analysis.
Preparation of the 2P-Fe3O4@SiO2-PdCl2 catalyst
A mixture of 3-(N,N-di(diphenylphosphinomethyl))aminopropyltriethoxysilane (0.928 g, 1.5 mmol) and Fe3O4@SiO2 (1.103 g) in dry toluene (50 mL) was stirred at reflux for two days under nitrogen. After the mixture was cooled to room temperature, the resulting product was magnetically separated, followed by washing with toluene to remove the unanchored phosphine ligand and dried at 120 °C in vacuo for 4 h to afford 1.322 g of the bidentate phosphine ligand-modified Fe3O4@SiO2 (2P-Fe3O4@SiO2). The phosphorus content of 2P-Fe3O4@SiO2 was found to be 0.96 mmol/g based on elemental analysis.
A mixture of palladium chloride (73 mg, 0.4 mmol) and 2P-Fe3O4@SiO2 (1.02 g) in dry acetone (50 mL) was stirred at reflux for 3 days under N2. After the reaction mixture was cooled to room temperature, the resulting product was magnetically separated, followed by washing with acetone repeatedly and dried at 80 °C in vacuo for 5 h to afford 1.037 g of 2P-Fe3O4@SiO2-PdCl2. The palladium content of 2P-Fe3O4@SiO2-PdCl2 was found to be 0.38 mmol/g based on ICP-AES.
Synthesis of polymers
A 100 mL, oven-dried reaction tube equipped with a CO inlet and a magnetic stirrer was charged with 4,4′-di(3-iodophenoxy)diphenyl sulfone 1a (0.3273 g, 0.5 mmol), DBU (0.184 g, 1.2 mmol), 2P-Fe3O4@SiO2-PdCl2 (79.5 mg, 0.03 mmol), 9,9-bis[4-(4-aminophenoxy)phenyl]fluorene 2a (0.2715 g, 0.51 mmol), and DMAc (2.5 mL) under an atmosphere of nitrogen. After being evacuated, the reaction tube was backfilled with CO and sealed. The reaction mixture was then heated to 120 °C over 20 min and was stirred for 12 h at 120 °C. After being cooled to room temperature, the resulting viscous polymer solution was further diluted with DMAc (13 mL), and the catalyst was magnetically separated. The polymer solution was then trickled into MeOH (100 mL) with stirring to produce a precipitate. The white fiber-like precipitate was collected by filtration, washed with hot MeOH (3 × 20 mL) and water (2 × 20 mL) and dried at 150 °C in vacuo for 5 h to yield polymer 3aa. The recovered palladium catalyst was washed with DMAc (2 mL), deionized H2O (2 mL), acetone (2 mL), dried at 100 °C in vacuo for 2 h and employed directly in the next polymerization cycle. Other polymers 3ab–3bd were also synthesized by a similar procedure as described for polymer 3aa.
Results and discussion
Preparation of the 2P-Fe3O4@SiO2-PdCl2 catalyst
The bidentate phosphine ligand-modified magnetic nanoparticles-anchored palladium complex[2P-Fe3O4@SiO2-PdCl2] was prepared by referring to our previously reported route as illustrated in Scheme 1 [36]. The silica-coated Fe3O4 (Fe3O4@SiO2) was reacted with 3-(N,N-di(diphenylphosphinomethyl))aminopropyltriethoxysilane at 110 °C in toluene under nitrogen for 2 days to give the bidentate phosphine ligand-modified magnetic nanoparticles (2P-Fe3O4@SiO2). The 2P-Fe3O4@SiO2 was then complexed with palladium chloride in dry acetone at reflux under nitrogen for 3 days to afford the 2P-Fe3O4@SiO2-PdCl2 complex. The palladium content of 2P-Fe3O4@SiO2-PdCl2 was found to be 0.38 mmol/g by using ICP-AES analysis.
Preparation of aromatic diiodide monomers
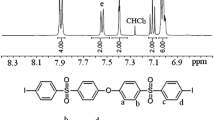
The synthetic routes to aromatic diiodides having ether sulfone linkages 1a and 1b are shown in Scheme 2 [38]. 4,4′-Di(3-iodophenoxy)diphenyl sulfone (1a) was obtained through the nucleophilic substitution reaction of 3-iodophenol with 4,4′-dichlorodiphenyl sulfone in toluene and DMAc with K2CO3 as base (Scheme 2a). On the other hand, 4,4′-di(4-iodophenylsulfonyl)diphenyl ether (1b) was prepared by the Friedel–Crafts acylation reaction of iodobenzene with 4,4′-oxydiphenyldisulfonyl chloride in nitrobenzene in the presence of FeCl3 (Scheme 2b). The 1H-NMR spectra shown in Fig. 1 were in good accordance with the proposed structures of monomers 1a and 1b.
Synthesis and characterization of polymers
Over the past decades, significant efforts have been made to design and synthesize wholly aromatic polyamides containing cardo structures. Generally, the incorporation of cardo structures into the main chains of polyamide imparts good solubility and processability, and better mechanical and thermal behaviors [39,40,41,42,43,44,45]. However, to our knowledge, relatively little attention has been paid to the synthesis of cardo poly(ether sulfone amide)s. To further examine the combined effects of sulfone linkages and cardo groups on the properties of aromatic polyamides, a series of novel aromatic polyamides containing both sulfone linkages and cardo groups were synthesized through a carbonylation and condensation reaction of aromatic diiodides bearing ether sulfone linkages, carbon monoxide, and aromatic diamines with cardo groups in the presence of 2P-Fe3O4@SiO2-PdCl2 as the catalyst. Initially, the carbonylation and condensation reaction of 4,4′-di(3-iodophenoxy)diphenyl sulfone 1a, carbon monoxide, and 9,9-bis[4-(4-aminophenoxy)phenyl]fluorene 2a were studied to screen the optimal reaction conditions including solvents, bases, reaction temperatures, and palladium quantities; the results are given in Table 1. At first, various organic bases including 1,4-diazabicyclo[2,2,2]octane (DABCO), n-Bu3N, 4-(dimethylamino)pyridine (DMAP) and 1,8-diazabicyclo[5,4,0]-7-undecene (DBU) were tested at 120 °C in DMAc. The use of DBU as base was found to be the most efficient and furnished the polymer 3aa with high inherent viscosity of 0.77 dL/g (entries 1–5). Compared to the other tertiary amines used, DBU swells the polyamide apart from acting as an acid acceptor, thereby enhancing the propagation reaction [22]. Replacement of DMAc as solvent with NMP, HMPA, DMF, DMSO, or DMI did not enhance the inherent viscosity of the polymer 3aa (entries 6–10). Lowering reaction temperature to 100 or 110 ºC generated the polymer 3aa with lower inherent viscosities because of lower reaction rates (entries 11 and 12). The reaction rate should increase with the rise in reaction temperature; however, the solubility of carbon monoxide in DMAc decreased gradually with increasing the reaction temperature. The reaction run at 130 or 140 °C also produced the polymer 3aa with a comparatively low inherent viscosity since the actual rate of the reaction was lower than that of the reaction performed at 120 °C (entries 13 and 14). We next studied the influence of the palladium catalyst quantity on the model reaction and found that the use of 6 mol% palladium catalyst was the best choice. Reducing the catalyst quantity to 3 mol% resulted in a lower inherent viscosity of polymer 3aa and demanded a longer reaction time (entry 16). Increasing the catalyst quantity to 10 mol% could enhance the reaction rate, but no significant increase in the inherent viscosity of the polymer was observed (entry 17). It was reported that the synthesis of aromatic polyamides via PdCl2(PPh3)2-catalyzed carbonylative polycondensation generally requires the use of an excess of PPh3 as ligand to prohibit the production of palladium black [22,23,24,25,26,27,28,29,30]. We found that employment of 6 mol% PdCl2(PPh3)2 and 24 mol% PPh3 as the catalytic system gave a low inherent viscosity of the polymer and the generation of palladium black was observed clearly (entry 18). However, when 6 mol% PdCl2(PPh3)2 and 96 mol% PPh3 were used, the polymer 3aa with higher molecular weight could also be generated (entry 19). Thus, the optimal polymerization reaction conditions were the use of 6 mol% 2P-Fe3O4@SiO2-PdCl2 with DBU as base at 120 °C in DMAc as solvent under 1 atm of CO for 12 h without addition of PPh3 (Table 1, entry 4).
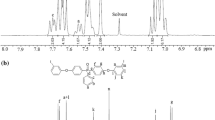
Having established the optimized polycondensation reaction conditions, we next investigated the heterogeneous carbonylation and condensation reaction of aromatic diiodides bearing ether sulfone linkages 1a–b, carbon monoxide, and aromatic diamines with cardo groups 2a–d as presented in Scheme 3, and the results are provided in Table 2. The novel aromatic polyamides containing both sulfone linkages and cardo groups 3aa–3bd were prepared in high yields of 92–95% with higher inherent viscosities between 0.70 and 0.77 dL/g and could be readily converted into flexible, transparent, and tough polyamide films via casting from their solutions in DMAc, revealing that the polyamides with comparatively higher molecular weights could be easily synthesized via the heterogeneous palladium-catalyzed carbonylation and condensation reaction. The molecular weight of polymer 3aa having inherent viscosity of 0.77 dL/g was determined by GPC in DMF relative to narrow polystyrene standards. The GPC trace was unimodal with polydispersity of 1.8. The chromatogram demonstrated that the relative Mn and Mw values were 85,000 and 154,000, respectively. The elemental analysis results of these polyamides are also provided in Table 2. In general, the carbon values determined were slightly lower than the calculated ones for the proposed polymer structures because of moisture intake, and the elemental analysis values are close to the calculated ones. The chain structures of the polymers 3aa–3bd were identified by applying FTIR and 1H NMR spectroscopy. The FTIR spectra of all the polyamides display characteristic absorption peaks at 3320–3450 cm−1 (N–H stretching) and 1650–1670 cm−1 (C=O stretching) based on amide linkages, at 1220–1235 cm−1 (C–O–C stretching) corresponding to aryl ether linkages, as well as 1318 and 1153 cm−1 (-SO2- stretching) due to sulfone linkages, which supporting their chain structures. Besides, the FTIR spectra of polymers 3ab, 3ad, 3bb, and 3bd show an absorption peak at 1135–1145 cm−1 (C–F stretching) owing to CF3 groups. The FT-IR spectra of the polymers 3aa–3bd are presented in Fig. 2. In the 1H-NMR spectra of polymers 3aa–3bd, the proton resonance signals of amide linkages appear as a sharp singlet at δ = 10–11 ppm, and other aromatic proton peaks could also be assigned in the polymer structures proposed. Figure 3 presents a typical 1H-NMR spectrum of the polymer 3ab. The above results indicate that the polyamides containing both sulfone linkages and cardo groups 3aa–3bd have the expected chemical structures.
Polymer solubility and crystallinity
The solubility behavior of the polyamides containing both sulfone linkages and cardo groups 3aa–3bd was evaluated by dissolving 10 mg of the powdery samples in 1 mL of various organic solvents at room temperature, and the results are listed in Table 3. As presented in Table 3, polymers 3aa–3bd could easily be dissolved in polar aprotic organic solvents including DMAc, NMP, DMF, DMSO and even dissolved in less polar pyridine and tetrahydrofuran at room temperature within 3 h. In addition, the polyamides 3bc and 3bd derived from aromatic diiodide 1b and aromatic diamines 2c–d exhibited better solubility than other polymers and could be swelled in acetone at room temperature due to the existence of relatively higher contents of sulfone and ether linkages in the polymer backbone. However, all these polyamides could not be dissolved or swelled in chloroform, toluene, or ethanol even on heating. The excellent solubility of polyamides 3aa–3bd should be attributed to the combined effects of flexible ether and sulfone linkages and bulky cardo structures in the polymer main chain, which increased the distance between polymer chains and the free volume, thereby resulting in an enhanced solubility.
The crystallinity of the polyamides containing both sulfone linkages and cardo groups was evaluated by using wide-angle X-ray diffraction (WAXD). The diffraction patterns illustrated in Fig. 4 indicate that the polymers 3aa–3bd are completely amorphous, which could be attributed to the incorporation of flexible ether and sulfone linkages and bulky cardo structures into polymer backbone, which led to poor packing of polymer chains. The outstanding solubility of the polymers 3aa–3bd in organic solvents should arise from their amorphous structures.
Thermal properties
The thermal behavior of the polyamides containing both sulfone linkages and cardo groups was investigated with DSC and TGA, and the results are provided in Table 4. Figure 5 presents DSC traces of polymers 3aa–3bd. It was evident that there were no melting endothermic peaks on their DSC traces, which revealing very little crystalline structures. The results of DSC analysis were in accordance with the WAXD measurements of the polymers 3aa–3bd. The polymers 3aa–3bd had higher glass transition temperatures (Tg) between 241 and 283 °C owing to the presence of strong polar sulfone linkages and rigid cardo groups in the polymer chain. In general, the polymers 3aa–3ad derived from aromatic diiodide 1a exhibited relatively lower Tg values of 241–254 °C due to the existence of higher content of flexible ether linkages, while polymers 3ba–3bd derived from aromatic diiodide 1b displayed relatively higher Tg values of 256–283 °C owing to the presence of higher content of strong polar sulfone linkages. In addition, polymers 3ba and 3bb displayed high Tg values of 280–283 °C, which may be attributed to the combined effects of higher content of strong polar sulfone linkages and more rigid fluorene cardo groups in the polymer backbone, which resulted in an increase in the interaction of polymer chains.
As seen from Table 4, the design of aromatic polyamides with the incorporation of both polar sulfone linkages and bulky cardo groups imparts not only outstanding solubility but also high thermal stability. From TGA traces of the polymers 3aa–3bd shown in Fig. 6, we found that these polyamides did not exhibit significant weight losses before 425 ºC in nitrogen. However, when the temperature was over 450 °C, a rapid thermal decomposition reaction occurred. Polymers 3aa–3bd had the temperatures at 5 and 10% weight loss in the range of 438–475 and 477–505 °C, respectively, in nitrogen. In addition, the polyamides 3aa–3bd remained 47–63% of original weight at 800 °C in nitrogen.
Mechanical properties
Polyamides 3aa–3bd could be easily converted into transparent, strong, and flexible polymer films via casting their DMAc solutions (15 wt% solid content) on glass plates, followed by evaporating DMAc and drying at 110 °C for 2 h, at 170 °C for 1 h, and at 190 °C in vacuo for 2 h. The unoriented cardo poly(ether sulfone amide) films were then utilized to measure the mechanical properties, and the results are given in Table 5. The tensile strengths, tensile moduli, and elongations at breakage of these polyamide films were found to be in the range of 78.8–84.4 MPa, 2.08–2.57 GPa and 10.2–12.5%, respectively. Polymer 3ad derived from 1a and 2d exhibited the lowest tensile strength and tensile modulus due to the presence of higher content of flexible ether linkages and bulky CF3 groups, which resulted in a decrease in the interaction of polymer chains. In contrast, polymer 3ba based on 1b and 2a showed the highest tensile strength and tensile modulus owing to the existence of higher content of strong polar sulfone linkages and more rigid fluorene cardo groups, which led to an increase in the interaction of polymer chains.
Optical properties
The optical transparency of these polymer films with thickness of 20–25 μm was evaluated by using UV–vis spectroscopy, and their UV–vis spectra are illustrated in Fig. 7. The transmittances of the polymer films were measured at the wavelengths ranging from 300 to 800 nm, and their transmittances at several wavelengths are provided in Table 6. As seen from Table 6, the cut-off wavelengths of these new polyamide films were ranged from 338 to 368 nm and the 80% transmission wavelengths were in the range of 439–536 nm. In comparison, the polyamide films derived from aromatic diiodide 1a showed slightly shorter cut-off wavelengths than the corresponding ones derived from aromatic diiodide 1b due to the presence of relatively higher content of flexible ether linkages in the polymers 3aa–3ad, which led to a decrease in the interaction of polymer chains. Polymer 3ad showed the best transparency with cut-off wavelength at 338 nm and transmittance of 80% at 439 nm owing to the combined effects of high content of ether linkages and fluorinated substituents in the polymer backbone. This can be explained by the decrease in the intermolecular interaction caused by the presence of high content of flexible ether linkages and bulky CF3 groups. The optical transmittance of these polyamides is generally below 80% at 450 nm, which is lower than that of the polyimides containing spirobifluorene structure in the side chain [46]. As shown in Table 6, the in-plane (nTE) and out-of-plane (nTM) refractive indices of the polymer films determined at 632.8 nm ranged from 1.6754 to 1.6995 and 1.6682 to 1.6938, respectively. All the polymer films exhibited higher nTE values than nTM, revealing that the macromolecular chains were preferentially aligned in the film plane. The average refractive index values (nAV) of these polymers ranged between 1.6730 and 1.6976. Polymer 3ad displayed the highest nAV value among the prepared polymers due to the presence of both high content of flexible ether linkages and bulky CF3 groups in the polymer chain.
Palladium leaching and recycle of the catalyst
Recyclability and palladium leaching of this immobilized palladium catalyst were evaluated in the carbonylation and condensation reaction of aromatic diiodide 1a, carbon monoxide, and aromatic diamine 2a for the synthesis of polymer 3aa under the optimal polycondensation reaction conditions. After completion of the carbonylative polymerization reaction, the reaction solution was further diluted with DMAc and the immobilized palladium catalyst could be conveniently separated from polymer 3aa by simply placing a magnet near the reaction tube. The content of residual palladium in polymer 3aa was measured to be 5.8 ppm by ICP-AES analysis. Thus, the leaching of palladium species into the desired polymer was not absolutely ruled out, but appeared to be negligible. After being washed with DMAc (2 mL), deionized water (2 mL), acetone (2 mL), and dried at 100 °C in vacuo for 2 h, the recovered catalyst was directly utilized in the next polymerization cycle. The recovered palladium catalyst was then employed for seven consecutive polymerization cycles with fresh monomers under the identical polymerization conditions, and the results are provided in Table 7. As seen from Table 7, almost consistent ηinh value and the yield of polymer 3aa were observed in eight consecutive polymerization cycles, which implying that 2P-Fe3O4@SiO2-PdCl2 could be reused at least 7 times without any apparent loss of catalytic efficiency. The recycle rate of the catalyst was over 98% for the eight consecutive polymerization cycles. As seen from the TEM images of the recovered palladium catalyst (Fig. 8b) and the fresh one (Fig. 8a), no obvious differences in the morphology and dispersion of particles were observed, indicating that no palladium black was formed during the process of polycondensation. The excellent reusability and negligible Pd leaching of 2P-Fe3O4@SiO2-PdCl2 might be mainly due to the stronger chelating action between palladium atom and the bidentate phosphine ligand.
Conclusion
A new, efficient and practical synthetic route to aromatic polyamides containing both sulfone linkages and cardo groups with higher molecular weights has been developed through heterogeneous palladium-catalyzed carbonylation and condensation reaction of aromatic diiodides bearing ether sulfone linkages, carbon monoxide, and aromatic diamines with cardo groups by using a bidentate phosphine ligand-modified magnetic nanoparticles-anchored palladium complex [2P-Fe3O4@SiO2-PdCl2] as the catalyst. The resulting cardo poly(ether sulfone amide)s displayed outstanding solubility and high thermal stability with the glass transition temperatures between 241 and 283 °C, the temperatures at 5% weight loss ranging from 438 to 475 °C in nitrogen. These polymer films exhibited good mechanical properties with tensile strengths of 78.8–84.4 MPa, tensile moduli of 2.08–2.57 GPa, elongations at breakage of 10.2–12.5%, and optically high transparency with cut-off wavelengths in the range of 338–368 nm. More importantly, the 2P-Fe3O4@SiO2-PdCl2 catalyst can be facilely separated from the desired polymer simply by placing a magnet near the reaction tube and reused at least seven times without any apparent decrease in the catalytic activity, thus making the current methodology economically and environmentally more acceptable.
References
Reglero Ruiz JA, Trigo-Lopez M, Garcia FC, Garcia JM (2017) Functional aromatic polyamides. Polymers 9(12):414–414
Liou GS, Hsiao SH (2002) Synthesis and properties of new soluble aromatic polyamides and polyimides on the basis of N, N′-bis(3-aminobenzoyl)-N, N ′- diphenyl-1,4-phenylenediamine. J Polym Sci Part A Polym Chem 40:2564–2574
Liaw DJ, Hsu PN, Chen WH, Lin SL (2002) High glass transitions of new polyamides, polyimides, and poly(amide-imide)s containing a triphenylamine group: synthesis and characterization. Macromolecules 35:4669–4675
Wu SC, Shu CF (2003) Synthesis and properties of soluble aromatic polyamides derived from 2,2’-bis(4-carboxyphenoxy)-9,9’-spirobifluorene. J Polym Sci Part A Polym Chem 41:1160–1166
Garcia JM, Garcia FC, Serna F, de laPena JL (2010) High-performance aromatic polyamides. Prog Polym Sci 35:623–686
Bera D, Padmanabhan V, Banerjee S (2015) Highly gas permeable polyamides based on substituted triphenylamine. Macromolecules 48:4541–4554
Dewilde S, Hoogerstraete TV, Dehaen W, Binnemans K (2018) Synthesis of poly-p-phenylene terephthalamide (PPTA) in ionic liquids. ACS Sustainable Chem Eng 6:1362–1369
Pascual BS, Trigo-Lopez M, Ramos C, Sanz MT, Pablos JL, Garcia FC, Reglero Ruiz JA, Garcia JM (2019) Microcellular foamed aromatic polyamides (aramids). Structure, thermal and mechanical properties. Eur Polym J 110:9–13
Trigo-Lopez M, Garcia JM, Reglero Ruiz JA, Garcia FC, Ferrer R (2018) Aromatic polyamides. In: Mark HF (ed) Encyclopedia of polymer science and technology. Wiley, New Jersey, pp 1–51
Liou G-S, Hsiao S-H, Ishida M, Kakimoto M, Imai Y (2002) Synthesis and characterization of novel soluble triphenylamine-containing aromatic poly- amides based on N, N’-bis(4-aminophenyl)-N, N’-diphenyl-1,4-phenylenediamine. J Polym Sci Part A Polym Chem 40:2810–2818
Liou G-S, Hsiao S-H (2002) Polyterephthalamides with naphthoxy-pendent groups. J Polym Sci Part A Polym Chem 40:1781–1789
Hsiao S-H, Chen W-T (2003) Syntheses and properties of novel fluorinated polyamides based on a bis(ether-carboxylic acid) or a bis(ether amine) extended from bis(4-hydroxyphenyl)phenyl-2,2,2-trifluoroethane. J Polym Sci Part A Polym Chem 41:420–431
Liaw D-J, Liaw B-Y (1998) Synthesis and properties of new polyamides derived from 1,4-bis(4-aminophenoxy)-2,5-di-tert-butylbenzene and aromatic dicarboxylic acids. J Polym Sci Part A Polym Chem 36:1069–1074
Espeso JF, Ferrero E, de la Campa JG, Lozano AE, de Abajo J (2001) Synthesis and characterization of new soluble aromatic polyamides derived from 1,4-bis(4-carboxyphenoxy)-2,5-di-tert-butylbenzene. J Polym Sci Part A Polym Chem 39:475–485
Johnson RN, Farnham AG, Clendinning RA, Hale WF, Merriam CN (1967) Poly(aryl ethers) by nucleophilic aromatic substitution. I. Synthesis and properties. J Polym Sci Part A Polym Chem 5:2375–2398
Attwood TE, King T, Leslie VJ, Rose JB (1977) Poly(arylene ether sulphones) by polyetherification: 2. Polycondensations Polymer 18:359–364
Harris JE, Johnson RN (1985) Polysulfone. In: Mark HF, Bikales NB, Overberger CG, Menges G (eds) Encyclopedia of polymer science and engineering, vol 11, 2nd edn. Wiley, New York, p 196
Knight J, Wright WW (1983) Heat-resistant polymers. Plenum, New York, p 170
Brode GL, Kwiatkowski GT, Bedwin AW (1974) High temperature polymers. II. High temperature polymers from 4,4′-[sulfonylbis(p-phenyleneoxy)]dianiline. J Polym Sci Part A Polym Chem 12:575–587
Chiriac C, Stille JK (1977) Polyaramides containing sulfone ether units. Macromolecules 10:712–713
Mehdipour-Ataei S, Sarrafi Y, Hatami M, Akbarian-Feizi L (2005) Poly(sulfone ether amide amide)s as a new generation of soluble, thermally stable polymers. Eur Polym J 41:491–499
Yoneyama M, Kakimoto M, Imai Y (1988) Novel synthesis of aromatic poly- amides by palladium-catalyzed polycondensation of aromatic dibromides, aromatic diamines, and carbon monoxide. Macromolecules 21:1908–1911
Yoneyama M, Kakimoto M, Imai Y (1989) Synthesis of aliphatic-aromatic polyamides by palladium-catalyzed polycondensation of aliphatic diamines, aromatic dibromides, and carbon monoxide. J Polym Sci Part A Polym Chem 27:1985–1991
Turner SR, Perry RJ, Blevins RW (1992) High molecular weight aromatic polyamides from aromatic diiodides and diamines. Macromolecules 25:4819–4820
Perry RJ, Turner SR, Blevins RW (1993) Synthesis of linear, high molecular weight aromatic polyamides by the palladium-catalyzed carbonylation and condensation of aromatic diiodides, diamines, and carbon monoxide. Macromolecules 26:1509–1513
Perry RJ, Turner SR, Blevins RW (1994) Palladium-catalyzed formation of poly(imide-amides). 1. reactions with diiodo imides and diamines. Macromolecules 27:4058–4062
Ueda M, Yokoo T (1994) Synthesis of poly(ether-ketone-amide)s by palladium- catalyzed polycondensation of aromatic dibromides containing ether ketone structure, aromatic diamines, and carbon monoxide. J Polym Sci Part A Polym Chem 32:2065–2071
Ueda M, Yokoo T, Nakamura T (1994) Synthesis of poly(ether-sulfone-amide)s by palladium-Catalyzed Polycondensation of aromatic dibromides containing ether sulfone structure, aromatic diamines, and carbon monoxide. J Polym Sci Part A Polym Chem 32:2989–2995
Perry RJ, Turner SR, Blevins RW (1995) Palladium-catalyzed formation of poly(imide-amides). 2 reactions with chloroiodophthalimides and diamines. Macromolecules 28:2607–2610
Rabani G, Kraft A (2002) Synthesis of poly(ether-esteramide) elastomers by palladium-Catalyzed Polycondensation of aromatic diiodides with telechelic diamines and carbon monoxide. Macromol Rapid Commun 23:375–379
Stevens PD, Li G, Fan J, Yen M, Gao Y (2005) Recycling of homogeneous Pd catalysts using superparamagnetic nanoparticles as novel soluble supports for Suzuki, Heck, and Sonogashira cross-coupling reactions. Chem Commun 35:4435–4437
Baruwati B, Guin D, Manorama SV (2007) Pd on surface-modified NiFe2O4 nanoparticles: a magnetically recoverable catalyst for Suzuki and Heck reactions. Org Lett 9:5377–5380
Jin M-J, Lee D-H (2010) A practical heterogeneous catalyst for the Suzuki, Sonogashira, and Stille coupling reactions of unreactive aryl chlorides. Angew Chem Int Ed 49:1119–1122
Shylesh S, Wang L, Thiel WR (2010) Palladium(II)-phosphine complexes supported on magnetic nanoparticles: filtration-free, recyclable catalysts for Suzuki-Miyaura cross-coupling reactions. Adv Synth Catal 352:425–432
Li P, Wang L, Zhang L, Wang G-W (2012) Magnetic nanoparticles-supported palladium: a highly efficient and reusable catalyst for the Suzuki, Sonogashira, and Heck reactions. Adv Synth Catal 354:1307–1318
Tang H, Huang B, Zhu X, Cai M (2018) Synthesis of poly(ether ketone amide)s containing 4-aryl-2,6-diphenylpyridine moieties by a heterogeneous palladium- catalyzed polycondensation of aromatic diiodides, aromatic diamines, and carbon monoxide. Polym Adv Technol 29:2204–2215
Liu L, Zou F, Zhang R, Cai M (2019) Synthesis of new fluorinated aromatic poly (ether ketone amide)s containing cardo structures by a heterogeneous palladium-catalyzed carbonylative polycondensation. Polym Adv Technol 30:58–69
Liu L, Li J, Yan T, Cai M (2020) Novel preparation of poly(arylene ether sulfone amide)s via supported palladium-catalyzed carbonylative polymerization. Polym Bull 77:1951–1968
Hu Z, Li S, Zhang C (2007) Synthesis and properties of polyamide-imides containing fluorenyl cardo structure. J Appl Polym Sci 106:2494–2450
Yang CP, Su YY, Hsu MY (2006) Synthesis and properties of fluorinated polyamides and poly(amide imide)s based on 9,9-bis[4-(4-amino-2-trifluoro- methylphenoxy)phenyl]fluorene, aromatic dicarboxylic acids, and various monotrimellitimides and bistrimellitimides. Colloid Polym Sci 284:990–1000
Sheng S, Li T, Jiang J, He W, Song C (2010) Synthesis and properties of novel polyamides containing sulfone-ether linkages and xanthene cardo groups. Polym Int 59:1014–1020
Sheng S-R, Ma C-X, Jiang J-W, Li Q, Song C-S (2011) Optically high transparency and light color of organosoluble fluorinated polyamides with bulky xanthene pendent groups. Polym Adv Technol 22:2523–2532
Yang CP, Lin JH (1995) Syntheses and properties of aromatic polyamides and polyimides based on 3,3-bis[4-(4-aminophenoxy)phenyl]phthalimidine. Polymer 36:2607–2614
Liaw DJ, Liaw BY, Chung CY (1999) Synthesis and characterization of new cardo polyamides derived from 8,8-bis[4-(4-aminophenoxy)phenyl]tricycle- [5.2.1.02,6]decane. Macromol Chem Phys 200:1023–1027
Liaw DJ, Liaw BY, Chung CY (2000) Synthesis and characterization of new cardo polyamides and polyimides containing tert-butylcyclohexylidene units. Macromol Chem Phys 201:1887–1893
Wen P, He R, Li X-D, Lee M-H (2017) Synthesis and characterization of high refractive index and low birefringence polyimides containing spirobifluorene in the side chain. Polymer 117:76–83
Acknowledgements
We thank the National Natural Science Foundation of China (Project 21664008), Natural Science Foundation of Jiangxi Province in China (Project 20181BAB203011) and Key Laboratory of Functional Small Organic Molecule, Ministry of Education (No. KLFS-KF-201704) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Huang, B., Tang, H. et al. Synthesis of novel aromatic polyamides containing both sulfone linkages and cardo groups by a recyclable palladium-catalyzed carbonylation and condensation polymerization. Polym. Bull. 79, 3333–3352 (2022). https://doi.org/10.1007/s00289-021-03675-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03675-0