Abstract
Conducting polymers remain as a key invention to the researchers in the last 3 decades. Among them, polyaniline serves as a potential candidate with feasible strategies to solve the current problems. Polyaniline is known for its extraordinary features such as ease of synthesis, low cost, considerable electrical conductivity, rich chemistry and strengthened biocompatibility. The scientific world has now diverged to the area of substituted polyanilines in the recent past owing to the efficient solubility, processability and extended applications in different fields. This review highlights the application aspects of the derivatives of polyanilines and their blends and composites in recent years. The wide application potentials of substituted polyanilines and their blends and composites in diverse fields such as in sensors, electrochromic display devices, solar cells, supercapacitors, batteries, semiconductors and anticorrosion materials, and in a variety of biological applications, have been highlighted. This review would bring new insights into polymer researchers to unravel novel applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
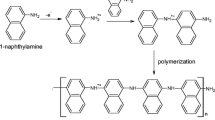
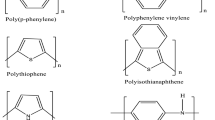
Polyaniline, the topic of interest has a historical background dating back to the 1830s. In 1834, it was F. Ferdinand Runge who discovered the oxidative polymerization of aniline for the first time [1], and in 1862, the electrochemical oxidation of aniline was demonstrated by Henry Lethe [2]. Several reactions with aniline were performed by scientists in the mid-eighteenth century, and polyaniline was known with different names such as krystalline, kyanol, aniline, benzidam and aniline black. [3]. Polyacetylene was found to be conducting in the mid–late 1970s by the scientists MacDiarmid, Shirakawa and Heeger, for which they were awarded the Nobel Prize in Chemistry in 2000 [4,5,6]. The puzzling polymer of the eighteenth century had a turning point after this discovery. Polyaniline was found to possess high conductivity in addition to a few other intrinsically conducting polymers (ICPs) such as polyacetylene, polypyrrole, polythiophene, poly(p-phenylenevinylene) and poly(p-phenylene) as shown in Fig. 1 [7]. Conjugated π electron system in their structure makes the polymers conducting. During the 1980s, the main focus of the researchers was to synthesize, characterize and study the electrical conductivity of polyaniline. But now the scientific world is interested in utilizing this material in a wide variety of applications. As we have stepped into the twenty-first century and now with contemporary science and technology, polyaniline, substituted polyanilines and their blends and composites are renowned for their versatility.
Structure of a number of intrinsically conducting polymers (ICPs) [7]
Polyaniline: superiority of polyaniline over other conducting polymers
Among all the other polymers, polyaniline is of great interest among the researchers worldwide. Based on its oxidation level, polyaniline exists in three different forms as the fully oxidized pernigraniline, the half-oxidized emeraldine and the fully reduced leucoemeraldine base [8,9,10]. Of the three, emeraldine base is known to be the most stable, non-toxic and conductive form of polyaniline (PANI) [8, 9, 11].
PANI is found to be thermally stable and known for its processability and conductivity. In addition to these properties, the aniline monomer is inexpensive and easily synthesized in various forms such as powders, thin films and pellets, its properties can readily be tuned, and it has many possible applications. These qualities of PANI make it superior to other ICPs [12].
Polyaniline: properties and applications
The commercial production of polyaniline is simple and cost-effective when compared to the complexities of its properties and its applications in diverse fields such as sensors, supercapacitors, solar cells, photocatalytic degradation and wastewater treatment, and hence the cost–benefit ratio is very high. PANI is extensively researched due to its excellent environmental stability, fascinating electroactivity and interesting chemical redox property. These features of PANI make it employed in multiple areas such as protective coatings, sensors, superhydrophobic/hydrophilic surfaces, actuators and biological fields [13,14,15,16,17]. The ingenious PANI is known for its extremely high stability, unique electronic and optical properties [18,19,20] and excellent high oxygen over nitrogen separation factor [21].
The unique properties of polyaniline make it applicable in diverse fields. The electrical conductivity of PANI renders help in the field of a conductive adhesive, conductive ink, conductive paint [22,23,24,25,26], antistatic textile [27] and electrostatic discharge materials [28,29,30]. In the presence of an electric field, viscosity in solution increases in PANI which makes it useful as an electrorheological material [31,32,33,34,35]. PANI is applied in sensors, detectors and indicators as it is capable of changing the electrical conductivity and colour when exposed to acidic, basic and some neutral vapours or liquids [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53].
PANI has potential applications in the field of supercapacitors and energy storage devices as it shows very high capacitance values [54,55,56,57]. PANI is used to shield electromagnetic interference as it is utilized to absorb and reflect electromagnetic radiation [58,59,60]. As PANI can collect and transfer energy, it also finds use as an electrode in rechargeable batteries, in electrochromic display devices, in photoelectric devices, in photovoltaic cells and transistors, and as electromembranes, etc. [61,62,63,64,65,66,67,68,69]. PANI is also attracted by polymer chemists in the fascinating field of organic or polymer light-emitting diodes on account of its property of emitting colour under various excitations [70, 71]. These numerous application possibilities of polyaniline enable us to understand the scope of it to explore new applications.
Polyaniline blends and composites
When the talented polymer, polyaniline could find extraordinary applications in almost every field of science, the next subject of interest to the researchers was aimed at the application possibilities of polyaniline blends and composites. Polymers were regarded as the best host matrices for composite materials [72]. On blending the polymer with the nanoparticles, several physiochemical properties such as enhanced electrical conductivity, increased mechanical and magnetic properties were identified. Furthermore, the researchers could find that the material was light in weight and the processability of the polymer was also found to be cost-effective [73]. Literature also reports that even better replicability of the form was observed on blending the polymer with the composite materials [74, 75]. Reports also prove that the presence of PANI chains in their matrices has a great impact on the improvement in the physico-chemical properties of the nanocomposites. Physico-chemical properties include the processability, chemical stability and thermal, magnetic, optical, catalytic, and electronic properties of the nanocomposite [76].
Wang et al. [77] demonstrated ferrite-grafted polyaniline nanofibres as electromagnetic shielding materials. Wang et al. [78] reviewed the graphene-based polyaniline nanocomposites and found an enormous number of applications in the field of supercapacitors, sensing platforms, fuel cells, solar cells, electrochromic devices, lithium-ion batteries, etc. Kumar et al. [79] synthesized and characterized polyaniline membranes with secondary amine additive containing N,N′-dimethyl propylene urea and offered a new path for hydrogen fuel cell application. El-Sayed et al. [80] synthesized polyaniline/tosyl cellulose stearate composites in 2018 and found them to be potentially useful as semiconducting materials. Kumar et al. [81] synthesized polyaniline-filled phenol divinyl benzene composite for potential lightning strike protection application.
Reddy et al. [82] synthesized polyaniline/TiO2 nanocomposite catalysts using chemical oxidative polymerization method for the application in wastewater treatment. The authors observed that these catalysts exhibited higher photocatalytic activity for the degradation of organic pollutants like rhodamine B, methylene blue and phenol than the unmodified TiO2 nanoparticles, which is attributed to the sensitizing effect of polyaniline. Anirudhan et al. [83] fabricated a novel zinc oxide nanorod-incorporated carboxylic graphene/polyaniline composite for the effective removal and subsequent photodegradation of the pesticide diuron from aqueous solutions. Dakshayini et al. [84] reviewed conducting polymer polyaniline and metal oxide-based hybrids for application in amperometric sensors and biosensors.
It is important to note that polyaniline and their blends and composites have placed their significant role not only in electronic devices but also in biological applications. Owing to their outstanding biocompatibility and eco-friendly nature, the biodegradable polymers have become more essential in almost all areas such as tissue engineering, packaging materials, sensors, water decontamination, disinfection, artificial skin and electrically conducting materials [85]. Electrical signal towards the cells is rendered due to the conducting nature of PANI.
Poyraz et al. [86] studied the PANI nanofibre/silver nanoparticle composite as an antibacterial agent. Shahadat et al. [87] presented a review on polyaniline-grafted biodegradable nanocomposite where a biodegradable content of cellulose, chitin, chitosan, etc., is incorporated in the PANI matrix. Pelin et al. [88] investigated the biomedical applications of lincomycin-embedded PANI-based coatings in 2018. Polyaniline and its drug composites with neomycin, trimethoprim and streptomycin were synthesized by oxidative polymerization and were probed to have antituberculosis activity by G. Kashyap and his team in 2019 [89].
PANI is also found to be insoluble in most of the solvents, infusibles and hygroscopic substances and has lower electrical conductivity than metals. Literature suggests that these properties of PANI limit its applications [90]. The rigid backbone of PANI is responsible for its poor solubility [91, 92]. Another drawback is the presence of a strong conjugated π electron system. Thus, to widen the possible applications of polyaniline, researchers have moved a step ahead synthesizing and characterizing the derivatives of polyaniline.
Substituted polyanilines/blends/composites
Owing to the limitations of polyanilines, researchers have turned their attention towards the substituted polyanilines around the 1990s. People could observe that the processability was enhanced by modifying the structure of the monomer [93, 94], by using a soluble precursor [95] and by developing a copolymer or composite [96,97,98,99]. Doped polyaniline and substituted derivatives of polyaniline are employed to enhance the processability and solubility of PANI. Introducing substituted groups on the benzene rings and amino N of PANI leads to the substituted derivatives of PANI. The most intensely investigated substituents are the electron-donating substituents and hydrophilic substituents. Substituted polyanilines have lower conductivity than polyanilines.
Substitution of electron-donating groups such as methyl, ethyl, methoxy, ethoxy and amino, on the hydrogen of the benzene ring of PANI reduces the rigidity and the interchain force in such a way that the solubility of the polymer is enhanced. Sharma and Kumar [100] studied the solvatochromic behaviour of polyanilines and alkyl-substituted polyanilines in different solvents such as propionic acid, acetic acid, formic acid and N-methyl-2-pyrrolidone. The authors suggested that the hypsochromic shifts and bathochromic shifts in the UV–visible spectral data depend not only on the solvents but depend greatly on the substituents in polyaniline. Hypsochromic shift followed the order as polyaniline < poly(o-toluidine) < poly(o-ethylaniline) < poly(o-propylaniline). Thus, Sharma and Kumar concluded that the hypsochromic shift increases as the alkyl substituent becomes bulky. The hydrophilic groups such as sulphonic group, carboxyl group and hydroxyl group when substituted on the benzene ring of PANI makes them highly soluble in water. These groups doped with imine N in the primary chain, such as sulphonic group, can form five-membered ring or six-membered ring in the molecular chain, thereby enhancing their thermal stability [101, 102].
Different homopolymers and copolymers prepared from anilines substituted with electron-withdrawing groups have been reported. Halogen (fluorine, chlorine, bromine and iodine)-substituted polyanilines are of great interest to the authors. Gok and Yavuz synthesized poly(2-halogenanilines) and its composites with Al2O3, SiO2 and red mud. Chloro-substituted polyaniline/SiO2 composite is found to have greater conductivity compared to the other halogen composites [103]. These distinct substituents attached to the aromatic ring of polyaniline can change their conductivity. The nature and substituent positions on the ring affect both the conjugation length and redox potential [104]. Reports suggest that the bromo-substituted polyaniline is an antibacterial agent which is found to be efficient and environmentally friendly [105]. A wide variety of applications were explored in substituted polyanilines only in the recent past.
In recent years, substituted polyanilines, their blends and composites have paved a new way in the field of conducting polymers. This review aims at presenting the novel and unique applications of substituted polyanilines, their blends and composites in the past 5 years. The increased processability and solubility of substituted derivatives of polyaniline make them potentially useful in various fields such as sensors, supercapacitors, electrochromic display devices, batteries, semiconductors, solar cells, light-emitting diodes and corrosion protection. In addition to this, the biological applications of substituted polyanilines provide a promising platform to the recent researches. These materials are also useful for their biomedical applications including antimicrobial performance, antifouling performance, biosensors, etc.
Synthesis and characterization of substituted polyanilines and their blends and composites
A summary of the reported literature in substituted polyanilines and their blends and composites in the recent past is presented in Table 1.
Applications of substituted polyanilines and their blends and composites
Sensors
A sensor is a device which detects a physical quantity and records responses and converts them into a signal which can be read by an observer or by an instrument. The derivatives of polyaniline along with their blends and composites have found an enormous number of applications in the field of sensors. When conducting polymers were used as supporting materials to prepare biosensors, fast response times and high level of storage and operational stability were observed by the authors [115]. Composites were prepared by including a rigid conductive polymer (such as PANI) into a flexible matrix (such as chitosan) which merges the matrix’s good processability with the conductive polymer’s electrical conductivity [116].
In 2017, the films of substituted polyaniline/chitosan composites were electrochemically synthesized in 0.10 M H2SO4 using cyclic voltammetric technique by Yavuz et al. [117]. Different chitosan composites were prepared with various substituents like N-methylaniline, N-ethylaniline and 2-ethylaniline. Porous surfaces were observed in the SEM images of substituted polyaniline/chitosan/H2SO4 composites which helped to facilitate the immobilization of biomolecules. The enzyme glucose oxidase (GOD) was incorporated onto the composites. The response of the GOD enzyme electrode to glucose was investigated by amperometric technique. The response current of poly(N-methylaniline)/chitosan/H2SO4/GOD increases linearly with glucose concentration from 0.06 to 1.83 Mm with a response time of 70 s as shown in Fig. 2. Yavuz and his co-workers could find that poly(N-methylaniline)/chitosan/H2SO4/GOD composite biosensor had a bigger linear range and higher sensitivity than free enzyme, other substituted polyaniline/chitosan/GOD and chitosan/GOD biosensors.
Changes in the response of free enzyme, chitosan/GOD, and sPANI/chitosan/H2SO4/GOD enzyme electrodes with glucose concentration (at 0.6 V in 0.10 M phosphate buffer solution (pH 7.4), 5 mg/mL GOD, 25 °C) [117]
Poly(N-methylaniline)/chitosan/H2SO4/GOD bioelectrode film onto ITO electrode was confirmed by the SEM and AFM studies which showed a more porous morphology and rough surface topology, respectively.
The biocompatibility of poly(N-methylaniline)/chitosan/H2SO4 offers a new path for glucose sensing applications.
The current research on H2O2 detection focuses primarily on electrode changes to reduce overpotential and boost electron transfer kinetics, which would influence sensing efficiency [118]. A biosensor has been developed by M. Baghayeri and team in 2014 to determine hydrogen peroxide [119]. They made use of emulsion polymerization to synthesize the poly(p-phenylenediamine)–Fe3O4 nanocomposite. Owing to the conducting nature of the polymer and the magnetic nature of Fe3O4 nanoparticles, the bioelectrochemical behaviour and magnetic behaviour of the composite were studied by the authors. The glassy carbon electrode surface was deposited with poly(p-phenylenediamine)–Fe3O4 nanocomposite followed by the immobilization of heme proteins on the composite surface. The authors remarked that the Hb-poly(p-phenylenediamine)–Fe3O4 nanocomposite modified glassy carbon electrode exhibited good electrocatalytical response and the constructed biosensor responded with good reproducibility and high sensitivity.
Pandule et al. [120] synthesized methane sulphonic acid-doped poly(2-chloroaniline) and the authors investigated its ammonia gas-sensing applications. They could observe that the electrical conductivity of the polymer remarkably enhanced with increased temperature from the electron hopping mechanism. The authors suggest poly(2-chloroaniline) as a chemical gas sensor material from the sensing responses of poly(2-chloroaniline) against different concentrations of ammonia as shown in Fig. 3.
Response of poly(2-chloroaniline) against different ammonia concentrations [120]
Pandule et al. [121] also have studied the ammonia gas-sensing applications of poly(2-chloroaniline) with a variety of inorganic acid dopants such as HCl, H2SO4 and HClO4. In response to their previous study, they could observe the linear increase in electrical conductivity with increase in temperature and the electrical conductivity was found to be higher with the inorganic acid dopant HClO4 and lesser with HCl. The resistance of poly(2-chloroaniline) increased linearly with increased concentrations of ammonia.
Rajabi and Noroozifar [122] offered a new path for the determination of uric acid and folic acid in urine and human serum, respectively. The authors were successful in fabricating an electrochemical sensor from poly(2- methoxyaniline) nanostructures. The enhanced electrochemical performance, low cost, ease of preparation, high sensitivity, long-time stability and good reproducibility helped the polymer to perform dual functions.
Tian in 2017 studied the gas-sensing properties of sulphur dioxide using the methoxy-substituted polyaniline, and the authors studied that at low concentrations of sulphur dioxide, the poly(2-methoxyaniline) was used as a sensor to determine the concentrations and at high concentrations of sulphur dioxide, poly(2-methoxyaniline) was used as a filter to adsorb the toxic SO2 from the environment [123].
A sensitive immunosensor for cancer diagnosis was fabricated by Wang et al. [124] by using the gold-decorated polyaniline derivatives. They synthesized Au-poly(o-aminophenol) and Au-poly(p-phenylenediamine). Electrical conductivity was observed in the composite due to the presence of gold nanoparticles. These composites played an important role in the electrochemical detection of three biomarkers of lung cancer, namely carcinoembryonic antigen (CEA), cytokeratin 19 fragment antigen 21-1 (CYFRA21-1) and neuron-specific enolase (NSE). These composites proved to be another feasible strategy to solve the current problems of lung cancer.
A soft biocompatible actuator was made available by Kongkaew et al. [125] by blending poly(2-chloroaniline) with pectin hydrogel. The electromechanical properties of the blend were investigated. The storage modulus sensitivity of the blend increased until the concentration of poly(2-chloroaniline) was 0.10% v/v and decreased above 0.10% v/v. The deflection responses in the blend in the presence and absence of the electric field strengths were examined. The free lower end of the hydrogel is seen to deflect towards the anode electrode in the presence of the applied electric field as shown in Fig. 4.
Bending of pectin hydrogel and 0.10%v/v P2ClAn/pectin hydrogel blend at electric field strengths of 0 and 500 V/mm [125]
Light-emitting diodes
Light-emitting diodes are the electrical components which emit light when current passes through it. LEDs work when the electrons in the semiconductor recombine with the electron holes releasing energy in the form of photons. Linganathan et al. [126] investigated the photoconductivity of poly(2-chloroaniline)/copper oxide nanocomposite and the composite proved to be a potentially useful candidate in the field of light-emitting diodes. The composite was thermally stable with an increased weight percentage of copper oxide nanoparticles. The conductivity of the nanocomposite was studied both in the presence and absence of light. Enhanced conductivity of 2.05 × 10−4 S cm−1 was observed by the authors in the presence of light with a 25% weight percentage of copper oxide.
Corrosion protection
One of the most important applications of conducting polymer is the protection of metals against corrosion. The function of a conductive polymer layer is to avoid contact with a corrosive setting and to decrease the rate of corrosion [127]. Metallic surfaces have been coated with conductive polymers to safeguard metals such as carbon steel [128,129,130,131], aluminium [132, 133] and stainless steel [134, 135] from corrosion. Chloro-substituted polyaniline has an excellent anticorrosion ability as reported by Jafari et al. [136]. The electrochemically synthesized poly(2-chloroaniline) coated on an aluminium alloy 3105 proved to be a protective layer in 3.5% NaCl solution. The AFM images conveyed the fact that poly(2-chloroaniline) was fixed to the aluminium alloy 3105 and the protection efficiency increased with an increase in current density to 15 mA cm−2 as shown in Fig. 5. The corrosion rate was 20 times lower for the aluminium alloy 3105 when compared with the poly(2-chloroaniline) coating on aluminium alloy which was found to be 4.1 × 10−4 mm year−1 and promised to be a potential candidate against corrosion protection.
Atomic force micrographs of pre-treated AA3105 after corrosion (a), and PClAni coatings grown by an applied current density of 15 mA cm−2 after corrosion (b) [136]
Cai et al. [137] investigated the anticorrosion properties of dedoped bromo-substituted epoxy resin composite coating by electrochemical impedance spectroscopy. Even after 100 days of immersion in 12.0 wt% NaCl solution, these coatings proved to have excellent protection. The composite coating is found to have good corrosion protection properties owing to the excellent redox ability, stronger adhesion strengths and enhanced hydrophobic properties of the dedoped bromo-substituted polyaniline. Authors concluded that greater the content of bromine, greater is the anticorrosion property. The corrosion inhibition efficiency of the copolymer poly(aniline-co-orthotoluidine) on the carbon steel has been reported, and it shows enhanced performance at highest rotation rate [138].
Batteries
Substituted polyanilines find potential applications in the field of rechargeable batteries, i.e. lithium-ion batteries. The use of these materials in batteries has not been explored much. In 2019, Vani and Jhancy Mary explored the use of lithium-ion batteries by synthesizing poly(2-chloroanilines) along with the starch and silk blends via interfacial polymerization [139].
Semiconductors
Substituted polyanilines due to enhanced solubility and processability have decreased electrical conductivity. So their conductivities lie between the metal and the insulator showing a wide range of applications as semiconductors. Mahudeswaran and his co-workers in 2016 [140] were able to synthesize a copolymer poly(aniline-co-o-bromoaniline), and the absorption, surface morphology, crystallinity and conductivity were studied. The electrical conductivity of the copolymer was reported to be 10−5 S cm−1 which is in the semiconducting range. The conventional inorganic semiconductors can be replaced by these organic semiconductors in optoelectronic devices.
The authors also synthesized the copolymer poly(aniline-co-o-ethylaniline) in 2015 [141]. Amorphous, spherical-shaped copolymer showed excellent conductivity in the semiconducting range of 1.143 × 10−3 S cm−1. This novel copolymer would be made good use in the area of organic semiconductors. They also observed that as the monomer concentration increased, the conductivity decreased to 6.39 × 10−5 S cm−1, and this may be attributed mainly due to the ethyl group which hindered the charge transfer process.
In addition to this, a copolymer and its nanocomposite, poly(2-methoxyaniline-co-2-chloroaniline)/ferric oxide nanocomposite, were synthesized by chemical oxidative polymerization in 2017 by Lakshmi and Jhancy Mary [142], and the composite proved to have higher thermal stability and greater electrical conductivity than the copolymer. This composite finds good application in the field of semiconductors as the electrical conductivity of the composite measured is 3.36 × 10−4 S cm−1 which is in the semiconducting range.
Linganathan et al. [143] synthesized poly(2-chloroaniline)/DBSA/silk blend by in situ chemical oxidative polymerization and reported that the polymer and blends are semiconducting and presented that poly(2-chloroaniline)/DBSA has increased conductivity than the poly(2-chloroaniline)/DBSA/silk blend and poly(2-chloroaniline)/silk blend. Among the silk blends, conductivity is greater in the blend with DBSA.
Lakshmi et al. [144] were able to prepare a blend, poly(2-chloroaniline-co-2-methoxy aniline)-blend-sodium alginate, by chemical oxidative polymerization using HCl as the dopant and ammonium persulphate as the oxidant which had a conductivity of 2.15 × 10−4 S cm−1. Thermal and electrical properties analysed were better in the case of copolymer blend than the pure copolymer.
In 2018, the author also studied the electrical conductivity of the same copolymer, poly(2-chloroaniline-co-2-methoxy aniline), by varying the composite and the blend. The composite material used was copper oxide nanoparticles, and the blend material used was polypropylene glycol. The copolymer composite and blend had better thermal stability and electrical conductivity than the pure copolymer. They estimated the electrical conductivity in the range of 10−5 S cm−1 [145].
Supercapacitors
The supercapacitors have drawn increasing attention as a novel energy storage device due to some prospective applications in hybrid electric vehicles, home appliances and mobile communications [146,147,148]. Zhang et al. [149] studied the nucleophilic ring-opening reaction by the bonding of aniline to graphene oxide along with the partial reduction in graphene oxide. The oxidative polymerization of N-phenyl glycine monomer and reduced graphene oxide modified with aniline through covalent grafting gave rise to the N-substituted carboxyl polyaniline covalent grafting-modified graphene oxide hybrid. The hybrid proved to be a nanocomposite since the studies determined the nanorod structure with the diameter of 40–50 nm and a length of tens to hundreds of nanometres.
Poly(2-chloroaniline) and its blend with silk were reported in 2014 by Linganathan et al. [143]. They studied the effect of the dodecylbenzene sulphonic acid on the polymer and blends. Activation energy of 0.1 eV was reported for the polymer and the blends. They could observe a remarkably higher dielectric constant for the poly(2-chloroaniline)/DBSA/silk blend than the polymer poly(2-chloroaniline)/DBSA. They suggested the application of this poly(2-chloroaniline)/DBSA/silk blend in the energy storage devices.
Sevil et al. [150] synthesized poly(2-chloroaniline)/polyaniline copolymer and the copolymer blend with polyvinyl chloride which was found to have enhanced conductivity in the range of 10−5 S cm−1 when the blends were exposed to gamma rays.
Electrochromic display devices
Electrochromism is the phenomenon in which there is a change in colour when the potential is varied. An electrochromic material is one in which a reversible colour change occurs upon oxidation (loss of electrons) or reduction (gain of electrons) [151, 152]. Electrochromic strips as battery state-of-charge indicators, anti-glare car rear-view mirrors and electrochromic sunglasses were employed for the materialistic applications of electrochromic devices [153,154,155]. Shahhosseini et al. [156] synthesized a conducting polymer comprised of ethylenedioxythiophene and nitroaniline as the monomer. The conducting polymer and the polymer composite with graphene were used for the fabrication of dye-sensitized solar cells substituting Pt counter electrode. At reductive potentials, the polymer exhibited a colour change from yellow to red and a colour change from green to dark colour was observed at oxidative potentials. This indicated that the corresponding polymer is a suitable material for making the electrochromic devices.
Saharan et al. [157] studied the applications of poly(2-methoxyaniline) in electrochromic display devices. They synthesized the polymer and also the copolymer poly(2-methoxyaniline-co-3-aminobenzenesulphonic acid) by electrochemical and chemical oxidative polymerization. The switching behaviour of the samples was observed from cyclic voltammogram and chronoamperometric studies. The switching stability of the electrochromic devices was determined from the switching times between their oxidized and reduced states. They also observed the colour change at varying potentials as shown in Fig. 6 and proved to pave the way in the field of electrochromic devices.
Electrochromic devices of poly(o-methoxy aniline) [157]
Solar cells
Solar cell is an electrical device that converts light energy into electricity. Substituted polyaniline took the place of Pt counter electrode in dye-sensitized solar cells. Shahhosseini et al. [156] synthesized an efficient material for replacing the Pt counter electrode in dye-sensitized solar cells (DSSC) by using a new monomer 4-(2,3-dihydrothieno[3,4-6][1,4][dioxin-5-yl) aniline. This novel monomer was prepared from para-nitroaniline and ethylene dioxythiophene. The conducting polymer and its composite with graphene were electrochemically synthesized and they were used for the fabrication of DSSC owing to the high electrocatalytic activity of aniline and high conductivity and chemical stability of EDOT giving a 21% greater energy conversion efficiency when compared to the Pt counter electrode.
Water decontamination
Many countries have contaminated water sources. In water treatments, chitosan as a natural polyelectrolyte is screened as a coagulant. Furthermore, chitosan is responsible for the chelation of many transition metal ions [158]. Abd El-Salam et al. [159] found a solution for the contamination of the environment by heavy metals. They were able to remove divalent metal ions like Cr, Fe, Mn, Cu and Zn from contaminated groundwater samples by grafting chitosan onto poly-2-hydroxy aniline. Authors studied the concentration effect of 2-hydroxyaniline, APS and acetic acid and also the effect of temperature on grafting copolymerization. 21.1116 kJ mol−1 was the observed activation energy of the copolymerization reaction. Thermodynamically, the values of ΔH* and ΔS* were found to be 22.8630 kJ mol−1 and − 109.4290 J mol−1 K−1, respectively, from the slope and intercept. For the removal of heavy metal ions, Langmuir and Freundlich adsorption studies were carried out. All the studied metal ions except Mn(II) showed greater monolayer adsorption capacity for poly(2-hydroxyaniline) and chitosan-grafted-poly(2-hydroxyaniline) when compared to chitosan. Monolayer adsorption capacity is greater for the removal of Fe metal ion in case of the graft which is about 10,000 (mg/g).
Biological applications of substituted polyanilines
Antibacterial activity
Polyaniline exhibits biocidal property due to its redox activity, charge transfer capacity and the existence of N+ groups in its chain [160,161,162]. Conductive PANI can communicate with the negatively charged bacterial cell wall electrostatically and trigger oxidative stress in bacteria, leading to bacterial cell disturbance and some degree of gene mutation in the bacteria. All of these eventually lead to bacteria’s death [161, 163, 164]. In 2013, Al Hussaini and Eldars [165] synthesized a thermally stable copolymer and nanocomposite, poly(aniline-co-o-phenylenediamine)/bentonite nanocomposite. The authors scrutinized the antibacterial efficiency of the composite with gram-positive bacteria Staphylococcus aureus and Staphylococcus epidermidis and gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa. Reasonably good antibacterial activity was witnessed with the composite with a higher concentration of nanoclay.
Nirmala Kumari Jangid and team in 2014 evaluated the antibacterial and antifungal activities of the dye substituted polyanilines. The polyanilines substituted with dyes acriflavine, rhodamine B and neutral red dye were found to have good conductivity in the range of 10−3–10−2 S cm−1. The dyes exhibited better antibacterial properties and antifungal properties when examined against the bacteria Escherichia coli (MTCC 442), Pseudomonas aeurginosa (MTCC 441), Staphylococcus aureus (MTCC 96) and Staphylococcus pyogenus (MTCC 443), and antifungal properties against Candida albicans (MTCC 227), Aspergillus niger (MTCC 282) and Aspergillus clavatus (MTCC 1323) [166].
Quan et al. [167] studied the antibacterial property of bromine-benzyl-disubstituted polyaniline against both gram-negative Escherichia coli and gram-positive Bacillus subtilis. They could find that bromine-benzyl-disubstituted polyaniline had high antibacterial activity compared to polyaniline and benzyl-substituted polyaniline. All the bacteria were found to be dead when the concentration of bromine-benzyl-disubstituted nanoparticles was 4.0 mg/mL. Thus, it is evident that the bromine groups substituted in the benzene rings are responsible for the high antibacterial activity of bromine-benzyl-disubstituted polyaniline. In addition to that, binary epoxy coatings containing bromine-benzyl-disubstituted polyaniline were prepared with bisphenol A/poly(ethylene glycol) using cardanol-based phenalkamine as curing agent. When the mixed epoxy coatings containing bromine-benzyl-disubstituted polyaniline were exposed to the Escherichia coli suspension for 2 weeks, fewer bacteria was observed on the surface by Quan et al.
In 2017, polyorthochloroaniline/copper nanocomposites were found to have antibacterial activity against gram-positive bacteria Bacillus subtilis and gram-negative bacteria Escherichia coli by Ahmad et al. [168]. Copper nanofiller with varying concentrations were injected in the polymer matrix to prepare the composites. Poly(2-chloroaniline)/copper nanocomposites had better antibacterial property than poly(2-chloroaniline). The authors reported that the antibacterial activity of the nanocomposite depends directly on the release of Cu2+ ions. Both the strains had a minimum zone of inhibition at 1% and a maximum zone of inhibition at 3% due to the maximum release of Cu2+ ions by copper nanoparticles as shown in Fig. 7.
Images of antibacterial activity of POC and its nanocomposites containing CuNPs (1–5%) against a E. coli and b B. subtilis [168]
The same team in 2018 [169] evaluated the antibacterial efficiency and the electrical conductivity of poly(2-chloroaniline) with chromium nanocomposite to the copper nanocomposite previously studied. They synthesized poly(2-chloroaniline)/chromium nanocomposites by chemical oxidative polymerization. By varying the load percentage of chromium nanofiller (1–10%), they probed the antibacterial properties against Escherichia coli and Bacillus subtilis and also the electrical conductivity of the composites. The authors could find that enhanced antibacterial activity and electrical conductivity were observed with enhanced load percentage of the chromium nanofiller as shown in Figs. 8 and 9, respectively.
Size of inhibitory zones versus load percentage of chromium in nanocomposites [169]
Plot of electrical conductivity versus load percentage of Cr NPs in nanocomposites [169]
Kashyap et al. [170] were able to study the antibacterial activities of the ascorbic acid-doped oligoaniline drug composites. Levofloxacin, cefaclor and cefuroxime were the drugs from which the oligoaniline composites were prepared. The antibacterial studies of the composites were explored against four gram-positive bacteria (Staphylococcus aureus MTCC 96, Streptococcus pyogenes MTCC 442, Bacillus subtilis MTCC 441 and Streptococcus mutans MTCC 890) and against four gram-negative bacteria (Escherichia coli MTCC 443, Pseudomonas aeruginosa MTCC 1688, Klebsiella pneumoniae MTCC 109 and Salmonella typhi MTCC 98). G. Kashyap and his co-workers could observe that the synthesized composites in comparison with the standard drugs like gentamicin, ampicillin, chloramphenicol, ciprofloxacin and norfloxacin showed better or equivalent antibacterial activities as shown in Fig. 10.
Antibacterial activity of ascorbic acid-doped OANI, drugs, OANI–drug composites and standard drugs [170]
To destroy the sulphate-reducing bacteria, Abd El-Salam et al. [171] synthesized a polymer and its composite with silver nanoparticles. Poly(2-aminothiophenol)/silver nanoparticle, poly(2-methylaniline)/silver nanoparticle and the copolymer poly(2-aminothiophenol-co-2-methylaniline)/silver nanoparticle were prepared and the antibacterial efficiency of the samples were tested against the standard bacterium Desulfovibrio sapovorans ATCC 33892. The team observed that the sulphate-reducing bacteria were easily killed with poly(2-aminothiophenol)/silver nanoparticles and poly(2-methylaniline)/silver nanoparticles than with the copolymer poly(2-aminothiophenol-co-2-methylaniline)/silver nanoparticles.
Bromine is found to play an efficient role as an environmental friendly antibacterial agent. Cai et al. [105] explored the antibacterial activity of bromo-substituted polyaniline against the gram-positive bacteria Bacillus subtilis and gram-negative bacteria Escherichia coli. With the increase in bromine content, remarkable antibacterial activity was achieved in doped/dedoped bromo-substituted polyaniline. The distribution of electrons in polyaniline becomes irregular due to the electron-withdrawing nature of bromine, and this brings stronger electrostatic contact with the bacteria thus destroying it.
Antibacterial activity was examined in dedoped bromo-substituted polyaniline epoxy resin composite coating by Cai et al. [137]. The bacteria Escherichia coli was brought into contact with the composite coating for 4 h, and a remarkable antibacterial activity with killing rate of 97.2% was noticed in the composite coating with higher bromine content as shown in Fig. 11.
Corresponding pictures of E. coli colonies after contacting with coating films (immersed in 12.0 wt% NaCl solution for 100 days) for 4 h [137]. EP—epoxy coating containing dedoped PANI. EBP I—epoxy coating containing dedoped Br PANI [KBr mass—4.284 g]. EBP II—epoxy coating containing dedoped Br PANI [KBr mass—5.712 g]. EBP III—epoxy coating containing dedoped Br PANI [KBr mass—7.140 g]
Antifouling
In public utilities, marine industries and medical devices, biofouling continues to be a significant problem for the surface applications [172, 173]. Strategies such as fouling discharge, fouling resistance and antimicrobial treatment could be used to avoid fouling [174, 175]. For antifouling applications, matrix materials such as epoxy coatings, fluorine-based coatings, silicone-based coatings, polyurethane coatings, polyacrylate coatings and PEG-based coatings were explored by the authors [176,177,178,179,180]. The coating surfaces containing the mixed epoxy coatings of bisphenol A/polyethylene glycol with bromine-benzyl-disubstituted polyaniline immersed in bacterial suspension for 2 weeks and in a static river for 30 days were found to have good antifouling property. The excellent antifouling performance of the coatings was due to the fouling resistance effect of PEG segments and the sterilization activity of bromine-benzyl-disubstituted polyaniline nanoparticles as shown in Fig. 12 [167].
Antifouling performance images of the coatings after immersion in the river [167]
Cai et al. [137] evaluated the antifouling property of the dedoped bromo-substituted polyaniline coated with epoxy resin. The steel plates brushed with the composite coatings were immersed in the river for 60 days. They found that the fouling behaviour gradually reduced with the increase in bromine content in the composite. The unrinsed surfaces were covered with fouling products and the rinsed surfaces with higher bromine content had the excellent antifouling property free from mud and algae as shown in Fig. 13.
Pictures of unrinsed surfaces (above) and rinsed surfaces (below) of different coatings after 60 days of field test (a pure epoxy; b EP; c EBP I; d EBP II; e EBP III coatings) [137]. EP—epoxy coating containing dedoped PANI. EBP I—epoxy coating containing dedoped Br PANI [KBr mass—4.284 g]. EBP II—epoxy coating containing dedoped Br PANI [KBr mass—5.712 g]. EBP III—epoxy coating containing dedoped Br PANI [KBr mass—7.140 g]
Summary and conclusion
The present paper thus brings an overview of the numerous applications of the derivatives of polyaniline and their blends and composites in diverse fields such as sensors, supercapacitors, batteries, electrochromic display devices, light-emitting diodes, solar cells, semiconductors, corrosion protection and water decontamination due to their ease of synthesis, low cost, environmental stability and enhanced solubility and processability. Polyanilines and their biocompatible nanocomposites have broadened the biological applications in tissue engineering, bone regeneration, targeted drug delivery, etc. The recent researchers have remarked excellent antimicrobial and antifouling performance in the substituted polyanilines and their blends and composites. Not much work has been reported so far in the other applications in biosciences with substituted polyanilines and their blends and composites. So, it is a great challenge to the researchers in exploring novel materials which could replace the conventional materials in the field of medicine. There is good scope in the field of substituted polyanilines and their blends and composites to widen the biomedical applications which can open up innovations in the near future.
References
Runge FF (1834) On some products of coal distillation. Ann Phys Chem 107:513–524
Letheby H (1862) On the production of a blue substance by the electrolysis of sulphate of aniline. J Chem Soc 15:161–163
Hofmann AW (1843) Chemical investigation of organic bases in coal tar oil. Ann Chem Pharm 47:37–87
MacDiarmid AG (2001) A novel role for organic polymers. Angew Chem Int 40:2581–2590
Shirakawa H (2001) The discovery of polyacetylene film: the dawning of an era of conducting polymers (Nobel lecture). Angew Chem Int 40:2574–2580
Heeger AJ (2001) Semiconducting and metallic polymers: the fourth generation of polymeric materials (Nobel lecture). Angew Chem Int 40:2591–2611
Ramussen SC (2017) The early history of polyaniline: discovery and origins. Substantia 1(2):99–109
Balint R, Caassidy NJ, Cartmell SH (2014) Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater 10:2341–2353
Paalanna OG (2009) Engineering chemistry. Tata McGraw Hill Education Private Limited, New Delhi
Molapo KM, Ndangili PM, Ajayi RF, Mbambisa G, Mailu SM, Njomo N, Masikini M, Baker P, Iwuoha EI (2012) Electronics of conjugated polymers (I): polyaniline. Int J Electrochem Sci 7:11859–11875
Sangamithirai D, Narayanan V, Muthuraman B, Stephen A (2015) Investigations on the performance of poly(o-anisidine)/graphene nanocomposites for the electrochemical detection of NADH. Mater Sci Eng C Mater Biol Appl 55:579–591
Genies EM, Boyle A, Lapkowski M, Tsintavis C (1990) Polyaniline: a historical survey. Synth Met 36:139–182
Li D, Huang JX, Kaner RB (2009) Polyaniline nanofibers: a unique polymer nanostructure for versatile applications. Acc Chem Res 42(1):135–145
Tian Z, Yu H, Wang L, Saleem M, Ren F, Ren P, Chen Y, Sun Y, Hung L (2014) Recent progress in the preparation of polyaniline nanostructures and their applications in anticorrosive coatings. RSC Adv 4:28195–28208
Wang L, Liu N, Ma ZJ (2015) Novel gold decorated polyaniline derivatives as redox active species for simultaneous detection of three biomarkers of lung cancer. J Mater Chem B 3:2867–2872
Zhang H, Wang J, Chen Y, Wang Z, Wang S (2013) Long-term cycling stability of polyaniline on graphite electrodes used for supercapacitors. Electrochim Acta 105:69–74
Hui X, Jiayue L, Yong C, Jang T, Zeting Z (2016) Facile fabrication of superhydrophobic polyaniline structures and their anticorrosive properties. J Appl Polym Sci. https://doi.org/10.1002/app.44248
Riul JA, So AMG, Mello SV, Bone S, Taylor DM, Mattoso LHC (2003) An electronic tongue using polypyrrole and polyaniline. Synth Met 132:109–116
Ma X, Shi W (2003) Investigation of quantum size effect of laser induced CdS quantum dots in sulphonic group polyaniline (SPAn) film. Microelectron Eng 66:153–158
Saxena V, Malhotra BD (2003) Prospects of conducting polymers in molecular electronics. Curr Appl Phys 3:293–305
Li XG, Huang MR, Zhu LH, Yang YL (2001) Synthesis and air separation of soluble terpolymers from aniline, toluidine and xylidine. J Appl Polym Sci 82:790–798
Roth S, Graupner W (1993) Conductive polymers: evaluation of industrial applications. Synth Met 57:3623–3631
Hino T, Taniguchi S, Kuramoto N (2006) Syntheses of conductive adhesives based on epoxy resin and polyanilines by using N-tert-butyl-5-methylisoxazolium perchlorate as a thermally latent curing reagent. J Polym Sci Part A Polym Chem 44:718–726
Hosoda M, Hino T, Kuramoto N (2007) Facile preparation of conductive paint made with polyaniline/dodecylbenzenesulfonic acid dispersion and poly(methyl methacrylate). Polym Int 56:1448–1455
Barros RA, Martins CR, Azevedo WM (2005) Writing with conducting polymer. Synth Met 155:35–38
Yoshioka Y, Jabbour GE (2006) Desktop inkjet printer as a tool to print conducting polymers. Synth Met 156:779–783
Bowman D, Mattes BR (2005) Conductive fibre prepared from ultra-high molecularweight polyaniline for smart fabric and interactive textile applications. Synth Met 154:29–32
Ohtani A, Abe M, Ezoe M, Doi T, Miyata T, Miyke A (1993) Synthesis and properties of high-molecular-weight soluble polyaniline and its application to the 4 MB-capacity barium ferrite floppy disk’s antistatic coating. Synth Met 57:3696–3701
Schoch KF Jr, Byers WA, Buckley LJ (1995) Deposition and characterization of conducting polymer thin films on insulating substrates. Synth Met 72:13–23
Kulkarni VG, Campbell JC, Mathew WR (1993) Transparent conductive coatings. Synth Met 57:3780–3785
Cho MS, Cho YH, Choi HJ, Jhon MS (2003) Synthesis, Electrorheological characteristics of polyaniline-coated poly(methyl methacrylate) microsphere: size effect. Langmuir 19:5875–5881
Lee IS, Cho MS, Choi HJ (2005) Preparation of polyaniline coated poly(methylmethacrylate) microsphere by graft polymerization and its electrorheology. Polymer 46:1317–1321
Lee IS, Lee JY, Sung JH, Choi HJ (2005) Synthesis and electrorheological characteristics of polyaniline–titanium dioxide hybrid suspension. Synth Met 152:173–176
Cho MS, Choi HJ, Ahn WS (2004) Enhanced electrorheology of conducting polyaniline confined in MCM-41 channels. Langmuir 20:202–207
Choi HJ, Kim TW, Cho MS, Kim SG, Jhon MS (1997) Electrorheological characterization of polyaniline dispersions. Eur Polym J 33:699–703
Bai H, Chen Q, Li C, Lu C, Shi G (2007) Electrosynthesis of polypyrrole/sulfonated polyaniline composite films and their applications for ammonia gas sensing. Polymer 48:4015–4020
Irimia-Vladu M, Fergus JW (2006) Suitability of emeraldine base polyaniline–PVA composite film for carbon dioxide sensing. Synth Met 156:1401–1407
Yan XB, Han ZJ, Yang Y, Tay BK (2007) NO2 gas sensing with polyanilines nanofibers synthesized by a facile aqueous/organic interfacial polymerisation. Sens Actuator B 123:107–113
Dixit V, Misra SCK, Sharma BS (2005) Carbon monoxide sensitivity of vacuum deposited polyaniline semiconducting thin films. Sens Actuator B 104:90–93
Jain S, Samui AB, Patri M, Hande VR, Bhoraskar SV (2005) FEP/polyanilines based multilayered chlorine sensor. Sens Actuator B 106:609–613
Ando M, Swart C, Pringsheim E, Mirsky VM, Wolfbeis OS (2005) Optical ozone-sensing properties of poly(2-chloroaniline), poly(N-methylaniline) and polyaniline films. Sens Actuator B 108:528–534
Kim JS, Sohn SO, Huh JS (2005) Fabrication and sensing behavior of PVF2 coated-polyaniline sensor for volatile organic compounds. Sens Actuator B 108:409–413
Hosseini SH, Entezami AA (2001) Preparation and characterization of polyaniline blends with polyvinyl acetate, polystyrene and polyvinyl chloride for toxic gas sensors. Polym Advan Technol 12:482–493
Joshi SS, Lokhande CD, Han SH (2007) A room temperature liquefied petroleum gas sensor based on all-electrodeposited n-CdSe/ppolyaniline junction. Sens Actuator B 123:240–245
Zou Y, Sun L, Xu F (2007) Prussian Blue electrodeposited on MWNTs-PANI hybrid composites for H2O2 detection. Talanta 72:437–442
Nohria R, Khillan RK, Su Y, Dikshit R, Lvov Y, Varahramyan K (2006) Humidity sensor based on ultrathin polyaniline film deposited using layer-by-layer nano-assembly. Sens Actuator B 114:218–222
Huang J, Virji S, Weiller BH, Kaner RB (2003) Polyaniline nanofibers: facile synthesis and chemical sensors. J Am Chem Soc 125:314–315
Muthukumar C, Kesarkar SD, Srivastava DN (2007) Conductometric mercury [II]; sensor based on polyaniline–cryptand-222 hybrid. J Electroanal Chem 602:172–180
Talaie A, Lee JH, Lee YK, Jang J, Romagnoli JA, Taguchi T et al (2000) Dynamic sensing using intelligent composite: an investigation to development of new pH sensors and electrochromic devices. Thin Solid Films 363:163–166
Arora K, Sumana G, Saxena V, Gupta RK, Gupta SK, Yakhmi JV et al (2007) Improved performance of polyaniline-uricase biosensor. Anal Chim Acta 594:17–23
Ren J, He F, Zhang L, Su C, Liu Z (2007) A newB-PAn-P system for the detection of bacteria population. Sens Actuator B 125:510–516
Andreu Y, Marcos S, Castillo JR, Galban J (2005) Sensor film for Vitamin C determination based on absorption properties of polyaniline. Talanta 65:1045–1051
Syed AA, Dinesan MK (1990) Polyaniline: reaction stoichiometry and use as an ion-exchange polymer and acid/base indicator. Synth Met 36:209–215
Lu J, Moon KS, Kim BK, Wong CP (2007) High dielectric constant polyaniline/epoxy composites via in situ polymerization for embedded capacitor applications. Polymer 48:1510–1516
Gupta V, Miura N (2006) Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim Acta 52:1721–1726
Sung JH, Kim SJ, Lee KH (2004) Preparation of compact polyaniline films: electrochemical synthesis using agar gel template and charge storage applications. J Power Sources 126:258–267
Meng C, Liu C, Fan S (2009) Flexible carbon nanotube/polyanilines paper-like films and their enhanced electrochemical properties. Electrochem Commun 11:186–189
Epstein AJ, Joo J, Kohlman RS, Du G, MacDiarmid AG, Oh EJ et al (1994) Inhomogeneous disorder and the modified Drude metallic state of conducting polymers. Synth Met 65:149–157
Joo J, Oh EJ, Min G, MacDiarmid AG, Epstein AJ (1995) Evolution of the conducting state of polyaniline from localized to mesoscopic metallic to intrinsic metallic regimes. Synth Met 69:251–254
Bhadra S, Singha NK, Khastgir D (2008) Semi-conductive composites from ethylene 1-octene copolymer and polyaniline coated nylon 6: studies on mechanical, thermal, processability, electrical and EMI shielding properties. Polym Eng Sci 48:995–1006
MacDiarmid AG, Yang LS, Huang WS, Humphrey BD (1987) Polyaniline: electrochemistry and application to rechargeable batteries. Synth Met 18:393–398
Somasiri NLD, MacDiarmid AG (1988) Polyaniline: characterization as a cathode active material in rechargeable batteries in aqueous electrolytes. J Appl Electrochem 18:92–95
Koga K, Yamasaki S, Narimatsu K, Takayanagi M (1989) Electrically conductive composite of polyaniline-aramid and its application as a cathode material for secondary battery. Polym J 9:733–738
Desilvestro J, Scheifele W, Haas O (1992) In situ determination of gravimetric and volumetric charge densities of battery electrodes: polyaniline in aqueous and nonaqueous electrolytes. J Electrochem Soc 139:2727–2736
Qiao Y, Li CM, Bao SJ, Bao QL (2007) Carbon nanotube/polyaniline composite as anode material for microbial fuel cells. J Power Sources 170:79–84
Watanabe A, Mori K, Iwasaki Y, Nakamura Y, Niizuma S (1987) Electrochromism of polyaniline film prepared by electrochemical polymerization. Macromolecules 20:1793–1796
Rodrigues MA, De Paoli MA, Mastragostino M (1991) Electrochromic properties of chemically prepared polyaniline. Electrochim Acta 36:2143–2146
Jelle BP, Hagen G, Hesjevik SM, Odegard R (1992) Transmission through an electrochromic window based on polyaniline, tungsten oxide and a solid polymer electrolyte. Mater Sci Eng B 13:239–241
Jelle BP, Hagen G, Sungle S, Obegard R (1993) Dynamic light modulation in an electrochromic window consisting of polyaniline, tungsten oxide and a solid polymer electrolyte. Synth Met 54:315–320
Chen SA, Chuang KR, Chao CI, Lee HT (1996) White-light emission from electroluminescence diode with polyaniline as the emitting layer. Synth Met 82:207–210
Gaponik NP, Talapina DV, Rogach AL (1999) A light-emitting device based on a CdTe nanocrystal/polyaniline composite. Phys Chem Chem Phys 1:1787–1789
Ruiz-Hitzky E, Darder M, Aranda P, Ariga K (2010) Advances in biomimetic and nanostructured biohybrid materials. Adv Mater 22:323–336
Sultana S, Khan MZ, Umar K, Muneer M (2013) Electrical, thermal, photocatalytic and antibacterial studies of metallic oxide nanocomposite doped polyaniline. J Mater Sci Technol 29:795–800
Hung M-T, Choi O, Ju YS, Hahn H (2006) Heat conduction in graphite-nanoplatelet-reinforced polymer nanocomposites. Appl Phys Lett 89:023117
Kuilla T, Bhadra S, Yao D, Kim NH, Bose S, Lee JH (2010) Recent advances in graphene based polymer composites. Prog Polym Sci 35:1350–1375
Jeon IY, Baek JB (2010) Nanocomposites derived from polymers and inorganic nanoparticles. Materials 3:3654–3674
Wang W, Gumfekar SP, Jiao Q, Zhao B (2013) Ferrite-grafted polyaniline nanofibers as electromagnetic shielding materials. J Mater Chem 1(16):2851–2859
Wang L, Lu X, Lei S, Song Y (2014) Graphene-based polyaniline nanocomposites: preparation, properties and applications. J Mater Chem A 2:4491–4509
Kumar A, Kumar V, Sain PK, Kumar M, Awasthi K (2018) Synthesis and characterisation of polyaniline membranes with secondary amine additive containing N,N′-dimethyl propylene urea for fuel cell application. Int J Hydrogen Energy 43:21715–21723
El-Sayeda NS, Abd El-Aziz ME, Kamel S, Turky G (2018) Synthesis and characterisation of polyaniline/tosylcellulose stearate composites as promising semiconducting materials. Synth Met 236:44–53
Kumar V, Zhou Y, Shambharkar G, Kunc V, Yokozeki T (2019) Reduced de-doping and enhanced electrical conductivity of polyaniline filled phenol- divinyl benzene composite for potential lightning strike protection application. Synth Met 249:81–89
Reddy KR, Karthik KV, Benaka Prasad SB, Soni SK, Jeong HM, Raghu AV (2016) Enhanced photocatalytic activity of nanostructured titanium dioxide/polyaniline hybrid photocatalysts. Polyhedron 120:169–174
Anirudhan TS, Shainy F, Mohan AM (2018) Fabrication of zinc oxide nanorod incorporated carboxylic graphene/polyaniline composite and its photocatalytic activity for the effective degradation of diuron from aqueous solutions. Sol Energy 171:534–546
Dakshayini BS, Reddy KR, Mishra A, Shetti NP, Malode SJ, Basu S, Naveen S, Raghu AV (2019) Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem J. https://doi.org/10.1016/j.microc.2019.02.061
Sheltami RM, Abdullah I, Ahmad I, Dufresne A, Kargarzadeh H (2012) Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr Polym 88:772–779
Poyraz S, Cerkez I, Huang TS, Liu Z, Kang L, Luo J, Zhang X (2014) One- step synthesis and characterisation of polyaniline nanofiber/silver nanoparticle composite networks as antibacterial agents. ACS Appl Mater Interfaces. https://doi.org/10.1021/am505571m
Shahadat M, Khan MZ, Rupani PF, Embrandiri A, Sultana S, Ahammad SZ, Ali SW, Sreekrishnan TR (2017) A critical review on the prospect of polyaniline-grafted biodegradable nanocomposite. Adv Colloid Int Sci 249:2–16
Pelin GP, Fufa O, Popescu RC, Savu D, Socol M, Zgura I, Holban AM, Vasile BS, Grumezescu V, Socol G (2018) Lincomycin- embedded PANI—based coatings for biomedical applications. Appl Surf Sci 455:653–666
Kashyap G, Ameta G, Ameta C, Ameta R, Punjabi PB (2019) Synthesis and characterisation of polyaniline-drug conjugates as effective antituberculosis agents. Bioorg Med Chem Lett 29(11):1363–1369
Nakajima T, Kawagoe T (1989) Polyaniline: structural analysis and application for battery. Synth Met 28:629–638
Kenry Liu B (2018) Recent advances in biodegradable conducting polymers and their biomedical applications. Biomacromolecules 19(6):1783–1803
Bharti M, Singh A, Samanta S, Aswal DK (2018) Conductive polymers for thermoelectric power generation. Prog Mater Sci 93:270–310
Sato M, Tanaka S, Kaeriyama K (1986) Soluble conducting polythiphenes. J Chem Soc, Chem Commun 11:873–874
Jen KY, Oboddi R, Elsenbaumer RL (1985) Processible and environmentally stable conducting polymers. Polym Mater Sci Eng 53:79–83
Gagnon DR, Capistran JD, Karasz FE, Lenz RW (1984) Conductivity anisotropy in oriented poly(p-phenylene vinylene. Polym Bull 12:293–298
Bjorklund RB, Lidberg B (1986) Electrically conducting composites of colloidal polypyrrole and methylcellulose. J Chem Soc Chem Commun 16:1293–1295
Depaolie MA, Walterman RJ, Diaz AF, Bargon J (1985) An electrically conductive plastic composite derived from polypyrrole and poly(vinyl chloride). J Polym Sci Polym Chem 23:1687–1698
Lindancy SE, Street GB (1984) Conductive composites from polyvinyl alcohol and polypyrrole. Synth Met 10:67–69
Bozkurt A, Akbulut U, Toppare L (1996) Conducting polymer composites of polypyrrole and polyindene. Synth Met 82:41–46
Sharma S, Kumar D (2010) Study on solvatochromic behaviour of polyanilines and alkyl substituted polyaniline. Indian J Eng Mater S 17:231–237
Nguyen MT, Kasai P, Miller JL, Diaz AF (1994) Synthesis and properties of novel water-soluble conducting polyaniline copolymers. Macromolecules 27:3625–3631
Chan HSO, Ng SC, Sim WS, Seow SH, Tan KL, Tan BTG (1993) Synthesis and characterisation of conducting poly(o-aminobenzylalcohol) and its copolymers with aniline. Macromolecules 26:144–150
Gok A, Yavuz AG (2007) Preparation and characterisation of poly(2-halogenaniline) composites with Al2O3, SiO2 and Red mud. Int J Polym Anal Charact 12:155–169
Leclerc M, Aprona GD, Zotti G (1993) Structure–property relationships in polyaniline derivatives. Synth Met 55:1527–1532
Cai W, Wang J, Quan X, Wang Z (2018) Preparation of bromo-substituted polyaniline with excellent antibacterial activity. J Appl Polym Sci. https://doi.org/10.1002/app.45657
Wankhede MG (2012) Chemical polymerisation of substituted derivatives of aniline in oxalic acid medium. Sci Revs Chem Commun 2(3):387–391
Porselvi L, Jhancy Mary S (2014) Synthesis, characterization and electrical conductivity of poly(2-chloroaniline)/MMT and poly(2-chloroaniline)/Na-Bentonite nanocomposites in the presence of surfactants. Int J Sci Technol Res 3(2):69
Vicentini DS (2014) Synthesis and characterization of carboxyl-substituted polyanilines doped with halogenated acids: combining conductivity with solubility. J Braz Chem Soc 25(11):1939–1947
Bissessur R, MacDonald J (2007) Synthesis and characterization of halo-substituted polyanilines/VOPO4 nanocomposites. Mater Chem Phys 106:256–259
Husain S, Shumaila Kumar A, Husain M (2017) Multiwall carbon nanotubes/polyaniline: poly-m-toulidine:poly-o-toulidine nanocomposites—synthesis, properties & field emission. Polym Compos. https://doi.org/10.1002/pc.24384
Amaya T, Kurata I, Inada Y, Hatai T, Hirao T (2017) Synthesis of phosphonic acid ring- substituted polyanilines via direct phosphonation to polymer main chains. RSC Adv 7:39306–39313
Jangid NK, Chauhan NPS, Pinki BP (2015) Preparation and characterization of polyanilines bearing rhodamine 6-G and Azure B as pendant groups. J Macromol Sci A 52:95–104
Sankar A, Kumaravel M, RameshKumar S, Vijayan M (2013) Chemical synthesis of conductive poly(methoxyaniline) in hydroxyethylidenediphosphonic acid. Asian J Chem 25(6):3001–3004
Yavuz AG, Uygun A, Bhethanabotla VR (2009) Substituted polyaniline/chitosan composites: synthesis and characterization. Carbohydr Polym 75:448–453
Mu S, Xue H (1996) Bioelectrochemical characteristics of glucose oxidase immobilised in a polyaniline film. Sensor Actuator B Chem 31(3):155–160
Kim SJ, Shin SR, Spinks GM, Kim IY, Kim SI (2005) Synthesis and characteristics of a semi interpenetrating polymer network based on chitosan/polyaniline under different pH conditions. J Appl Polym Sci 96(3):867–873
Yavuz AG, Uygun A, Bhethanabotla VR (2010) Preparation of substituted polyaniline/chitosan composites by in situ electropolymerisation and their application to glucose sensing. Carbohydr Polym 81:712–719
Chen W, Cai S, Ren QQ, Wen W, Zhao YD (2012) Recent advances in electrochemical sensing for hydrogen peroxide: a review. Analyst 137:49–58
Bhagayeri M, Zare EN, Lakouraj MM (2014) A simple hydrogen peroxide biosensor based on a novel electro-magnetic poly(p-phenylenediamine) @ Fe3O4 nanocomposite. Biosens Bioelectron 55:259–265
Pandule SS, Shisodia SU, Patil MR, Pawar RP, Chabukswar VV (2016) Synthesis and characterisation of methane sulphonic acid doped poly(2-chloroaniline) study of its physical properties and ammonia gas sensing application. J Macro Mol Sci Part A 53(12):768–772
Pandule SS, Patil MR, Keri RS (2018) Properties and ammonia gas sensing applications of different inorganic acid- doped poly(2-chloroanilines). Polym Bull. https://doi.org/10.1007/s00289-017-2263-0
Rajabi H, Noroozifar M (2017) New synthesis of poly ortho-methoxyaniline nanostructures and its application to construct modified multi-wall carbon nanotube/graphite paste electrode for simultaneous determination of uric acid and folic acid. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2017.02.133
Tian Y, Qu K, Zeng X (2017) Investigation into the ring- substituted polyanilines and their application for the detection and adsorption of sulphur dioxide. Sens Actuators B. https://doi.org/10.1016/j.snb.2017.04.057
Wang L, Liu N, Ma ZJ (2015) Novel gold- decorated polyaniline derivatives as redox- active species for simultaneous detection of three biomarkers of lung cancer. J Mater Chem B. https://doi.org/10.1039/c5tb00001g
Kongkaew W, Sangwan W, Lerdwijitjarud W, Sirivat A (2017) Soft poly(2-chloroaniline)/pectin hydrogel and its electromechanical properties. J Biomater Appl. https://doi.org/10.1177/0885328217739457
Linganathan P, Sundararajan J, Samuel JM (2014) Synthesis, characterisation and photoconductivity studies on poly(2-chloroaniline) and poly(2-chloroaniline)/CuO nanocomposites. J Compos Mater. https://doi.org/10.1155/2014/838975
Olad A, Rashidzadeh A (2008) Preparation and anticorrosive properties of PANI/Na-MMT and PANI/O-MMT nanocomposites. Prog Org Coat 62:293–298
Goncalves GS, Baldissera AF, Rodrigues LF, Martini EMA, Ferreira CA (2011) Alkyd coatings containing polyanilines for corrosion protection of mild steel. Synth Met 161:313–323
Martyak NM, McAndrew P (2007) Corrosion performance of steel coated with co-polyamides and polyaniline. Corros Sci 49:3826–3837
Cook A, Gabriel A, Laycock N (2004) On the mechanism of corrosion protection of mild steel with polyaniline. J Electrochem Soc 151:529–535
Gabriel A, Laycock N, Murray HN, Williams G, Cook A (2006) Oxidation states exhibited by in-coating polyaniline during corrosion-driven coating delamination on carbon steel. Electrochem Solid State Lett 9:57–60
Shreepathi S, Hoang HV, Holze R (2007) Corrosion protection performance and spectroscopic investigations of soluble conducting polyaniline-dodecylbenzenesulfonate synthesized via inverse emulsion procedure. J Electrochem Soc 154:67–73
Ogurtsov NA, Pud AA, Kamarchik P, Shapoval GS (2004) Corrosion inhibition of aluminium alloy in chloride mediums by undoped and doped forms of polyaniline. Synth Met 143:43–47
Huerta-Vilca D, Moraes SR, Motheo AJ (2004) Anodic treatment of aluminium in nitric acid containing aniline, previous to deposition of polyaniline and its role on corrosion. Synth Met 140:23–27
Zhong L, Xiao S, Hu J, Zhu H, Gan F (2006) Application of polyaniline to galvanic anodic protection on stainless steel in H2SO4 solutions. Corros Sci 48:3960–3968
Jafari Y, Nooshabadi MS, Ghoreishi SM (2014) Poly(2-chloroaniline) electropolymerisation coatings on aluminium alloy 3105 and evaluating their corrosion protection performance. Trans Indian Inst Met. https://doi.org/10.1007/s12666-013-0374-3
Cai W, Wang J, Quan X, Zhao S, Wang Z (2018) Antifouling and anticorrosion properties of one-pot synthesised dedoped bromo-substituted polyaniline and its composite coatings. Surf Coat Technol 334:7–18
Benchikh A, Aitout R, Makhloufi L, Benhaddad L, Saidani B (2009) Soluble conducting poly(aniline-co-orthotoluidine) copolymer as corrosion inhibitor for carbon steel in 3% NaCl solution. Desalination 249:466–474
Vani G, Mary SJ (2019) Synthesis and characterisation of poly(2-chloroaniline) its starch and silk blends and applications in lithium ion batteries. Mater Today Proc 8(1):176–181
Mahudeswaran A, Vivekanandan J, Vijayanand PS, Kojima T, Kato S (2016) A facile synthesis of poly(aniline-co-o-bromoaniline) copolymer: characterisation and application as semiconducting material. Int J Mod Phy B 30:165008
Mahudeswaran A, Vivekanandan J, Vijayanand PS (2015) Chemical oxidative polymerisation of aniline with o-ethyl aniline: their molecular structure, morphology and conducting properties. Asian J Chem 27(12):4501–4504
Lakshmi P, Mary SJ (2017) A comparative study on the electrical and thermal properties of the chemically synthesised copolymer, poly(2-methoxyaniline-co-2-chloroaniline) and its nanocomposite, poly(2-methoxyaniline-co-2-chloroaniline)-composite-Fe2O3. J Pharm 17–23
Linganathan P, Mary SJ (2014) Effect of dodecyl benzene sulphonic acid on the electrical conductivity behaviour of poly(2-chloroaniline)/silk blends. Am J Polym Sci 4(4):107–116
Lakshmi P, Mary SJ (2018) Chemical synthesis, characterisation and electrical conductivity behaviour of poly(2-chloroaniline-co-2-methoxyaniline)-blend-sodium alginate. J Appl Chem Sci Int 9:115–121
Lakshmi P, Mary SJ (2018) Chemical synthesis, spectral characterisation and electrical conductivity behaviour of poly(2-methoxyaniline-co-2-chloroaniline) and its Cuo nanocomposite and polypropylene glycol blend. J Polym Compos 6(3):1–13
Zhang Y, Feng H, Wu X, Wang L, Zhang A, Xia T, Dong H, Li X, Zhang L (2009) Progress of electrochemical capacitor electrode materials: a review. Int J Hydrogen Energy 34(11):4889–4899
Burke A (2007) R & D considerations for the performance and application of electrochemical capacitor. Electrochim Acta 53(3):1083–1091
Liu X, Long Q, Jiang C, Zhan B, Li C, Liu S, Zhao Q, Huang W, Dong X (2013) Facile and green synthesis of mesoporous Co3O4 nanocubes and their applications for supercapacitors. Nanoscale 5(14):6525–6529
Zhang J, Gao J, Song Q, Guo Z, Chen A, Chen G, Zhou S (2016) N-substituted carboxyl polyaniline covalent grafting reduced graphene oxide nanocomposites and its application in supercapacitor. Electrochim Acta 199:70–79
Sevil UA, Coskun E, Guven O (2014) Electrical conductivity and spectroscopic characterisation of blends of poly(2-chloroaniline)/polyaniline copolymer with PVC exposed to gamma rays. Radiat Phys Chem 94:45–48
Paddeu S, Ram MK, Carrara S, Nicolini C (1998) Langmuir-Schaefer films of a poly(o-anisidine) conducting polymers for sensors and displays. Nanotechnology 9:228–236
Bamfield P (2001) Chromic phenomena. Royal Society of Chemistry, Cambridge
Mortimer RJ, Dyer AL, Reynolds JR (2006) Electrochromic organic and polymeric materials for display applications. Displays 27:2–18
Rosseinsky DR, Mortimer RJ (2001) Electrochromic systems and the prospects for devices. Adv Mater 13:783–793
Zhang L, Lang Q, Shi Z (2010) Electrochemical synthesis of three-dimensional polyaniline network on 3-Aminobenzenesulphonic acid functionalized glassy carbon electrode and its application. Am J Anal Chem 1:102–112
Shahhosseini L, Nateghi MR, Kazemipour M, Zarandi MB (2016) Electrochemical synthesis of novel polymer based on (4-(2,3-dihydrothienol [3,4-6][1,4][dioxin-5-yl) aniline) in aqueous solution: characterisation and application. Mater Chem Phys 177:554–563
Saharan R, Kaur A, Dhawan SK (2015) Synthesis and characterisation of poly(o-methoxy aniline) and its copolymer for electrochromic device energy applications. Indian J Pure Appl Phy 53:316–319
Dutta PK, Dutta J, Tripathi VS (2004) Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res 63:20–31
Abd El-Salam HM, Kamal EHM, Ibrahim MS (2016) Synthesis and characterisation of chitosan-grafted-poly(2-hydroxyaniline) microstructures for water decontamination. J Polym Environ. https://doi.org/10.1007/s10924-016-0847-7
Abdolahi A, Hamzah E, Ibrahim Z, Hashim S (2014) Application of environmentally friendly coatings toward inhibiting the microbially influenced corrosion (MIC) of steel: a review. Polym Rev 54:702–745
Nikolaidis MRG, Pagnon JC, Ali N, Sum R, Davies N, Roddam LF, Ambrose M (2015) Functionalised polyanilines disrupt Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Colloids Surf B Biointerfaces 136:666–673
Bonilla AM, Garcia MF (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37:281–339
Nikolaidis MRG, Bennett JR, Swift S, Easteal AJ, Ambrose M (2011) Broad spectrum antimicrobial activity of functionalised polyanilines. Acta Biomater 7:4204–4209
Pandiselvi K, Thambidurai S (2015) Synthesis, characterisation and antimicrobial activity of chitosan-zinc oxide/polyaniline composites. Mater Sci Semicond Process 31:573–581
Al-Hussaini AS, Eldars W (2014) Non-conventional synthesis and antibacterial activity of poly(aniline-co-o-phenylenediamine)/bentonite nanocomposites. Des Monomer Polym 17(5):458–465
Jangid NM, Chauhan NPS, Punjabi PB (2014) Novel dye-substituted polyanilines: conducting and antimicrobial properties. Polym Bull 71:2611–2630
Quan X, Wang J, Souleymana T, Cai W, Zhaoa S, Wang Z (2018) Antibacterial and antifouling performance of bisphenol A/poly(ethylene glycol) binary epoxy coatings containing bromine-benzyl disubstituted polyanilines. Prog Org Coat 124:61–70
Ahmad MN, Anjum MN, Nawaz F, Iqbal S, Saif MJ, Hussain T, Mujahid A, Farooq MU, Nadeem M, Rahman A, Raza A, Shehzad K (2017) Synthesis and antibacterial potential of hybrid nanocomposites based on polyorthochloroaniline/copper nanofiller. Polym Compos. https://doi.org/10.1002/pc.24558
Ahmad MN, Rafique F, Nawaz F, Farooq T, Anjum MN, Hussain T, Hassan S, Batool M, Khalid H, Shehzad K (2018) Synthesis of antibacterial poly(o-chloroaniline)/chromium hybrid composites with enhanced electrical conductivity. Chem Cent J 12:46
Kashyap G, Meghawal K, Ameta C, Ameta R, Punjabi PB (2018) Synthesis of ascorbic acid doped oligoaniline, its drug composites and study of their antibacterial behaviour. Polym Bull. https://doi.org/10.1007/s00289-018-2502-z
Abd El- Salam HM, Azzam EMS, Aboad RS (2017) Synthesis and characterisation of poly(2-aminothiophenol-co-2-methylaniline)/silver nanoparticles as anti- sulphate reducing bacteria. Int J Polym Mater. https://doi.org/10.1080/00914037.2017.1354196
Callow JA, Callow ME (2011) Trends in the development of environmentally friendly fouling resistant marine coatings. Nat Commun 2:244
Peres RS, Armelin E, Aleman C, Ferreira CA (2015) Modified tannin extracted from black wattle tree as an environmrntally friendly antifouling pigment. Ind Crop Prod 65:506–514
Yang WJ, Neoh KG, Kang ET, Teo SLM, Rittschof D (2014) Polymer brush coatings for combining marine biofouling. Prog Polym Sci 39:1017–1042
Wang Y, Wang Z, Wang J, Wang S (2018) Triple antifouling strategies for reverse osmosis membrane biofouling control. J Membr Sci 549:495–506
Ming DWW, Benthem RV, With GD (2005) Superhydrophobic films from raspberry like particles. Nano Lett 5:2298–2301
Liu K, Su ZG, Miao SD, Ma GH, Zhang SP (2016) UV-curable enzymatic antibacterial waterborne polyurethane coating. Biochem Eng J 113:107–113
Mondal P, Purkait MK (2017) Effect of polyethylene glycol methyl ether blend humic acid on poly(vinylidene fluoride-co-hexafluropropylene) PVDF-HFP membranes: pH responsiveness and antifouling behaviour with optimization approach. Polym Test 61:162–176
Basu BJ, Kumar VD, Anandan C (2012) Surface studies on superhydrophobic and oleophobic polydimethylsiloxane–dssilica nanocomposite coating system. Appl Surf Sci 261:807–814
Sileika TS, Kim HD, Maniak P, Messersmith PB (2011) Antibacterial performance of polydopamine- modified polymer surfaces containing passive and active components. ACS Appl Mater Interfaces 3:4602–4610
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sebastian, J., Samuel, J.M. Recent advances in the applications of substituted polyanilines and their blends and composites. Polym. Bull. 77, 6641–6669 (2020). https://doi.org/10.1007/s00289-019-03081-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03081-7