Abstract
Copper is a fascinating metal with wide applications since ancient time. Although Copper is resistant to certain environmental factors and several chemicals, aggressive medium could deteriorate the metal leading to its complete damage. The need for an eco-friendly corrosion inhibitor is much relevant in the present scenario. The inhibition efficiency of gum arabic (GA), sodium alginate (SA) and its blend is investigated using weight loss method, potentiodynamic polarization and electroscopic impedance spectroscopy (EIS) analysis in 1.0 M HCl solution. Higher inhibition efficiency is obtained at room temperature for the blend with 6666 ppm GA and 3333 ppm SA. The values of inhibition efficiency obtained are 88.8%, 80.5% and 86.2% using weight loss, potentiodynamic polarization and EIS analysis, respectively. The investigations are complemented by atomic force microscopy and field emission scanning electron microscopy which helped to analyze the physical adsorption of the biopolymer on the metal surface. The adsorption of the biopolymer on Copper obeyed Langmuir isotherm and inhibition efficiency has an inverse relation with temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper metal is extensively used in construction, electrical and transportation owing to its high thermal and electrical conductivity [1]. Copper is having face-centered cubic crystal structure with a thermal conductivity of 401 W/mK [2]. Corrosion adversely affects the performance of metal and periodic cleaning is necessary to maintain its efficiency. The cleaning of these oxide layers is done in industries by acid pickling [3]. Acidic medium for cleaning metals destroys its intrinsic properties. This can be prevented by addition of corrosion inhibition substances along with the cleaning medium [4].
Corrosion inhibitors are generally classified into anodic inhibitors, cathodic inhibitors and mixed inhibitors. Most anodic inhibitors contain CrO42−, NO2− or MoO42− that forms a passive layer on the surface of anode [5]. This type is not always recommended, because it may increase the corrosion rate if the treatment rate is not sufficient. Cathodic inhibitors are oxygen scavengers, cathodic poisoner or cathodic precipitates that precipitates on cathodic surface by limiting the cathodic chemical reaction. Mixed inhibitors work by adsorbing on metal surface by chemisorption or physisorption. Most used corrosion inhibitors are mixed type like sulfur-containing compounds, amines, mercaptobenzothiazole, imidazoline or nitrogen containing compounds [6]. Inorganic and organic coatings were widely used as corrosion inhibitors. However, the wide use of these coatings in contact with the environment creates severe environmental pollution [7]. These problems with synthetic or chemical based corrosion inhibitors can be solved by the use of environmental friendly materials as corrosion inhibitors [8,9,10]. The use of polymer, polymer blends or copolymers for various applications is not new [9, 11, 12]. In the present study, biomaterials like gum arabic (GA) [13]and sodium alginate (SA) [11, 14] are used as corrosion inhibitors and their anti-corrosion activity is attributed to the existence of heteroatoms like nitrogen, oxygen and cyclic rings in their heterostructures.
GA is a natural gum obtained from the hardened sap of Acacia trees belonging to Leguminosae family. Water soluble GA is made up of a mixture containing arabinogalactan, oligosaccharides, polysaccharides and glycoproteins [15]. Functional groups in GA form metal complexes with copper and behave as a protective coating shielding it from corrosion. Sucrose, polysaccharides, arabinogalactan, oligosaccharides and glycoprotein present in GA helps in adsorbing to the metal surface. Shen et al. recently reported the corrosion inhibition efficiency of GA in oil industry for carbon steel. GA forms a film on carbon steel by means of chemisorption and thereby prevents corrosion in the oil-filled synthetic brine environment achieving 94% corrosion inhibition efficiency at room temperature [12]. GA was also used to inhibit the corrosion caused by NaOH on aluminum. The results showed that the inhibition is caused by the adsorption of GA and followed Langmuir and Freundlich adsorption isotherms [13]. Umoren et al. studied the corrosion behavior of mild steel and aluminum under acidic medium in the presence of GA. Physical adsorption was observed for GA with aluminum and chemical adsorption was observed with mild steel. The inhibition efficiency increased with an increase in GA concentration at 0.5 g/L which results in 37.88% at 60 °C for mild steel and 79.69% at 30 °C for aluminum [16].
SA is a biopolymer obtained from the cell walls of brown algae which grows in sea, such as Laminaria or Fucus [17]. Obot et al. showed the inhibition effect of SA on the corrosion of API X60 steel in 3.5% NaCl solution [14]. Jmiai et al. reported that alginate biopolymer inhibited the corrosion of copper in 1 M HCl solution. They studied that alginate biopolymer adsorbed physically on copper surface with an inhibition efficiency of 83% at 0.1 mg/L−1 [18].
Separate works are available for the inhibition effect of SA and GA on various metals both under acidic and alkaline medium. The present study introduced a green polymer blend comprising of GA and SA as a corrosion inhibitor of the copper in dilute HCl (1.0 M). A single miscible phase of GA and SA is formed as a corrosion inhibitor and inhibition efficiency is compared with pure GA and SA. The corrosion inhibition efficiency was studied by carrying electrochemical analysis including electrochemical impedance spectroscopy (EIS). AFM and FE-SEM studies were performed to understand the morphology and adsorption effect of blend on the metal surface.
Methodology
Materials
Copper sheet of 35 Gauge (0.191 mm thickness) was purchased from ART IFACT company, India. Hydrochloric acid (37% w/w) was purchased from Sisco Research Laboratories, Mumbai. SA, GA, methanol (> 99.8%) and acetone (> 99%) were purchased from Sigma Aldrich, USA. Deionized (DI) water obtained from Isochem laboratories, Cochin, was used for preparing the solutions.
Preparation of solutions
1.0 M HCl is prepared by diluting 4.1 ml concentrated HCl in 45.9 ml DI water and stirred well using a glass rod. GA and SA in required concentration as mentioned in Table 1 is added to 50 ml of 1.0 M HCl solution and stirred continuously for about 1 h using a magnetic stirrer. Thus, copper coupons are dipped in this prepared solution for performing the potentiodynamic polarization studies and EIS analysis.
Material characterization
The electrochemical measurements were analyzed by CH Instruments Software version 18.01. AFM images were obtained with Park Systems XE-70, Germany, in non-contact mode using a cantilever tip. All the electrochemical measurements were taken in Electrochemical Workstation, 604 D, CH Instruments, USA. Morphological analysis of the copper coupons before and after corrosion was performed using FE-SEM integrated with EDS/EBSD, Supra 40 VP (Carl Zeiss), Germany.
Gravimetric corrosion measurements
Before performing all experiments, copper coupon was washed with distilled water and acetone followed by degreasing with methanol and dried at 50 °C for 5 min.
For mass loss experiment, copper coupon of dimensions (1 cm × 1 cm) was cut from the copper sheet with an exposed surface area of 1.0 cm2 by masking the other surface and used for the all experiments. The masses were measured using a Shimadzu electronic weighing balance of model ATY224 with a resolution of 0.0001 mg. The initial mass (Mi) of copper coupon after proper cleaning are obtained using the electronic balance. Copper coupons are then immersed in 1.0 M HCl for 24 h with and without the presence of GA, SA and blend. After immersion, copper coupon is removed from the solution, washed with distilled water, cleaned with methanol and dried at 50 °C. The final mass (Mf) is measured and the corrosion rate (CR) from mass loss is calculated from the equation given below.
where S is area of the copper coupon (1 cm2), and T is the time period of immersion (h).
The inhibition efficiency is evaluated by,
where CR is the corrosion rate without inhibitor and CR(inh) is the corrosion rate with inhibitor.
Electrochemical corrosion measurements
The working electrode, counter electrode and reference electrode used were copper coupon, platinum wire and Ag/AgCl rod, respectively. 1.0 M HCl solution with different concentration of blend was used as electrolyte in which the electrodes were immersed. The solutions were bubbled with nitrogen gas (99.99% purity) at the flow rate of 50 ml/min for 30 min. The working electrode after proper cleaning is connected to the workstation. The platinum electrode was polished with 1.0 µm, 0.3 µm and 0.05 µm α-alumina powder before each experiment. The electrochemical corrosion analysis was carried out between the potential range of 0.0 mV and − 0.2 mV at a scan rate of 10.0 mVs−1. Electrochemical impedance spectra were recorded in the range 105–1 Hz with an ac voltage amplitude of 0.005 V.
The CR was evaluated from the following equation
where “ew” is the equivalent weight in grams/equivalent, “A” is the area in cm2, “d” is the density in g/cm3 and Icorr is the corrosion current density in µA/cm2.
Results and discussions
Gravimetric analysis of corrosion rate
Effect of concentration
The CR, surface coverage and inhibition efficiency of GA, SA and blends on copper after an immersion time of 24 h at room temperature (29.85 °C) are shown in Table 2. The results proved that CR decreased with increase in inhibitor concentration and least value of CR is obtained for GASA3. The inhibition rate of SA investigated by Jmiai et al. on copper in 1.0 M HCl was 78% at a concentration of 10−1 mg/L [18]. The IE% obtained for 1 g/L of GA on mild steel in 1.0 M HCl is 95% at 303 K [10]. The ability to adsorb on metal surface has increased on blend formation. The changes happened to the spectral bands, crystallinity and intensity peaks on blend formation have been discussed in our previous work [19]. The biopolymers exhibit polar grouping nature and form metal complexes with copper coupon. The metal complexes formed increases with increase in concentration of biopolymers and are adsorbed on copper coupon preventing further deterioration [20].
Effect of temperature on corrosion activation energy
The corrosion activation parameters with and without the presence of inhibitor are investigated by gravimetric method for an immersion time of 24 h at different temperatures of 303 K, 308 K, 313 K and 318 K in a hot air oven. The results are summarized in Table 3 showing the inhibition efficiency of prepared samples decreases with increase in temperature which can be attributed to the desorption of inhibitor from copper coupon and thereby increasing the probability of acid attack leading to dissolution of the metal [21]. The activation energy value (Ea) was calculated from graphical plot of Log CR versus 1000/T as shown in Fig. 1a using the following form of Arrhenius equation
where T is the absolute temperature in Kelvin (K), R is the molar gas constant (8.314 J/mol·K) and C is the Arrhenius constant.
The value of Ea calculated from the plot is 24.409 kJ/mol for blank medium without inhibitor after 24 h of immersion time. Ea obtained for GA5, SA5, and GASA3 is found to be 45.56 kJ/mol, 34.89 kJ/mol and 56.93 kJ/mol, respectively. The minimum activation threshold energy needed for chemical adsorption was reported to be 80 kJ/mol [22]. The results obtained for Ea in the presence of inhibitor is greater than that without inhibitor. The molecules of polymer get physically adsorbed on the active sites of copper and form as a barrier preventing it from further corrosion [23]. It is observed that an increase in temperature increases the corrosion rate which is attributed to the faster electrochemical reaction at higher temperatures. The chemical bonds which join the inhibitor molecule to the metal surface gets impaired at higher temperature due to the rise in enthalpy and Gibbs free energy value [16]. The stability of the film formed on the surface of the metal gets reduced as a result of this. Increase in temperature adds on energy to the chemical reaction and thereby leads to higher corrosion rate.
Adsorption isotherm
In order to understand and investigate the adsorption of inhibitor medium on copper coupon, it is necessary to study the adsorption isotherm which is shown in Fig. 1b. There exist different adsorption isotherm plots like Freundlich, Langmuir, BET, Frumkin, etc., among which Langmuir adsorption fits best with the experimental data and provides a satisfactory explanation for adsorption of polymers on copper coupon in the present study. Adsorption of biopolymer molecules on copper coupon is the most probable technique by which metal corrosion is prevented. The Langmuir adsorption isotherm in its linear form is given by,
where Kads is the adsorption/desorption equilibrium constant, C is the concentration and θ is the degree of surface coverage given in Table 2. The graph of C/θ versus C plot fitted best with the experimental data at 303 K.
Gibbs adsorption energy (∆G) provides information about the nature of adsorption of molecules.
where R is the gas constant and T is the absolute temperature (K) and 55.5 is the molar concentration of water.
The sign of ∆G obtained greatly influence the corrosion behavior of the metal surface in the presence of inhibitor medium. When the anodic potential exceeds the cathodic potential, the reaction becomes endergonic and ∆G is positive for such reactions. The corroding behavior can be suppressed only when ∆G is positive and corrosion potential (Ecorr) value is an indicator for the stability of copper coupon against corrosion without the presence of an external current [24]. The values obtained for ∆G are 20.06 kJ/mol and 20.64 kJ/mol for SA and GA, respectively, indicating physical adsorption.
Morphological analysis of copper coupon
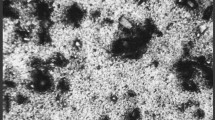
The surface and morphological analysis of copper coupon before and after immersion with and without the presence of inhibitor in 1.0 M HCl medium was investigated using FE-SEM analysis. The FE-SEM image of cleaned copper coupon before immersion shows smooth and clear surface, whereas the image after immersion shows abrasions on the surface. Figure 2a shows the image of copper coupon before immersion and Fig. 2b shows the image of corroded surface with small pits and rough scratches created by acidic medium. The highly corroded surface has triangular shaped crystalline aggregates in the absence of inhibitor medium. However, the presence of inhibitor relatively reduces abrasion marks and small pits created by the acidic medium. Copper coupon retrieved after immersing in the acidic medium containing inhibitor shows relatively smooth surface with less abrasions. The aggregates of inhibitor polymer molecules on metal surface are clearly visible in Fig. 2c–e.
The EDS spectra obtained provide information about the elementary composition of copper coupon before and after immersion in the acidic medium with and without the presence of inhibitor. The spectra of copper coupon prior to immersion in Fig. 2a show the presence of copper and zinc. Abraded copper coupon after immersion shows the presence of chlorine and oxygen in addition to copper and zinc. The percentage of chlorine is found to decrease in the presence of inhibitor (1.81 weight% for GASA3), whereas it is very high for metal coupon immersed in HCl (18.22 weight%) alone.
Figure 3 shows the AFM image of copper coupon with and without the presence of inhibitor after immersion in 1.0 M HCl solution. This technique provides information on the extend of corrosion and roughness of the metal surface. Figure 3a shows the image of smooth surface of copper coupon before immersion in the acidic medium and Fig. 3b shows the image of abraded copper surface after immersion in the acidic medium. Figure 3c–e shows the image of physically attached polymers on the surface of copper coupon.
Electrochemical method for corrosion parameters
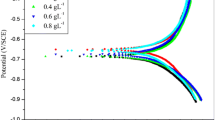
The potentiodynamic polarization curves of SA, GA, and its blend at different concentrations are shown in Fig. 4. The observed values of Icorr, Ecorr, CR and IE% for all the samples are depicted in Table 4. Icorr value is obtained using CH Instruments Software version 18.01 from their respective plots. The polarization curve permits us to understand and analyze the variation in corrosion parameters with and without the addition of inhibitors at different concentration. The corrosion current is directly related to the rate of corrosion by Eq. (3).as follows
The corrosion inhibition efficiency is calculated by,
where Icorr and Icorr(inh) are the corrosion current density in the absence and presence of inhibitor medium, respectively. The results obtained from Fig. 4 show that anodic and cathodic current densities decrease with increase in the addition of inhibitors and are shown in Table 5. It was reported that GA and alginate derivatives behaves as mixed type inhibitors [25, 12]. From the Tafel plot analysis of the GASA1, GASA2 and GASA3, it is clear that the addition of inhibitor medium shifted both cathodic and anodic curves to lower values of current density. This indicated that the three blend samples behave as mixed type of corrosion inhibitor. From Table 4, the least value of corrosion current is obtained for GASA3 indicating that it adsorbs more thereby decreasing the corrosion kinetics on the copper surface than GASA2 and GASA3. Thus, the improved inhibition efficiency of GASA3 is attributed to its amphiphilic nature due to the presence of GA and the subsequent formation of protective stable layer which prevented the exposure of metal surface to the acidic solution [12].
Electrochemical impedance measurements for inhibition efficiency
Electrochemical impedance spectroscopy measurements were carried out for determining the impedance characteristics of the metal/electrolyte interface. The Nyquist plot of copper coupon after 24 h of immersion time in GA5, SA5, GASA3 and acidic medium without inhibitor are depicted in Fig. 5. The electronic circuit corresponding to the best fit curve for the experimental data are shown in Fig. 6. The plots give depressed semicircular loops as a result of the non-ideal character of the interface of the metal coupon in the presence of corrosive acidic medium. The diameter of semicircular loops of GA5, SA5 and GASA3 are greater when compared to that of blank copper coupon. The inhibitor medium develops a protective thin coating on copper coupon and thereby increases the impedance of metal–electrolyte interface. The electrochemical parameters are given in Table 5. Chen et al. studied the inhibition rate of GA on carbon steel in synthetic brine and obtained 83.5% at 5 g/L by electroscopic impedance spectroscopy [12]. The inhibition rate obtained for SA on API X60 steel in 3.5% NaCl solution at 1000 ppm was 87.2% at 25 °C [14]. The resistance of electrolytic solution is given by RS; RT is the total sum of the resistance of film formed (RF) and polarization resistance (RP = RP1 + RP2) for equivalent circuit in Fig. 6a, b and (RP = RP1 + RP2 + RP3) for Fig. 6c. Q is called the constant phase element, CF and CDL are called the film capacitance and double layer capacitance, respectively. The inhibition efficiency is calculated by,
where RT is the total resistance in the absence of inhibitor medium and RT(inh) is the total resistance in the presence of inhibitor medium. The values of inhibition efficiency calculated using above equation listed in Table 5 are comparable with that obtained from electrochemical corrosion analysis by Tafel plot.
Conclusion
The green biopolymer blend GASA3 exhibited good inhibition efficiency against copper coupon corrosion in acidic medium (HCl) than GA and SA alone. The physical adsorption of biopolymers and its blend were proved from Arrhenius plot and Langmuir isotherm. Copper coupon before immersion shows smooth surface in the SEM image, whereas that after immersion shows rough scratches and pits in the absence of inhibitor medium. Copper coupon after immersion in the acidic medium with the inhibitor shows very less abrasions and scratches. The inhibition efficiency of the biopolymers and the prepared blends were investigated by gravimetric analysis, potentiodynamic polarization and EIS methods. The maximum inhibition efficiency is obtained for GASA3 which are 88.8%, 80.5%, 86.2% after gravimetric method, potentiodynamic polarization and EIS methods respectively.
Code availability
All data are available online in Mendeley dataset through the given code: 10.17632/mj6jd2cp75.4.
References
Sherif E-SM (2012) Corrosion behavior of copper in 0.50 M hydrochloric acid pickling solutions and its inhibition by 3-amino-1, 2, 4-triazole and 3-amino-5-mercapto-1, 2, 4-triazole. Int J Electrochem Sci 7:1884–1897
Saranarayanan R, Lakshminarayanan AK, Venkatraman B (2019) A combined full-field imaging and metallography approach to assess the local properties of gas tungsten arc welded copper–stainless steel joints. Arch Civ Mech Eng 19(1):251–267
Gong W, Xu B, Yin X, Liu Y, Chen Y, Yang W (2019) Halogen-substituted thiazole derivatives as corrosion inhibitors for mild steel in 0.5 M sulfuric acid at high temperature. J Taiwan Inst Chem Eng 97:466–479
Li X, Deng S, Xie X (2014) Experimental and theoretical study on corrosion inhibition of o-phenanthroline for aluminum in HCl solution. J Taiwan Inst Chem Eng 45(4):1865–1875
Palanisamy G (2019) Corrosion inhibitors. In: Singh A (ed) Corrosion inhibitors. IntechOpen, London
Lece HD, Emregül KC, Atakol O (2008) Difference in the inhibitive effect of some Schiff base compounds containing oxygen, nitrogen and sulfur donors. Corros Sci 50(5):1460–1468
Zarras P, Stenger-Smith JD (2015) Smart inorganic and organic pretreatment coatings for the inhibition of corrosion on metals/alloys. In: Tiwari A, Rawlins J, Hihara LH (eds) Intelligent coatings for corrosion control. Elsevier, Amsterdam, pp 59–91
Swaroop BS, Victoria SN, Manivannan R (2016) Azadirachta indica leaves extract as inhibitor for microbial corrosion of copper by Arthrobacter sulfureus in neutral pH conditions—a remedy to blue green water problem. J Taiwan Inst Chem Eng 64:269–278
Kim S et al (2020) Development of polyetherimide composites for use as 3D printed thermal protection material. J Mater Sci. https://doi.org/10.1007/s10853-020-04676-6
Azzaoui K et al (2017) Eco friendly green inhibitor Gum Arabic (GA) for the corrosion control of mild steel in hydrochloric acid medium. Corros Sci 129:70–81
Dang N, Wei YH, Hou LF, Li YG, Guo CL (2015) Investigation of the inhibition effect of the environmentally friendly inhibitor sodium alginate on magnesium alloy in sodium chloride solution. Mater Corros 66(11):1354–1362
Shen C, Alvarez V, Koenig JDB, Luo J-L (2019) Gum Arabic as corrosion inhibitor in the oil industry: experimental and theoretical studies. Corros Eng Sci Technol 54(5):444–454
Umoren SA, Obot IB, Ebenso EE, Okafor PC, Ogbobe O, Oguzie EE (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti Corros Methods Mater 53(5):277–282
Obot IB, Onyeachu IB, Kumar AM (2017) Sodium alginate: a promising biopolymer for corrosion protection of API X60 high strength carbon steel in saline medium. Carbohydr Polym 178:200–208
Verbeken D, Dierckx S, Dewettinck K (2003) Exudate gums: occurrence, production, and applications. Appl Microbiol Biotechnol 63(1):10–21
Umoren SA (2008) Inhibition of aluminium and mild steel corrosion in acidic medium using Gum Arabic. Cellulose 15(5):751
Papageorgiou SK, Kouvelos EP, Favvas EP, Sapalidis AA, Romanos GE, Katsaros FK (2010) Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr Res 345(4):469–473
Jmiai A, El Ibrahimi B, Tara A, El Issami S, Jbara O, Bazzi L (2018) Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: experimental and theoretical approaches. J Mol Struct 1157:408–417
Bindhu B, Nair RM (2020) A polymer blend from Gum Arabic and Sodium Alginate-preparation and characterization. J Polym Res. https://doi.org/10.1007/s10965-020-02128-y
Izadi M, Shahrabi T, Ramezanzadeh B (2017) Electrochemical investigations of the corrosion resistance of a hybrid sol–gel film containing green corrosion inhibitor-encapsulated nanocontainers. J Taiwan Inst Chem Eng 81:356–372
Sangeetha Y, Meenakshi S, Sundaram CS (2016) Corrosion inhibition of aminated hydroxyl ethyl cellulose on mild steel in acidic condition. Carbohydr Polym 150:13–20
Hamdy A, El-Gendy NS (2013) Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt J Pet 22(1):17–25
Kumar S et al (2016) Experimental and theoretical studies on inhibition of mild steel corrosion by some synthesized polyurethane tri-block co-polymers. Sci Rep 6:30937
Shukla SK, Ebenso EE (2011) Corrosion inhibition, adsorption behavior and thermodynamic properties of streptomycin on mild steel in hydrochloric acid medium. Int J Electrochem Sci 6(8):3277–3291
Tawfik SM (2015) Alginate surfactant derivatives as ecofriendly corrosion inhibitor for carbon steel in acidic environment. RSC Adv 5:104535–104550
Acknowledgement
We have to express our appreciation to J Joseph (Dept. of Chemistry, Noorul Islam Centre for Higher Education) for extending his research facilities to carry out our research work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Availability of data and material
Required data or original images obtained during the study will be available from the corresponding author on request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nair, R.M., Bindhu, B., Manikandanath, N.T. et al. An eco-friendly green biopolymer blend for copper corrosion inhibition. Polym. Bull. 79, 121–136 (2022). https://doi.org/10.1007/s00289-020-03491-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03491-y