Abstract
Some novel radiopaque biodegradable and biocompatible iodinated polymers based on poly-3-hydroxy butyrate (PHB) were obtained. Following the attachment of diethanol amine to PHB, the hydroxyl ends were capped with 4-iodobenzoic acid and 2,3,5-tri-iodobenzoic acid. In this manner, tri-novel radiopaque polymers were obtained. The resulting polymers were structurally characterized by NMR technique. They were evaluated with respect to their possible use as radiopaque implant biomaterials indicating X-ray visibility in a noninvasive manner using routine X-ray absorption imaging techniques. These polymers exhibited good radiopacity with conventional imaging X-ray techniques in vivo. Additionally, biocompatibility of these iodinated polymers was also evaluated. There were no signs of infection or abscess formation on the surgical area. These novel radiopaque PHBs should be promising biomaterials for a new-generation radiopaque materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(3-hydroxy alkanoate)s (PHAs) are storage materials for some microorganisms. They are hydrophobic biodegradable polyesters that their structures can be controlled by the carbon source [1,2,3,4,5]. PHAs are generally classified in two types: short-chain-length (scl) and medium-chain-length (mcl) PHAs. Both types of the PHAs need modification reactions [6,7,8,9] in order to improve hydrophilicity and mechanical properties [10,11,12,13,14,15,16,17]. Because of their biodegradability and biocompatibility, PHAs are potential candidates for medical applications [18,19,20,21,22]. Poly-3-hydroxy butyrate (PHB) is a member of PHAs. PHB is a crystalline, brittle polymer with high melting transition temperature (~ 170 °C). There are few chemical modification reactions of PHB. Functionalization of PHB was carried out by chlorination in our laboratories [23, 24]. The chlorinated PHB was blended with polymethyl methacrylate in view of its optical behavior [25]. Modified PHB can also be obtained by the anionic polymerization of α-methyl-α-pentyl-β-propiolactone [26]. Radiopaque materials absorb X-rays; therefore, they can be visualized and traced in the human body. There have been many attempts to modify the frequently used medical products to enhance visibility. In this manner, X-ray visible BaSO4 nanoparticles were used in trans-catheter arterial embolization procedures [27] and in bone contact [28]. In order to enhance radiopaque properties of the polymers, monomers containing heavy halogen atoms such as bromine and iodine were introduced. Iodine has been widely used to enhance radiopacity because of its great mass attenuation and documented low systemic toxicity [29,30,31,32]. Meng et al. reported the preparation and evaluation of radiopaque microspheres based on polyvinyl alcohol and lipiodol which is poppy seed oil that contains 38% iodine by weight [33]. Iodinated radiopaque methacrylate copolymer can be prepared via the ring-opening polymerization of glycidyl methacrylate [34]. van Hooy-Corstjens et al. reported the iodinated methacrylic polymer cages to restore the height between two adjacent vertebrae [35]. The iodine-containing methacrylic copolymers were also used into the cement powder [36]. Estep et al. synthesized novel radiopaque oils, the 1,3,5-trialkyl-2,4,6-tri-iodobenzenes, for mucosal coating in the gastrointestinal tract [37]. Koole and co-workers prepared monomers containing covalently bonded iodine and copolymerized with 2-hydroxyethyl methacrylate and methyl methacrylate for x-ray visible stent application indicating high stability in living body [38, 39]. Herein, we report first time the synthesis and characterization of novel radiopaque PHB derivatives. PHB is renewable, biodegradable and biocompatible microbial polyester. It is also commercially available. To gain radiopacity to this very valuable biomaterial is very important for the medical applications in vivo. The carboxylic acid end was reacted with diethanol amine in order to obtain PHB with three hydroxyl groups. Then, the hydroxyl groups were reacted with iodobenzoic acid derivatives in order to obtain novel biodegradable radiopaque biodegradable polymers. Structural characterization was performed by proton, carbon NMR and size exclusion chromatography. X-ray visibility of the obtained polymers was confirmed in vivo and in vitro.
Experimental
Materials
Poly-3-hydroxy butyrate (PHB), microbial polyester, was supplied from BIOMER (Germany). Dimethyl formamide (DMF), N,N′-dicyclohexylcarbodiimid (DCC), dimethyl amino pyridine (DMAP), stannous 2-ethyl hexanoate (Sn-oct), diethanol amine (DEA), 4-iodobenzoic acid (IB), 4-iodobenzoyl chloride (IB-Cl), triethyl amine (TEA), 2,3,5-tri-iodobenzoic acid (3IB) and the other chemicals were purchased from Sigma-Aldrich and used without further purification.
Synthesis of hydroxylated PHB (PHB-DEA)
Hydroxylated PHB was obtained by the reaction of PHB with DEA according to the procedure reported in cited Ref. [40].
The modified procedure is as follows: A mixture of 60.3 g of vacuum-dried PHB, 102.4 g of DEA and 2.1 g of Sn-oct in 250 mL of CHCl3 was stirred at room temperature for 24 h. Then, it was refluxed for 3 h. The solvent was distilled under atmospheric condition (not in the rotary evaporator). Then, the crude product was cured in 125 °C for 1.5 h. The product was washed with excess methanol and filtered. The crude product was dried under vacuum at 40 °C for 24 h. For further purification, the obtained polymer was dissolved in 600 mL of CHCl3 and filtered. The solvent was evaporated in a rotary evaporator. The obtained polymer was washed with excess methanol again. The pure diethanol amine derivative of PHB, three hydroxyl-terminated PHB, was filtered and dried under vacuum 40 °C for 24 h. Yield was 56 g. This was coded as PHB-DEA. Characteristic FTIR signals: 1567 cm−1 amide carbonyl; 3301 cm−1 primary hydroxyl groups of DEA; 1721 cm−1 belong to ester carbonyl of PHB. The characteristic chemical shifts of the PHB-DEA sample in 1H NMR spectrum were observed at 1.3 ppm for –CH3, 2.4–2.6 ppm for –CH2–COO–, 3.0 ppm for –N–CH2–, 3.5–3.8 ppm for –CH2–OH, 4.1 ppm for –CH–OH and 5.1–5.3 ppm for –CH–O– [40]. The GPC result was Mn 32,813 Da, Mw 47,700 Da, PDI 1.45; Mn 7800 Da, Mw 8706 Da, Đ 1.12.
Synthesis of iodinated PHBs
The iodinated PHB samples (PHB-DEA-IB-6, PHB-DEA-IB-12 and PHB-DEA-3IB-1) were obtained by the reaction between PHB-DEA and IB/3IB according to the Steglich esterification method [41].
PHB-DEA-IB-6
PHB-DEA (9.8 g) was dissolved in a mixture of CH2Cl2 (100 mL) and DMF (5 mL). To this solution were added under continuously stirring 4-iodobenzoic acid (6.3 g, 0.025 mol), DCC (9.2 g, 0.045 mol) and DMAP (0.81 g, 0.0066 mol) under argon.
Yield was 10.7 g. After stirred at room temperature for 24 h, the precipitated side product, dihexyl urea, was filtered. The solvent of the filtered solution was evaporated, the crude product was leached with excess methanol, and the iodinated PHB, PHB-DEA-IB-6, was purified via filtering of methanol. The product was dried under vacuum at 40 °C for 24 h. The GPC result was Mn 29,100 Da, Mw 38,500 Da, Đ 1.33.
PHB-DEA-3IB-1
The same procedure was repeated using the following reagents: PHB-DEA (4.85 g) was dissolved in a mixture of CH2Cl2 (50 mL) and DMF (5.0 mL). To this solution were added under continuously stirring 2,3,5-tri-iodobenzoic acid (3.42 g, 0.0068 mol), 50 mL of 1 M DCC in CH2Cl2 (0.050 mol) and DMAP (1.32 g, 0.010 mol) under argon. Yield was 5.78 g. The GPC result was Mn 30,800 Da, Mw 40,000 Da, Đ 1.30.
PHB-DEA-IB-1
PHB-DEA-IB-1 was obtained by the reaction between hydroxylated PHB and 4-iodobenzoyl chloride in conventional manner. Briefly, PHB-DEA (2.16 g) was dissolved in a mixture of CH2Cl2 (30 mL) and triethylamine (0.42 g, 0.004 mol). The solution was chilled under 10 °C using ice/water mixture. 4-Iodobenzoyl chloride (3.42 g) in 10 mL of CH2Cl2 was added into the cold solution with continuously stirring in 5 min. The solution was left to warm up to room temperature for overnight. The needle crystals of the triethylamine hydrochloride side product were removed via filtration process. The solvent was evaporated and precipitated with cold petroleum ether (100 mL). The product, PHB-DEA-IB-1, was dried under vacuum at 40 °C for 24 h. Yield was 2.88 g.
PHB-DEA-IB-3
PHB-DEA (1.75 g) was dissolved in a mixture of CH2Cl2 (30 mL) and triethylamine (0.50 g, 0.005 mol). To this solution was added solid 4-iodobenzoyl chloride (0.81 g) with continuously stirring in 5 min. The solution was continuously stirred at room temperature for 24 h. The needle crystals of the triethylamine hydrochloride side product were removed via filtration process. The solvent was evaporated and precipitated with cold petroleum ether (100 mL). The product, PHB-DEA-IB-3, was dried under vacuum at 40 °C for 24 h. Yield was 0.87 g.
Characterization of the polymers synthesized
Molecular weights were determined by size exclusion chromatography instrument, Viscotek GPCmax Auto sampler system, consisting of a pump, three ViscoGEL GPC columns (G2000H HR, G3000H HR and G4000H HR) and a Viscotek differential refractive index (RI) detector with a THF flow rate of 1.0 mL/min at 30 °C. A calibration curve was generated with three polystyrene (PS) green standards: 2960, 50,400 and 696,500 Da, of low polydispersity. The polymer sample solution containing 0.05 g in 10 mL of THF was filtered and injected automatically into the instrument. Data were analyzed using Viscotek Omni SEC Omni 01 software.
Proton NMR spectra in CDCl3 solutions of the samples were taken at a temperature of 25 °C with an Agilent NMR 600-MHz NMR (Agilent, Santa Clara, CA, USA) spectrometer equipped with a 3-mm broadband probe.
In vivo implantation
Two female albino Wistar rats with an average weight of 230 g were used in this study. The animals were housed and fed adlibitium and divided into two: rat I and rat II. All rats were anesthetized with intraperitoneal injection of a 0.1 mL/kg alphazyn and 0.3 mL/kg ketamine mixture. Similar to our previous studies [42,43,44], the biomaterial samples in sizes approximately 0.5 × 0.5 cm and 1.9 mm thickness were placed symmetrically as shown in Fig. 1 under the skin of the back of the rat, on top of the muscle fascia. In rat I, two different iodinated polymer samples were implanted: PHB-DEA-IB-6 (a), PHB-DEA-3IB-1 (b) and (c) PHB-DEA (control).
In vivo biocompatibility–X-ray studies of the PHAs
On the fifth day of the implantation, the rats were sedated with the same protocol as above, and X-ray images of the surgical area were taken by a conventional X-ray device. (Siemens, Luminos dRF Max, X-ray generator 65 kW). On the same day, the rats were killed. There were no signs of infection or abscess formation on the surgical area. Our study was approved by Animal Research Ethics Committee of Bezmialem Vakıf University Experimental Research Center (approval number; 2018/10).
Results and discussion
Iodinated PHB samples
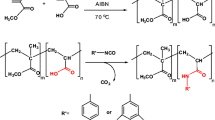
PHB is microbial polyester with one hydroxyl and one carboxylic acid end. Diethanol amine was capped with carboxylic end of PHB to obtain hydroxylated PHB with three hydroxyl ends. As precursor, tri-hydroxylated PHB (PHB-DEA) was synthesized more than twenty times. The obtained PHB-DEA samples were structurally characterized by the NMR technique. All samples indicated the characteristic signals. Reaction pathways of the iodinated PHB samples are shown in Scheme 1.
In order to evaluate the esterification syntheses, Steglich esterification and esterification with acid chloride methods were used to obtain iodinated PHB radiopaque derivatives. Some different molar ratios of the reagents resulted in iodinated products. Results and conditions of the iodinated PHB derivatives are shown in Table 1.
The hydroxyl ends of the PHB-DEA were reacted with 4-iodo- and 2,3,5-tri-iodobenzoic acids to obtain radiopaque iodinated PHB derivatives. The obtained polymers were purified by precipitating in excess methanol at least in three times. So, unreacted iodobenzoic acid starting materials were removed from the obtained product. Molar masses of the purified iodinated PHB derivatives were at around 30,000 g/mol (Mn) with polydispersity 1.30. The iodobenzene derivatives of PHB were characterized using proton and carbon NMR techniques. Figure 2 shows 1H NMR spectra of the 4-iodobenzene and 2,3,5-tri-iodobenzene derivatives of PHB: PHB: PHB-DEA-IB-6 and PHB-DEA-3IB-1, respectively. Typical chemical shifts of the iodobenzoyl protons have been localized in between 7.6 and 7.8 ppm while the tri-iodobenzoyl protons were localized in chemical shifts 8.3 and 7.75 ppm.
13C NMR spectrum of PHB-DEA-IB-6 is shown in Fig. 3. The characteristic iodobenzoyl groups of the iodinated PHB were observed in their 13C NMR spectra. The chemical shifts at 139, 132, 128 and 101 ppm (a, b, c and e, respectively) are assigned to the ring carbons [45]. As for tri-iodobenzoyl derivative of PHB, 13C NMR spectrum of PHB-DEA-3IB-1 contained the characteristic signals related to the ring carbons, as well. As shown in Fig. 4, the signals/ppm at 149, 145, 137, 114, 106 and 94 ppm were assigned to the ring carbons.
In vitro X-ray analysis of the PHB derivatives
The X-ray visibility of the iodinated PHB derivatives was studied under X-ray irradiation. The images of the PHB samples were taken under daylight and under X-ray irradiation. Figure 5 shows the images of the iodinated PHB derivatives: PHB-DEA-IB-6 (a-1) under daylight, (a-2) under X-ray irradiation; PHB-DEA-3IB-1 (b-1) under daylight, (b-2) under X-ray irradiation; PHB-DEA-IB-1 (c-1) under daylight, (c-2) under X-ray irradiation. Iodobenzoyl derivatives of PHB (a, b, c) all showed the X-ray visibility while PHB without iodine content did not show visibility. Interestingly, 4-iodobenzoate derivative of PHB (PHB-DEA-IB-6 and PHB-DEA-IB-1) showed the higher radiopacity than the tri-iodo derivative of PHB (PHB-DEA-3IB-1). Maybe, the less thickness of the rectangular mold affects of the opacity.
Biocompatibility of the novel polymers
Biocompatibility of implanted radiopaque microspheres was investigated in vivo (Fig. 6). The implanted samples were found to be well tolerated. There were no signs of infection or abscess formation on the surgical area.
X-ray analysis
In rat I, the polymer stated as control group (PHB-DEA-22 on left side) was not visualized under the X- ray as expected. All the other polymer samples implanted revealed radiopacity under the X-ray. Polymers implanted to the rat II (PHB-DEA-IB-6 on left side- PHB-DEA-3IB-1 on right side) seem to have a higher opacity than the rat I polymers. Additionally, the polymer sample PHB-DEA-IB-12 implanted to the rat I seem to have similar density of radiopacity with the vertebral bone of the rat, whereas the polymer samples implanted to the rat II reveal higher radiopacity compared to the skeleton of the rat.
PHB, a well-known biocompatible polymer, has wide variety of clinical applications. It has been used as micelles, hydrogels and micro-carrying system for drugs, and chemotherapeutics. In some cases, clinicians might need to trace the carrying system in vivo to see the therapeutic effect of the drug or the agents. There are ways to trace the carrying systems: adding a fluorescent property to the hydrogels or micelles or adding heavy metals such as Fe to be detected in magnetic resonance images in blood samples [46].
However, the most practical way to trace a polymer in vivo is with its radiopacity. If this material is visible under an X-ray image, then the material can be seen in any part of the body. Additionally, the quantity of the material can be detected with the degree of enhancement of the material in an X-ray image. In the literature, shape memory polyutherane foams were presented with enhanced radiopacity, which was used as filling in intracranial aneurysm treatment. These polyutherane foams seem to be visible through the skull also [47]. In our study, we have modified three hydroxylated PHB with iodobenzoic acid derivatives in order to add radiopacity. We could see that we can adapt the radiopacity of the polymer with the modification of the polymer.
With this radiopacity modification, these biopolymers can be use in clinical applications as a substitute for muscle facias in abdominal surgery, tendon substitutes or as a dura in spinal surgeries. This property will increase the clinical application diversity of the biomaterial.
Conclusion
These novel radiopaque polymeric materials have a potential as new bulking agents for radiological observation. Its advantage over the existing bulking agents lies in their X-ray visibility in situ. These novel polymers were also found to be biocompatible. This is encouraging with respect to the intended application, although it must be acknowledged that the biocompatibility studies should be performed with longer period of time resembling more of clinical application. PHB is a very unique biodegradable, biocompatible, microbial polyester obtained from ubiquitous renewable resources such as CO2, sugar, acetic acid and plant oils. Radiopaque PHB could be a very special biomaterial for biotechnology. To gain radiopacity to this very valuable biomaterial is very important for the medical applications in vivo. The first time obtained novel radiopaque PHB is the first example of the very large microbial polyester family. We believe that this first time synthesized novel radiopaque PHB will be very attractive and a promising biomaterial in tissue engineering and drug delivery systems.
References
Gross RA, DeMello C, Lenz RW, Brandl H, Fuller RC (1989) The biosynthesis and characterization of poly (β-hydroxyalkanoates) produced by Pseudomonas oleovorans. Macromolecules 22:1106
Lenz RW, Marchessault RH (2005) Bacterial polyesters: biosynthesis, biodegradable plastics and biotechnology. Biomacromolecules 6:1
Solaiman DKY, Ashby RD, Foglia TA, Marmer WN (2006) Conversion of agricultural feedstock and coproducts into poly(hydroxy alkanoates). Appl Microbiol Biotechnol 71:783
Steinbüchel A (2001) Perspectives for biotechnological production and utilization of biopolymers: metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol Biosci 1:1
Koray O, Koksal MS, Hazer B (2010) Simple production experiment of poly (3-hydroxy butyrate) for science laboratories and its importance for science process skills of prospective teachers. Energy Educ Sci Technol Part B Soc Educ Stud 2, 1–2:39
Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbial Lett 128:219
Ishii D, Takisawa K, Matsumoto K, Ooi T, Hikima T, Takata M, Taguchi S, Iwata T (2017) Effect of monomeric composition on the thermal, mechanical and crystalline properties of poly [(R)-lactate-co-(R)-3-hydroxybutyrate]. Polymer 122:169
Scholz C, Fuller RC, Lenz RW (1994) Growth and polymer incorporation of Pseudomonas oleovorans on alkyl esters of heptanoic acid. Macromolecules 27:2886
Tappel RC, Kucharski JM, Mastroianni JM, Stipanovic AJ, Nomura CT (2012) Biosynthesis of poly [(R)-3-hydroxyalkanoate] copolymers with controlled repeating unit compositions and physical properties. Biomacromolecules 13:2964
Toraman T, Hazer B (2014) Synthesis and characterization of the novel thermoresponsive conjugates based on poly (3-hydroxy alkanoates). J Polym Environ 22:159
Hazer DB, Kilicay E, Hazer B (2012) Poly (3-hydroxyalkanoate) s: diversification and biomedical applications: a state of the art review. Mater Sci Eng C 32:637
Scandola M, Focarete ML, Adamus G, Sikorska W, Baranowska I, Swierczek S, Gnatowski M, Kowalczuk M, Jedlinski Z (1997) Polymer blends of natural poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and a synthetic atactic poly (3-hydroxybutyrate). Characterization and biodegradation studies. Macromolecules 30:2568
Hazer B, Akyol E, Şanal T, Guillaume S, Çakmakli B, Steinbuchel A (2019) Synthesis of novel biodegradable elastomers based on poly [3-hydroxy butyrate] and poly [3-hydroxy octanoate] via transamidation reactio. Polym Bull. https://doi.org/10.1007/s00289-018-2410-2
Sparks J, Scholz C (2008) Synthesis and characterization of a cationic poly (β-hydroxyalkanoate). Biomacromolecules 9:2091
Lorenzini C, Versace DL, Renard E, Langlois V (2015) Renewable epoxy networks by photoinitiated copolymerization of poly (3-hydroxyalkanoate) s and isosorbide derivatives. React Funct Polym 93:95
Hazer B (2015) Simple synthesis of amphiphilic poly (3-hydroxy alkanoate) s with pendant hydroxyl and carboxylic groups via thiol-ene photo click reactions. Polym Degrad Stab 119:159
Abe H, Doi Y, Kumagai Y (1994) 3-hydroxybutyrate-β-6-hydroxyhexanoate] as a compatibilizer for a Biodegradable Blend of Poly [(R)-3-hydroxybutyrate] and Poly (6-hydroxyhexanoate). Macromolecules 27:6012
Hazer DB, Hazer B (2011) The effect of gold clusters on the autoxidation of poly (3-hydroxy 10-undecenoate-co-3-hydroxy octanoate) and tissue response evaluation. J Polym Res 18:251
Hazer B (2010) Amphiphilic poly (3-hydroxy alkanoate) s: potential candidates for medical applications. Int J Polym Sci. https://doi.org/10.1155/2010/423460
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio-and materials industry. Chem Soc Rev 38:2434
Hazer DB, Bal E, Nurlu G, Benli K, Balcı S, Öztürk F, Hazer B (2013) In vivo application of poly-3-hydroxyoctanoate as peripheral nerve graft. J Zhejiang Univ Sci B (Biomed Biotechnol) 14(11):993
Hazer B, Steinbüchel A (2007) Increased diversification of polyhydroxyalkanoates by modification reactions for industrial and medical applications. Appl Microbiol Biotechnol 74:1
Arkin AH, Hazer B, Borcakli M (2000) Chlorination of poly (3-hydroxy alkanoates) containing unsaturated side chains. Macromolecules 33:3219
Arkin AH, Hazer B (2002) Chemical modification of chlorinated microbial polyesters. Biomacromolecules 3(6):1327
Yalcin B, Cakmak M, Arkın AH, Hazer B, Erman B (2006) Control of optical anisotropy at large deformations in PMMA/chlorinated-PHB (PHB-Cl) blends: mechano-optical behavior. Polymer 47:8183
Arkin AH, Hazer B, Adamus G, Kowalczuk M, Jedlinski Z, Lenz RW (2001) Synthesis of poly (2-methyl-3-hydroxyoctanoate) via anionic polymerization of α-methyl-β-pentyl-β-propiolactone. Biomacromolecules 2(3):623
Wang Q, Qian K, Liu S, Yang Y, Liang B, Zheng C, Yang X, Xu H, Shen AQ (2015) X-ray visible and uniform alginate microspheres loaded with in situ synthesized BaSO4 nanoparticles for in vivo transcatheter arterial embolization. Biomacromolecules 16:1240
Nuutınen J-P, Clerc C, Törmälä P (2003) Mechanical properties and in vitro degradation of self-reinforced radiopaque bioresorbable polylactide fibres. J Biomater Sci Polym Ed 14:665
Lee BH, Leon C, McLemore R, Valdez Macias J, Vernon BL (2011) Synthesis and characterization of thermo-sensitive radio-opaque poly (N-isopropylacrylamide-co-PEG-2-iodobenzoate). J Biomater Sci Polym Ed 22:2357
Sang L, Wei Z, Liu K, Song K, Wang J, Qi M (2014) Radiopaque iodinated polyurethane for embolic agents. Acta Polym Sin 1:31
Wei Z, Song P, Sang L, Liu K, Zhou C, Wang Y, Li Y (2014) Radiopaque iodinated poly (ester–urethane) s based on poly (butylene succinate): retarded crystallization and dual recrystallization behaviour. Polymer 55:2751
Li S, Yu J, Wade MB, Policastro GM, Becker ML (2015) Radiopaque, iodine functionalized, phenylalanine-based poly (ester urea) s. Biomacromolecules 16:615
Meng WJ, Lu XJ, Wang H, Fan TY, Cui DC, Zhang SS, Zheng ZZ, Guan HT, Song L, Zou YH (2015) Preparation and evaluation of biocompatible long-term radiopaque microspheres based on polyvinyl alcohol and lipiodol for embolization. J Biomater Appl 30(2):133–146
Dawlee S, Jayakrishnan A, Jayabalan M (2009) Studies on novel radiopaque methyl methacrylate: glycidyl methacrylate based polymer for biomedical applications. J Mater Sci Mater Med 20:S243–S250
van Hooy-Corstjens CSJ, Aldenhoff YBJ, Knetsch MLW, Govaert LE, Arin E, Erli H, Koole LH (2004) Radiopaque polymeric spinal cages: a prototype study. J Mater Chem 14:3008
Boelen EJH, Lewis G, Xu J, Slots T, Koole LH, van Hooy-Corstjens CSJ (2008) Evaluation of a highly‐radiopaque iodine‐containing acrylic bone cement for use in augmentation of vertebral compression fractures. J Biomed Mater Res 86A:76
Estep KG, Josef KA, Bacon ER, Illig CR, Toner JL, Mishra D, Blazak WF, Miller DM, Johnson DK, Allen JM, Spencer A, Wilson SA (2000) 1,3,5-Trialkyl-2,4,6-triiodobenzenes: novel X-ray contrast agents for gastrointestinal imaging. J Med Chem 43:1940
Benzina A, Kruft MAB, Bar F, Vanderveen FH, Bastiaansen CW, Heijnen V, Reutelingsperger C, Koole LH (1994) Studies on a new radiopaque polymeric biomaterial. Biomaterials 15:1122
Aldenhoff YBJ, Kruft MAB, Pijpers AP, van der Veen FH, Bulstra SK, Kuijer R, Koole LH (2002) Stability of radiopaque iodine-containing biomaterials. Biomaterials 23:881
Tuzen M, Sahiner S, Hazer B (2016) Solid phase extraction of lead, cadmium and zinc on biodegradable polyhydroxybutyrate diethanol amine (PHB-DEA) polymer and their determination in water and food samples. Food Chem 210:115
Neises B, Steglich W (1978) Simple method for the esterification of carboxylic acids. Chem Int Ed 17:522
Hazer DB, Hazer B, Dinçer N (2011) Antibacterial effects of laser ablated Ni nanoparticles. J Biomed Biotechnol 2011:956169. https://doi.org/10.1155/2011/956169
Hazer DB, Hazer B, Kaymaz F (2009) Synthesis of microbial elastomers based on soybean oily acids. biocompatibility studies. Biomed Mater 4(3):035011. https://doi.org/10.1088/1748-6041/4/3/035011
Shishatskaya EI, Khlusov IA, Volova TGA (2006) A hybrid PHB–hydroxyapatite composite for biomedical application: production, in vitro and in vivo investigation. J Biomater Sci Polym Ed 17(5):481
Nygren CL, Wilson CC, Turner JFC (2005) On the solid state structure of 4-iodobenzoic acid. J Phys Chem A 109:2586
Zhao F, Yao D, Guo R, Deng L, Dong A, Zhang J (2015) Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials (Basel) 5(4):2054
Rodriguez JN, Yu YJ, Miller MW, Wilson TS, Hartman J, Clubb FJ, Gentry B, Maitland DJ (2012) Opacification of shape memory polymer foam designed for treatment intracranial aneurysms. Ann Biomed Eng 40(4):883
Acknowledgements
This work was supported by the Kapadokya University (#KÜN.2018-BAGP-001) and Bülent Ecevit University Research Funds (#BEU-2017-72118496-01). The authors thank to Fatih Pekdemir for taking FTIR spectra. This paper has been proofread by Bülent Ecevit University Article Proofreading and Editing Office.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erol, A., Rosberg, D.B.H., Hazer, B. et al. Biodegradable and biocompatible radiopaque iodinated poly-3-hydroxy butyrate: synthesis, characterization and in vitro/in vivo X-ray visibility. Polym. Bull. 77, 275–289 (2020). https://doi.org/10.1007/s00289-019-02747-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02747-6