Abstract
In this paper, the current advances in chemical injection method of polymer flooding are reviewed. The ultimate goal of polymer flooding for EOR process is to improve tertiary oil recovery by increasing the overall oil driving efficiency as a result of the improvements in injected fluid’s viscosity and mobility ratio. However, it was found that there were some limitations of polymer flooding. Hence, this paper will be reviewing studies on combinations of polymer flooding with other chemical methods by various researchers. Polymer flooding and its combinations with other flooding methods discussed in this paper are: polymer, alkaline polymer (AP), micellar polymer (MP), nanoparticles injection with polymers (NP) and alkaline surfactant polymer (ASP). The working principle, resistance, effectiveness and field application of each flooding method are also reviewed and compared.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background
Conventional oil extraction
Oil recovery can typically be broken down into three major sections depending on the production life of a reservoir, namely primary, secondary and tertiary recovery. In the oil and gas industry, primary and secondary oil recoveries are categorized as conventional oil extraction methods, whereas tertiary oil recovery is called enhanced oil recovery (EOR) method.
The primary recovery phase can be characterized by the natural driving energy that initially occurs in the reservoir being used as the medium to recover the crude oil to the surface as well as the additional artificial lift mechanism used together with the natural drive energy. Primary phase recovery is basically known as a low recovery process where it only recovers oil less than 30% of the original oil in place (OOIP), depending on the reservoir characteristics.
The secondary oil recovery phase or the widely known water flooding method is the injection of external fluid (water and/or gas) mainly for pressure maintenance. With secondary recovery, certainly more oil can be recovered, between 30 and 50%, depending on the reservoir characteristics. However, with only these two major methods of oil recovery, we are actually leaving behind almost 50–70% of crude oil in the reservoir; thus, providing a need to study the ways of recovering more crude oil through the third and final process, the tertiary oil recovery phase.
Tertiary recovery (enhanced oil recovery)
EOR is not just characterized by the injection of the fluids, but it is also engineered to change the physics of the down-hole system in a way that is favorable to us. Tertiary recovery phase (EOR) consists of three major flooding systems which are used to mobilize the crude oil trapped in the porous rocks (reservoir rocks) by increasing the sweep efficiency and reducing the viscosity of the crude oil. Based on the US Department of Energy, the three major techniques used are the chemical flooding, miscible flooding and thermal flooding. One of the advantages of the chemical flooding as compared to the other flooding method in general is the huge variety of different methods being used in the oilfield such as alkaline, surfactants, polymers, engineered water and foam methods. Despite all of the others, polymer flooding is the most efficient and broadly used method for the EOR in the oilfield.
Problem statement
In the face of declining oilfields and at the time where recovering hydrocarbon is becoming more difficult, effective technique is the key to recover more oils from a mature oilfield. Based on a survey, the average recovery factor of oilfields around the world is estimated to be 40%, which leads to a large amount of discovered oil to be left behind in the reservoir despite an existing production well. The current situation in the world explains the problem faced where the oil demand is increasing while the oil production capacity is decreasing. The growing demand in the petroleum industry and the crucial need for a better recovery method are the main reasons for interests on many EOR scheme implementations around the world.
Objectives and paper outline
Polymer flooding is carried out using several types of chemical injection such as the injection of water-soluble polymer alone or integration of polymer with a surfactant (surfactant polymer injection, SP) or/and alkaline solution (alkaline-surfactant polymer injection, ASP). From recent studies on nanotechnology, the injection of nanoparticles with the polymer had emerged as one of the effective polymer flooding method. Therefore, in this paper, various types of polymer injections are discussed in detail and the processes are reviewed in general. The reservoir characteristics are also important factors that affect the type of polymer flooding used in the EOR method applied. The type of polymer flooding used for EOR also has to be compatible with the reservoir properties and characteristics such as the oil trapping and facies type. The aim of this paper is to compare all of the compatible reservoir characteristics with respect to the type of polymer flooding method of EOR.
Literature review
EOR refers to the improved oil recovery with oil saturation reduction lower than the residual oil saturation [1]. Usually, EOR can yield up to 35% of oil recovery; depending on the reservoir drive type, viscosity of oil as well as permeability of the reservoir, and the yield may drop to about 5% if the condition is not favorable [2]. Various methods have been applied in the industry to obtain a high yield of EOR, such as miscible gas flooding [3, 4], chemical flooding [5,6,7], thermal flooding [8] and microbial flooding [9, 10].
Chemical flooding is an EOR method where chemical agents are injected into the oil reservoirs to distort the oil reservoir’s equilibrium after the conventional and physical recovery (primary and secondary recovery). Chemical flooding can be categorized according to the main chemical agents used in the flooding process [11]. The common chemical flooding applied in the industry are polymer flooding [12,13,14], alkaline flooding [15, 16] and surfactant flooding [17,18,19,20].
Polymer flooding
Polymer flooding is one of the most favorable EOR processes in many oil reservoirs [21]. Polymer flooding has commonly been applied in sandstone reservoir, and it is also the most applied chemical flooding method in sandstone reservoirs [22]. Kamal et al. [23] reviewed a polymer system that can be used in polymer flooding from the view of rheology, adsorption, stability and field applications. The potential polymer systems for EOR are polyacrylamide [24, 25], partially hydrolyzed polyacrylamide [26, 27], co-polymers of acrylamide [28, 29], hydrophobically modified associating polymers [30], thermoviscosifying polymers [31, 32], cationic polymers [33] and biopolymers [24].
Zaitoun et al. [34] presented their pilot studies on polymer flooding in the Pelican Lake pool, Alberta. They were able to increase about 5% of tertiary oil recovery in comparison to water flooding by using polymer flooding under the conditions of oil viscosity ranging from 1000 to 25,000 mPa.s and temperature of 15 °C. In 2005, another pilot study was done to study the effects of polymer flooding in the recovery of heavy oil of viscosities ranging from 2000 to 5000 mPa.s. The results reported that the pilot oil field has an increment of 15–21% of heavy oil recovery [35]. However, there are some limitations on polymer flooding, such as low tolerance of temperature, poor salinity resistance and high susceptibility to oxidative degradation [36]. Due to these limitations, the study on combinations of other flooding methods with polymer flooding are introduced, such as micellar polymer (MP) flooding, alkaline polymer (AP) flooding, alkaline-surfactant polymer (ASP) flooding and nanosilica polymer (NP) flooding.
Alkaline polymer (AP) flooding
Blehed and El-Sayed [37] studied the implementation of EOR in Safaniya oilfield in Saudi Aramco using AP flooding. A scaled quarter five-spot model with Safaniya crude oil was set up with the formulation of xanthan–NaOH with a concentration of 0.10/1.0, and the overall recovery yield was 70%. In [38], a study of AP flooding was also presented in EOR in David Pool, Lloydminster, Alberta, Canada. The results showed that the surfactant in the injected fluid was required to be of formulation 0.80 wt% Na2CO3 and 600 mg/L Pusher 1000E with softened water with 6660 mg/L total dissolved solids and not more than 10 mg/L hardness. With this formulation, an additional 21% of original OOIP was recovered as compared to the water injection.
Yang et al. [39] conducted a pilot study in the Yangsanmu oilfield to study AP flooding during freshwater supply shortage. Based on the research, usage of anti-salt polymer and using an active alkali were able to increase oil recovery with an additional 22.8% even when initiated at a water cut of 90%. In [40], laboratory-scale research was reported on AP flooding for heavy oil recovery using channeled sandpacks, with concentration of heavy oil recovery of 0.40% NaOH, 0.20% Na2CO3 and 1000 mg/L polymer, and the tertiary oil recovery in their study was found to be up to 30%.
The effects of the addition of alkali in polymer flooding had been studied by many researchers. Blehed and El-Sayed [37] and Wu et al. [40] reported that AP flooding improved the mobility ratio of injected fluid in oil reservoirs. In some studies, AP flooding showed better sweep efficiency in comparison to polymer flooding [37, 39,40,41]. Under high water-cut conditions, with some modifications, AP flooding still showed a positive result in EOR [39, 42]. The findings by [16, 40, 41] also showed that the low initial viscosity enhanced the injectivity of the injection fluid.
Nanoparticles injection with polymer (NP) flooding
Zhu et al. [43] studied the rheological and EOR properties of nanosilica particles hybridized with hydrophobic association of partially hydrolyzed polyacrylamide (HAHPAM) under simulated conditions of high-temperature and high-salinity oil reservoir. The results had shown that a combination of nanosilica particles–HAHPAM has a higher viscosity and elastic modulus compared to HAHPAM alone. The shear resistance and long-term thermal stability in synthetic brine were improved by the addition of nanosilica to the HAHPAM. The core test also showed that nanosilica–HAHPAM combination had a 5.13% higher recovery than HAHPAM alone.
Yousefvand and Jafari [44] had also done a study on the performance of nanosilica in polymer flooding of heavy oil reservoir with the presence of salt. The research was set up with a strongly oil-wet five-spot glass micromodel saturated with heavy oil and analyzed by an image processing technique. The displacement mechanism was analyzed and the oil recovery efficiency of flooding test was determined from the experiment. The results showed that with the presence of nanosilica in the polymer, the viscosity of the injection fluid was improved. The wettability to water wet was able to be changed by nanosilica particles in some of the micromodels. The application of NP flooding resulted in an additional 10% increase in tertiary oil recovery in heavy oil reservoir under brine condition for this research.
Cheraghian [45] had investigated the effect of NP flooding in heavy oil industry under brine condition. The aim of the study was to analyze the effects of nanosilica particles on the viscosity of the injection fluid and recovery increment in enhanced heavy oil recovery. The sample of the heavy oil was obtained from a South Iranian oilfield, with a viscosity of 1320 mPa.s. The results indicated that nanosilica particles were able to provide higher viscosity, and the flooding test showed a very positive result where there was an 8.3% increment in the EOR in comparison with the common polymer flooding after one pore volume injection. These results showed the potential of NP flooding to be applied in the EOR to reduce the composition of nanosilica used to increase the recovery efficiency.
Micellar polymer (MP) flooding
One of the major characteristics of polymer flooding is its synergistic iteration with surfactant flooding, creating a polymeric surfactant also known as micellar polymer (MP) flooding. Polymer functions to increase the viscosity of floodwater, while the surfactant acts as the emulsifier that increases the interfacial tension (IFT) of the floodwater.
An initial test was done in the Shengli oilfield by the implementation of dilute MP flooding to determine the efficiency of this flooding technique and further development of EOR for future application in the Shengli oilfield. Since its first injection in June 2004, trial results have shown a rapid decrease in water cut and an increase in oil production rate. Based on the latest field results, water cut has diminished from 98.2% in 2004 to 85.2% in 2007, a 13% shrink from the initial value. Meanwhile, oil production has been rising rapidly at an initial rate of 34 tons per day to 193 tons per day, with a growth of 159 tons per day [46].
Holm and Robertson [47] reported that MP flooding has the potential to improve the sweeping efficiency of solutions due to high brine permeability as it was found from their research that MP flooding had recovered about 70% of the residual oil. Thomas and Ali [48] also demonstrated the potential of MP flooding for EOR as the recovery of MP flooding was able to recover original oil of 50% or more for most cases in their study. Das et al. [49] also demonstrated the feasibility of MP flooding to be applied in Upper Assam Basin in India, as their core sample test with black liquor as micelle showed significantly higher maximum recovery of 56% as compared to surfactant flooding with maximum recovery of 40%.
Alkaline-surfactant polymer flooding
Another prominent type of flooding is ASP flooding. In Daqing oilfield, this technology is used on the industrial scale with a recovery rate of oil found to be over 20% [50]. The other chemical oil recovery methods possess several disadvantages such as reduced adsorptive value in surfactant flood and diluted alkaline flood after long periods [51]. Conversely, ASP flooding does not encounter this problem. In addition, ASP flooding has the advantage of the multitude of chemical phase properties as well as monitoring of natural surfactant generation, which makes it much preferable to the other existing tertiary recovery [52].
Currently, Daqing oilfield is arguably the largest oilfield implementing ASP flooding in large scale since September 1994 [53, 54]. The result has shown that ASP flooding increased 60% of the oil production rate under a sandstone layer with porosity of 26% and permeability of 1.426 μm2. Propositions made in [55] suggested for the development of weak alkali ASP flooding or alkali-free SP flooding to lessen the impact of strong alkali such as that shown in pilot test results in Daqing oil field [56,57,58]. Other oilfields in Gudong, Karamay and Shengli are some examples of Chinese ASP flooding projects at the present today which showed favorable EOR rate [59, 60]. In Gudong, recovery efficiency increases with oil production rate amassing up to 1490 barrels per day at peak conditions as compared to the initial rate of 630 barrels per day [61]. Laboratory tests had also indicated the technical feasibility of using organic alkali in ASP flooding to be applied in high-temperature condition of 95 °C in Shuanghe oilfield with total recovery percentage of 66.3% [62].
ASP flooding applications in Canada such as in Alberta (a medium gravity oil pool) increased the production from 300 barrels per day to 1502 barrels per day in December 2007. In the USA, ASP projects established in the state of Wyoming are flourishing [63]. In West Kiehl oil plant in Wyoming, the project had shown an increase in oil recovery up to 26% OOIP in only 2.5 years. In Cambridge, the oil increments that resulted from the injection was estimated to be 1.143 million barrels. In Tanner field which consists of Minneulsa B sand with 20% porosity and 200 mD permeability, ASP flooding increased up to 17% oil recovery [64]. The result of ASP flooding in Lawrence oilfield, Illinois, with Bridgeport sand with 20% porosity and 200 mD permeability, was satisfactory as the oil well production increased from 1 to 12% [65].
Viraj oilfield in India is composed of sandstone and siltstone of 30% porosity. Results have shown that water cut was diminished from 83.5 to 71.4%, while oil production rate increased from 24.4 to 98.23 m3/day [66]. In another pilot ASP flooding in Jhalora, an additional displacement efficiency of about 23% of oil initially in place (OIIP) with the initial production rate of the oilfield of 2030 bbls per day had increased to a cumulative oil gain of approximately 47,000 bbls after injection of about 0.17 PV ASP slug [67]. Lagomar LVA-6/9/21 oilfield in Lake Maracaibo was studied for suitability for ASP flooding, with 24% reservoir porosity and permeability in the range of 58–1815 mD which resulted in oil recovery of 48% after the injection [68, 69].
In general, the average for oil recovery factor had increased by 21.8% of OOIP with average oil cut reduction of 18% of the initial oil recovered. The desirable achievement was due to the excellent results of oil displacing agents, as well as desirable reservoir properties and oil displacement ability which are patterned and spaced well for ideal sweep efficiency [70]. The reservoirs with ASP flooding application also range from low temperature to high temperature, as well as low salinity to high salinity [71].
However, a few drawbacks of ASP flooding are the alkaline nature of the solution used in ASP flooding which causes scaling and corrosion as well as the emulsification of oil and water that causes difficulty in wastewater treatment [16, 57, 72].
Another venture that had been in discussion by several papers is the possibility of growth in offshore applications for ASP flooding in oil recovery. A number of researches had been done in the design and preparation of pilot projects for ASP flooding in Malaysia’s St. Joseph oilfield and Venezuela’s La Salina oilfield [68, 73,74,75].
Methodology
Polymer flooding
Polymer flooding has been applied in the petroleum industry for more than 40 years [24] and can increase recovery up to 5–30% of OOIP [76].
Due to the research efforts contributed by researchers for the past two decades, there had been new developments on the knowledge of reservoir engineering, oil recovery mechanisms, solution properties, physical and numerical simulations and efficient prediction method of the polymer flooding technology [77].
Working principle of polymer flooding
Polymer is the material that plays a vital role in the application of enhanced oil recovery technology. A typical polymer flooding project involves mixing and injection of polymer over an extended period of time until about one-third to half of the reservoir pore volume has been injected. The polymer “slug” is followed by continued long-term water flooding to drive the polymer slug and the oil bank in front of it toward the production wells. The polymer would then be injected continuously over a period of years to reach the desired pore volume. When water is injected into a reservoir, it will find the path of least resistance to the lower pressure region of the offset producing wells. If in any situation the oil in the place has higher viscosity than the injected water, the water will finger through the oil which would result in low sweep efficiency or bypassed oil [24]. One of the routine screening parameters used for preliminary analysis of reservoir is the mobility ratio that represents the effects of relative permeability and viscosity of water and oil on fractional flow based on Darcy’s Law [78].
The addition of polymer into the reservoir will increase the viscosity of water and reduce the relative permeability of the water in the reservoir and then increases oil recovery due to increase of fractional flow [14]. The displacement of the oil by the water will be efficient and piston-like if the mobility ratio is 1 or less, whereby, if the mobility ratio is more than one, the more mobile water will finger through the oil and leave the regions of in-swept oil behind [79].
According to the principle of mobility ratio, water-soluble polymers can be used to increase the viscosity of the water phase, while reducing the permeability of water to the porous rock and thereby creating a more efficient and uniform front to displace oil from the reservoir [14]. The effect can be obtained at various reservoir conditions where mobile oil saturation is greater than zero. However, the significant effect can only be obtained in the reservoir if the value of permeability, k, is big, which indicates high mobile oil saturation. In the oil characteristics aspect, the value will be significant in light oil reservoir as its viscosity is low and its permeability is great [80]. Mobility ratio (M) is defined as the ratio of mobility (λ) of the displacing fluid (water) to the mobility of the displaced fluid (oil), where mobility is permeability (κ) divided by viscosity (μ) [81], as can be seen in Eq. 1 (Source: [81]):
The ideal properties for mobility control agents can be summarized as follows: low cost or high cost-effectiveness, tolerance of high injection activity, effective when mixed with reservoir brines which is up to 20% of the total dissolved solid, resistance to mechanical degradation, 5–10 years’ stability at reservoir temperature, resistance to microbial degradation, low retention in porous rock, effective in the presence of oil or gas and low sensitivity to oxygen gas, hydrogen sulfide, pH or oilfield chemicals.
Type of polymers used in polymer flooding
Recently, the most used polymers are hydrogel polymer, polyacrylamides, HPAM, Xanthan Gum and biopolymer. Hydrogel polymers have been used for many years in EOR applications to control the mobility of injected water. These polymers are non-Newtonian fluids which are also called as pseudo-plastic fluids, because their viscosity is a function of the cut rate [76].
Hydrogel polymers are usually used with surfactants and alkali agents for increasing the sweep efficiency of tertiary recovery floodings [24].
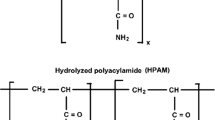
Polyacrylamide is a synthetic polymer that relies on the combination of high molecular weight and chain expansion due to repulsion of pendant ionic groups along the polymer chain to thicken and viscosify aqueous fluids [82]. There is a variety of this polymer available from several manufacturers. Generally, the performance of a polyacrylamide depends on its molecular weight and its degree of hydrolysis. Partially hydrolyzed polyacrylamide (HPAM) is one of the polyacrylamide groups that has the shape of a straight chain polymer of acrylamide monomers, some of which has been hydrolyzed. The molecule is a flexible chain structure known as a random coil and, since it is a polyelectrolyte, it will interact with ions in the solution.
HPAM, hydrolyzed polyacrylamides, is the most often used polymer in EOR applications, especially because of its relatively low price with good viscosity properties, and is well known for its psychochemical characteristics [83]. The implementation of HPAM flooding is relatively easy and can significantly improve oil recovery rate under standard reservoir conditions [84]. This polymer is available in various molecular weights up to 30 million and can be used for temperatures up to 99 °C depending on the hardness of the brine. Meanwhile, modified type such as HPAMAMPS co-polymers and sulfonated polyacrylamide can be used up to temperatures of 104 and 120 °C. It is produced generally as free-flowing powders or as self-inverting emulsions. Previous experiences indicate that it shows high sensitivity to salinity, presence of oil or surfactant and other chemicals. Precipitation can also occur if there is a presence of significant concentration of divalent cations such as Ca2+ or Mg2+ due to the high degree of hydrolysis in the polymer [85]. Increased mechanical degradation can also occur in the presence of the divalent cations during injection into porous media [86].
Xanthan Gum is a polysaccharide or usually called a biopolymer produced by the microbial action of Xanthomonas campestris [87] on a substrate of carbohydrate media, with a protein supplement and an inorganic source of nitrogen [88]. The biopolymer is an extracellular slime, which forms on the surface of the cells. The fermented broth is pasteurized to kill the microbes and precipitated from the broth by alcohol, then concentrated. Xanthan Gum is well known to have excellent performance in high-salinity brine. It is relatively compatible with most surfactants and other injection fluid additives used in tertiary oil recovery formulations. This type of biopolymer usually produced as broth and concentrated form that can be easily diluted to working concentrations without elaborate shear-mixing equipment. It is important that both of the forms must be highly pseudo-plastic solutions and are easily pumped. Some experience indicates that xanthan-type polymer usually has cellular debris that can cause plugging. Other than that, it also has significant hydrolytic degradation above the temperature of 70 °C. Currently, some companies are developing new special manufacturing techniques that are able to increase its thermal stability up to the temperature of 105 °C. Since the polymer originates from microbial activity, it is usually injected coincidentally with an effective biocide to prevent microbial degradation.
Alkaline polymer (AP) flooding
Although polymer flooding has been applied in the industry since the early 1960s, polymer flooding has its own setback in which its limitation is in removing trapped oil beads and oil layer at the surface of the rock to decrease residual oil saturation. Due to the suitability of polymer flooding properties, a number of studies on alkaline solution injection before the flooding of polymer solution had been done extensively in the 1980s, and has led to the development of AP flooding [89].
In the early stage, AP flooding is applied by injecting alkali before polymer solution flooding. The alkali injected functions to mobilize the trapped oil, then the polymer solution is injected to provide better mobility control and efficiency of volumetric sweep in the process of EOR [89].
AP flooding is the combination of alkaline flooding and polymer flooding technology used in EOR. To get good yield of recovery, the polymer solution used has to be of high viscosity. The addition of alkali will lower the viscosity of the polymer solution due to the salt effect of the alkali. However, the hydrolysis of polymer is catalyzed by the presence of alkali; thus, there is also a possibility that the addition of alkali may increase the viscosity [90].
Field application of AP flooding
One of the suitable applications of AP flooding is in heavy oil reservoirs. To recover oil from heavy oil reservoirs, the technique required to be able to plug the high-permeability channels to increase the sweep efficiency, the fluid has to be of high viscosity to increase the mobility or reduce the IFT of emulsification of heavy oil with brine. In this case, common alkaline flooding and polymer flooding have limited effects on EOR. When an alkaline solution is injected, an emulsion is formed in situ. However, the emulsion is weak and unstable, which only penetrates through the high-permeable and low-pressure area, yet the massive layer of heavy oil which has low permeability remains untouched. For polymer flooding, high oil viscosity, higher concentration and larger molecule of the polymer are used to control the mobility, which are ineffective [40]. Besides, under brine condition, the degradation of the polymer is increased. The disadvantages of alkaline flooding and polymer flooding methods can be overcome by the synergic effects of alkaline flooding and polymer flooding, making the AP flooding method feasible for enhanced heavy oil recovery. Using the AP flooding method, Wu et al. [40] was able to recover up to 30% of heavy oil in channeled sandpack flood test of sample from the Pelican Lake pool.
Working principle of AP flooding
In AP flooding, the alkali added will react with the acidic component which is found in some crude oil to form soaps. The interfacial tension (IFT) between oil and water is reduced by the soaps formed from the reaction between the oil and alkali, which will further decrease the residual oil saturation (ROS). The addition of water-soluble polymer in AP flooding is to control the mobility of the fluid to enhance the effect of sweep and oil recovery [91]. Thus, more residual oil is displaced by AP flooding as compared to polymer flooding and alkaline flooding.
AP flooding has an advantage over polymer flooding and alkaline flooding as the fluid viscosity will be lowered by the addition of alkali in the polymer solution. The low viscosity leads to low injectivity, which is utilized in the injection of the fluid. The fluid viscosity can also be increased to a higher viscosity by the reaction of alkali with water and rocks. The starting injection and the later sweep efficiency are enhanced; however, the injected polymers will be reduced by adsorption on the rocks. Thus, the overall effect is the equilibrium of both the reduction of alkaline and polymer concentrations [92].
Synergy of AP flooding
According to [16], a combination of alkali flooding and polymer flooding generates synergic effects, and they are compiled as follows:
-
Reduction of polymer adsorption and alkali consumption due to the mixing effect of alkaline polymer solution.
-
The collaboration of alkaline and polymer improve the sweep efficiency and displacement efficiency of EOR. The polymer increases the viscosity of the AP solution, which contributes to improved sweep efficiency, by transporting alkali to the area which the alkaline solution would be unable to reach alone without the polymer, while the alkali generates soaps to lower the IFT to displace more oil on the rock surfaces.
-
Biodegradation effect is reduced by the alkaline polymer environment generated by AP flooding.
-
The addition of alkali resulted in reduction of polymer viscosity, and having tight formation the reduction in viscosity may enhance the injectivity near the wellbore.
Nanoparticles injection with polymers (N/P)
The latest development in the nanotechnology field has shown us an enlightened path to improve the recovery factor of oil in the reservoir by the injection of nanoparticles along with the polymer. Nanotechnology is applied in the construction of functional materials, devices, and systems by regulating matter at the nano-scale level, and the exploitation of their original properties and phenomena that developed at that scale [93]. Commonly, a nanoparticle can be found in size of typically less than 100 nm. A core and a thin shell are the two major characteristics of nanoparticles [94].
Working principle of NP flooding
The core and thin shell might have basic structures and might consist of more than one entity. The molecular shell consists of three different regions, tail group, hydrocarbon chain and active head group. However in certain cases, one or more of these regions might be missing (as shown in Fig. 1). As a polymer, the hydrocarbon chain might be long or entirely missing, as in an ion, protecting the nanoparticles [93]. The solubility of the nanoparticles is determined by the chemical nature of the shell such as lipophobic and hydrophilic nanoparticles (LHP) dissolved in polar solvent (water), and hydrophobic and lipophilic nanoparticles (HLP) dissolved in non-polar solvents (toluene).
Nanoparticle (not to scale) schematic diagram showing molecular shell with hydrocarbon chain, and tail group, with missing active head group (from [93])
The unique properties of designed nanoparticles have shown promising applications in a diverse range of fields, spanning from medicine, drug delivery, biology, food additives, polymer composite, metal ion removal, corrosion protection, heterogeneous catalysis and improved surface properties in EOR [95].
The addition of nanoparticles can increase the ultimate oil recovery rate approximately up to 38% [96]. Previous researches had also shown that the addition of nanoparticles can increase the viscosity of the injected fluid approximately 35 times more than water [44]. Therefore, the mobility of the injected fluid decreases and the sweep efficiency improves. The wettability of the medium is an essential mechanism in oil recovery. In relation with that, the addition of nanoparticles is able to change the wettability condition to water wet and enhance the oil recovery. The most commonly used material to prepare the nano-fluid is silicon dioxide nano-powder.
To evaluate the effectiveness of nanoparticle-stabilized emulsions as a displacing fluid for EOR, flooding experiments have been carried out using silica beads [7], and the movement at the emulsion–water phase and emulsion–oil phase interface was investigated during nanoparticle-stabilized emulsion flooding [97]. These studies suggested the prospect of substantial additional oil recovery as compared to the conventional water flooding.
Micellar polymer flooding
Micellar flooding is also called as micro-emulsion flooding and surfactant polymer flooding [48]. The operation employs a micellar solution that disperses the surfactant in an oleic or aqueous solvent which can solubilize large amounts of water or oil to form either oil-in-water or water-in-oil micro-emulsions, respectively. A micro-emulsion which consists of oil, surfactant and water exists in the form of droplets with the size that is less than about 1 µm. Hence, this method improves the microscopic efficiency of the reservoir [98]. A micro-emulsion is defined as a stable, translucent micellar solution of oil, water that may contain electrolytes and one or more amphiphilic compounds [99]. Micellar polymer flooding method was first used and patented for Marathon oil company in the early 1960s [100] and simple models for prediction of MP flooding were used [101]. The injection profile of the method consists of injecting a pre-flush to achieve the desired salinity environment, followed by micellar slug of polymer solution along with drive water which is graded into waterflood [48]. Figure 2 shows the injection profile of the micellar polymer flooding method.
Injection profile of the micellar polymer flooding method (from [48])
Working principle of MP flooding
Basically, micellar polymer processes had been developed by two different concepts using surfactants in EOR. The first concept is a solution containing a selectively low concentration of surfactant that is soluble in water and is injected into the reservoir in relatively large pore volumes which are from 10 to 60% for the purpose of reducing the IFT between the oil and water, and thereby increasing oil recovery, while the second concept is the injection in a relatively smaller pore volume from 3 to 20% of oil–external surfactant solution with a relatively higher concentrated surfactant solution injected into the reservoir for the purpose of miscible displacement of the tertiary oil. Much effort has also been devoted in the recent years to the theoretical understanding of multicomponent surfactant mixture [102]. The mixtures of component solutions categorized by IFT [103] are due to the free energy of the creation of the micellar core–solvent interface and also because of the steric and electrostatic interactions among the head groups at the micellar surface [104] where a co-surfactant was added to the system along with low molecular weight alcohol and an electrolyte, normally (NH4)2S04 or NaCl below 0.01 dyne/cm.
The illustration of the working principles of micellar polymer flooding can be seen in Fig. 3. The process of injecting a pre-flush of low salinity brine followed by micellar slug into a reservoir provides mobilizing control to move it toward production wells. The slug is a solution which is a mixture of a co-surfactant, surfactant, brine and oil that reduces the capillary and interfacial forces between water and oil. The oil discharge holds out the pores where they are trapped, so that it can be thrown away by water moving. The co-surfactant in a micellar slug matches the viscosity of the solution, where they will stabilize and the absorption by the reservoir rock can be prevented. An electrolyte is added to assist in adjusting the viscosity. To boost the production, polymer-thickened water was injected for mobility control after the micellar slug. A buffer of freshwater is usually injected following the polymer flooding and ahead of the drive water to reduce contamination of the chemical solutions and improve oil recovery of the chemicals that dissolve in water and they are pumped into a reservoir through injection wells. This method has one of the highest recovery efficiencies of the current EOR methods, but it is also one of the costliest to implement.
Graphic illustration of the working principles of the micellar polymer flooding method (from [13])
Advantages and disadvantages of MP flooding
An important property of micellar systems is their ability to solubilize a variety of substances ranging from hydrocarbons to inorganic ions. Physical models describing solubilization processes in micellar environment consider the solubilization site dependence on the relative hydrophobic or lyophilic nature of the substrate [105]. Strong evidence has been presented that solubilization of relatively hydrophobic molecules or ions in the micelle–water interface is an entropically favored process with the release of bound or interfacial water molecules as the chief driving force. Other than that, micellar solutions enhance the solubility of hydrophobic solutes that are otherwise only sparingly soluble in water. Also, the MP process is technically applicable for secondary recovery or tertiary recovery [103].
However, the disadvantage of the use of MP flooding is the creation of an extremely stable oil-in-water type of emulsion at the producing well. The presence of even small concentrations of surfactant can produce very stable emulsions with water-to-oil ratios (WOR) of as high as 10 or more. Such emulsions are not amenable to breaking using conventional oilfield technology and represent both a lost revenue to the micellar project and also an extremely difficult to treat environmental hazard. They are also limited in use in carbonate reservoirs or where reservoir brines contain excessive calcium or magnesium ions.
Alkaline-surfactant polymer flooding
As the name suggests, ASP flooding derives from the technology of the alkali flooding, surfactant flooding and polymer flooding to recover residual oil in water floods. With the injection of ASP flood, IFT reduction, mobility ratio enhancement and sweep efficiency improvement throughout the water floods can be achieved [106]. These improvements lead to a speedy decrease of oil–water IFT which escalates capillary numbers by orders of the degree to the favored range for efficient oil recovery [107].
Working principle of ASP flooding
Soap is formed from the injection of alkali that reacts with the organic acid that is contained in crude oil, which positively counters to create ultralow IFT between the oil and water phase with the addition of surfactant. The IFT can be controlled by pH values as well as the ionic strength of the surfactant [108]. The surfactant infused with alkali can decrease adsorption percentage of the surfactant and lower the IFT. With the addition of the polymer, the viscosity of its aqueous phase rises so that the mobility of the aqueous phase lowers. This decrease in mobility ratio greatly increases the sweep efficiency.
Field application of ASP flooding
In the case of ASP flooding, caustic or alkali solutions are injected into the reservoir. The caustic solution reacts with the natural acids that are present in crude oil (naphthenic acids) that will then in turn form into surfactants in the form of sodium naphthenate that has similar working principles to synthetic surfactants. This allows the reduction of IFT of oil and water phase and increases the mobility of additional amount of oil to the oil reservoir. The alkali functions to reduce the adsorption of the surfactant and increase the wettability of oil to an oil-wet state [109].
In alkaline flooding, the most suitable oil to be extracted is oil that has high composition of organic acids and possesses large American Petroleum Institute (API) gravity value. Reservoir or well formation structure that is favored for alkaline flooding is sandstone over common carbonate formations. This is because carbonate formations usually comprise calcium sulfate or calcium sulfate dehydrate (generally known as gypsum). These compounds are alkaline in nature and thus an alkaline injection will not cause any major differences to the formation.
However, precipitation can form from these carbonate reservoirs that can make the extraction and recovery of oil more difficult. To solve this problem, sodium sulfate can be used to decrease the concentration of carbonate ions and calcium ions [52] Alkaline injections can be easily absorbed by clays, minerals or silica. The temperature of the reservoir also affects the alkali consumption. High temperatures can cause the alkali consumption to escalate.
While ASP flooding showed superior results than its individual counterpart flooding of alkaline, surfactant and polymer flooding, the dangers of scaling and corrosion occurring in the pipeline can cause the industry to search for and prefer a method that does not use alkali solution and thus resort to utilization of weak alkali instead of strong alkali for ASP injections. After the injection of ASP slug, it was recorded that the viscosity of the displacing liquid as well as the emulsification and scaling increase with the decrease of liquid production [55].
Comparison between methods
The comparisons between the each of the flooding methods are presented in Table 1. Performance-wise, micellar polymer flooding is the best technology as it can yield the highest oil recovery efficiency of 60%. However, it is only limited to the carbonate reservoir. Therefore, the selection of the best polymer flooding method relies heavily on and has to be tailored in accordance with the reservoir conditions.
Each flooding method has its own strength and weakness; to apply these methods in EOR, it is crucial to determine the properties of the reservoir and oil properties before deciding on the method to be used. As each method is designed to be applied in different conditions, by taking consideration of the properties of reservoir and oil, the most suitable method can be determined. However, the economics should also be taken into considerations, so that the method applied is sustainable.
Conclusion
The application of EOR technology has been used widely, as it increases the yield to of oil from the reservoir by up to 30%. For the past two decades, a lot of research has been done to improve the efficiency of polymer flooding with other chemicals to recover more oil from the reservoir. Therefore, EOR technology is urgently needed because of several reasons, such as the declining oil production since 1995, non-productive primary and secondary recovery, high crude oil price, increasing energy demand and significant oil remaining after secondary recovery which is up to 60% of the OOIP. After comparing four of the methods that have been used in current technologies, each technology showed which application field works the best for each particular polymer. Some researches state that the polymer itself is not good enough to recover more oil from the reservoir and it needs other chemical or technology like alkaline polymer, micellar polymer, nanoparticles injection with polymer and ASP, but still each of these new technologies has its own limitations and some of them have less favored cost-effectiveness, but somehow these polymers recover more oil. Performance-wise, micellar polymer flooding is the best technology, as it yielded the highest oil recovery efficiency of up to 60%. However, it is only limited to the carbonate reservoir. Ultimately, the selection of the best polymer flooding method relies heavily on and has to be tailored in accordance with the reservoir conditions. As a recommendation, more research needs to be done on polymer flooding so that in the future we could use the new technology of polymer that would possess all of the ideal criteria and also be able to satisfy the global demands.
Abbreviations
- EOR:
-
Enhanced oil recovery
- AP:
-
Alkaline polymer
- MP:
-
Micellar polymer
- NP:
-
Nanoparticles injection with polymers
- HAHPAM:
-
Hydrophobic association of partially hydrolyzed polyacrylamide
- OOIP:
-
Original oil in place
- IFT:
-
Interfacial tensions
- ASP:
-
Alkaline-surfactant polymer
References
Thomas S (2008) Enhanced oil recovery—an overview. Oil Gas Sci Technol Rev IFP 63(1):9–19
Reddy AD (2015) Enhanced oil recovery. Int J Sci Res IJSR 4(4):2252–2256
Blunt M, Fayers FJ, Orr FM (1993) Carbon dioxide in enhanced oil recovery. Energy Convers Manag 34(9):1197–1204
Lake LW, Schmidt RL, Venuto PB (1992) A niche for enhanced oil recovery in the 1990s. Oilfield Rev 4(1):55–61
Dong M, Ma S, Liu Q (2009) Enhanced heavy oil recovery through interfacial instability: a study of chemical flooding for Brintnell heavy oil. Fuel 88(6):1049–1056
Yang HT, Britton C, Liyanage PJ, Solairaj S, Kim DH, Nguyen QP, Weerasooriya U, Pope GA (2010) Low-cost, high-performance chemicals for enhanced oil recovery. Soc Pet Eng. doi:10.2118/129978-MS
Zhang H, Dong M, Zhao S (2010) Which one is more important in chemical flooding for enhanced court heavy oil recovery, lowering interfacial tension or reducing water mobility? Energy Fuels 24(3):1829–1836
Ali SMF, Thomas S (1996) The promise and problems of enhanced oil recovery methods. Pet Soc Canada. doi:10.2118/96-07-07
Brown LR (2010) Microbial enhanced oil recovery (MEOR). Curr Opin Microbiol 13(3):316–320
Maudgalya S, Knapp RM, McInerney M (2007) Microbially enhanced oil recovery technologies: a review of the past, present and future. Soc Pet Eng. doi:10.2118/106978-MS
Gurgel A, Moura MCPA, Dantas TNC, Neto ELB, Neto AAD (2008) A review on chemical flooding methods applied in enhanced oil recovery. Braz J Pet Gas 2(2):83–95
Huseynli P (2013) Evaluation of polymer flooding for enhanced oil recovery in the Norne field E-segment, Center for Integrated Operations in the Petroleum Industry
Bera A, Mandal A (2015) Microemulsions: a novel approach to enhanced oil recovery: a review. J Pet Explor Prod Technol 5:255–268
Wassmuth FR, Green K, Arnold W, Cameron N (2009) Polymer flood application to improve heavy oil recovery at East Bodo. J Can Pet Technol 48(2):55–61
Sedaghat MH, Ahadi A, Kordnejad M, Borazjani Z (2013) Aspects of alkaline flooding: oil recovery improvement and displacement mechanisms. Middle East J Sci Res 18(2):258–263
Sheng JJ (2017) Critical review of alkaline-polymer flooding. J Pet Explor Prod Technol 7(1):147–153
Chahardahcherik M, Gholamzadeh MA (2012) Economical comparison of surfactant and water flooding for enhanced oil recovery. Int J Sci Eng Res 3(8):35–40
Gogoi SB (2014) Effluent as surfactant for enhanced oil recovery. Innov Energy Policies 3:109. doi:10.4172/2090-5009.1000109
Mwangi P (2010) An experimental study of surfactant enhanced waterflooding. LSU Masters Theses
Sheng JJ (2015) Status of surfactant EOR technology. Petroleum 1(2):97–105
Silva IG, Melo D, Aparecida M, Luvizotto JM, Lucas EF (2007) Polymer flooding: a sustainable enhanced oil recovery in the current scenario. In: Presented at the Latin American and Caribbean petroleum engineering conference
Manrique EJ et al (2010) EOR: current status and opportunities. In: Presented at the SPE improved oil recovery symposium
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA (2015) Review on polymer flooding: rheology, adsorption, stability, and field applications of various polymer systems. Polym Rev 55(3):491–530
Abidin AZ, Puspasari T, Nugroho WA (2012) Polymers for enhanced oil recovery technology. Procedia Chem 4:11–16
Lee LT, Lecourtier J, Chauveteau G (1989) Influence of calcium on adsorption properties of enhanced oil recovery polymers. Oil-field chemistry, vol 396. American Chemical Society, Washington, pp 224–240
Gao C (2013) Viscosity of partially hydrolyzed polyacrylamide under shearing and heat. J Pet Explor Prod Technol 3(3):203–206
Samanta A, Ojha K, Sarkar A, Mandal A (2013) Mobility control and enhanced oil recovery using partially hydrolysed polyacrylamide (PHPA). Int J Oil Gas Coal Technol 6(3):245–258
Afolabi RO, Afolabi RO (2015) Effect of surfactant and hydrophobe content on the rheology of poly(acrylamide-co-N-dodecylacrylamide) for potential enhanced oil recovery application. Am J Polym Sci 5(2):41–46
Thomas A, Gaillard N, Favero C (2012) Some key features to consider when studying acrylamide-based polymers for chemical enhanced oil recovery. Oil Gas Sci Technol Rev D’IFP Energ Nouv 67(6):887–902
Zhou W, Zhang J, Han M, Xiang W, Feng G, Jiang W (2007) Application of hydrophobically associating water-soluble polymer for polymer flooding in China offshore heavy oilfield. Int Pet Technol Conf. doi:10.2523/IPTC-11635-MS
Chen Q, Wang Y, Lu Z, Feng Y (2013) Thermoviscosifying polymer used for enhanced oil recovery: rheological behaviors and core flooding test. Polym Bull 70(2):391–401
Wang Y, Lu ZY, Han YG, Feng YJ, Tang CL (2011) A novel thermoviscosifying water-soluble polymer for enhancing oil recovery from high-temperature and high-salinity oil reservoirs. Adv Mater Res 306–307:654–657
Zou C, Zhao P, Ge J, Lei Y, Luo P (2012) β-Cyclodextrin modified anionic and cationic acrylamide polymers for enhancing oil recovery. Carbohydr Polym 87:607–613
Zaitoun A, Tabary R, Fossey J-P, Boyle T (1998) Implementing a heavy-oil horizontal-well polymer flood in Western Canada. In: Presented at the seventh UNITAR International conference on heavy crude and tar sands, Beijing, China
Levitt D, Bourrel M, Bondino I, Jouenne S, Gingras J-P (2011) The interpretation of polymer coreflood results for heavy oil. Soc Pet Eng. doi:10.2118/150566-MS
Al-Sabagh AM, Kandile NG, El-Ghazawy RA, El-Din MRN, El-Sharaky EA (2016) Solution properties of hydrophobically modified polyacrylamides and their potential use for polymer flooding application”. Egypt J Pet 25(4):433–444
Al Blehed MS, El-Sayed AAH (2000) Feasibility of oil recovery by polymer/alkaline flooding through horizontal well. Eng J Univ Qatar 13:13–28
Pitts MJ, Wyatt K, Surkalo H (2004) Alkaline-polymer flooding of the David Pool, Lloydminster Alberta. Soc Pet Eng. doi:10.2118/89386-MS
Yang D, Wang J, Jing L, Feng Q, Ma X (2010) Case study of alkali—polymer flooding with treated produced water. Soc Pet Eng. doi:10.2118/129554-MS
Wu Y, Dong M, Shirif E (2011) Study of alkaline/polymer flooding for heavy-oil recovery using channeled sandpacks. SPE Reserv Eval Eng 14(3):310–319
Lin FFJ, Besserer GJ, Pitts MJ (1987) Laboratory evaluation of crosslinked polymer and alkaline-polymer-surfactant flood. J Can Pet Technol 26(6). doi:10.2118/87-06-04
Zhang J, Wang K, He F, Zhang F (1999) Ultimate evaluation of the alkali/polymer combination flooding pilot test in XingLongTai oil field. Soc Pet Eng. doi:10.2118/57291-MS
Zhu D, Wei L, Wang B, Feng Y (2014) Aqueous hybrids of silica nanoparticles and hydrophobically associating hydrolyzed polyacrylamide used for EOR in high-temperature and high-salinity reservoirs. Energies 7(6):3858–3871
Yousefvand H, Jafari A (2015) Enhanced oil recovery using polymer/nanosilica. Procedia Mater Sci 11:565–570
Cheraghian G (2016) Application of nano-fumed silica in heavy oil recovery. Pet Sci Technol 34(1):12–18
Hongyan W, Xulong C, Jichao Z, Aimei Z (2009) Development and application of dilute surfactant–polymer flooding system for Shengli oilfield. J Pet Sci Eng 65(1–2):45–50
Holm LW, Robertson SD (1981) Improved micellar/polymer flooding with high-ph chemicals. J Pet Technol 33(1):161–172
Thomas S, Ali SMF (1992) Micellar-polymer flooding: status and recent advances. J Can Pet Technol 31(8). doi:10.2118/92-08-05
Das BM, Gogoi SB, Mech D (2017) Micellar-polymer for enhanced oil recovery for Upper Assam Basin. Resour Effic Technol 3(1):82–87
Deng S, Yu G, Jiang Z, Zhang R, Ting YP (2005) Destabilization of oil droplets in produced water from ASP flooding. Colloids Surf Physicochem Eng Asp 252(2–3):113–119
Stoll M et al (2010) Alkaline-surfactant-polymer flood: from the laboratory to the field. In: Presented at the SPE EOR conference at oil and gas West Asia
Liu S, Miller CA, Li RF, Hirasaki G (2010) Alkaline/surfactant/polymer processes: wide range of conditions for good recovery. SPE J 15(2):282–293
Shutang G, Huabin L, Zhenyu Y, Pitts MJ, Surkalo H, Wyatt K (1996) Alkaline/surfactant/polymer pilot performance of the West Central Saertu, Daqing oil field. SPE Reserv Eng 11(3):181–188
Alvarado V, Manrique E (2010) enhanced oil recovery: an update review. Energies 3(9):1529–1575
Zhu Y, Hou Q, Weng R, Jian G, Luo Y, Li J (2013) Recent progress and effects analysis of foam flooding field tests in China. In: Presented at the SPE enhanced oil recovery conference
Guo H, Li Y, Gu Y, Wang F, Yuliang Z (2016) Comparison of strong alkali and weak alkali ASP flooding pilot tests in Daqing oilfield. In: Presented at the SPE improved oil recovery conference
Zhu Y, Lei M (2016) Studies on surfactant-polymer combination flooding formulations for a high salinity reservoir. In: Presented at the SPE EOR conference at oil and gas West Asia
Zhu Y, Zhang Y, Niu J, Liu W, Hou Q (2012) The research progress in the alkali-free surfactant-polymer combination flooding technique. Pet Explor Dev 39(3):371–376
Gao C, Shi J, Zhao F (2014) Successful polymer flooding and surfactant-polymer flooding projects at Shengli oilfield from 1992 to 2012. J Pet Explor Prod Technol 4(1):1–8
Zhijian Q, Yigen Z, Xiansong Z, Jialin D (1998) A successful ASP flooding Pilot in Gudong oil field. In: Presented at the SPE/DOE improved oil recovery symposium
Li et al H (2008) Performance analysis of ASP commercial flooding in central Xing2 area of Daqing oilfield. In: Presented at the SPE symposium on improved oil recovery
Zhao F, Ma Y, Hou J, Tang J, Xie D (2015) Feasibility and mechanism of compound flooding of high-temperature reservoirs using organic alkali. J Pet Sci Eng 135:88–100
Meyers JJ, Pitts MJ, Wyatt K (1992) Alkaline-surfactant-polymer flood of the West Kiehl, Minnelusa Unit. In: Presented at the SPE/DOE enhanced oil recovery symposium
Pitts MJ, Dowling P, Wyatt K, Surkalo H, Adams KC (2006) Alkaline-surfactant-polymer flood of the tanner field. In: Presented at the SPE/DOE symposium on improved oil recovery
Sharma A et al (2013) The design and execution of an alkaline/surfactant/polymer pilot test. SPE Reserv Eval Eng 16(4):423–431
Pratap M, Gauma MS (2004) Field implementation of alkaline-surfactant-polymer (ASP) flooding: a maiden effort in India. In: Presented at the SPE Asia Pacific oil and gas conference and exhibition
Jain AK, Dhawan AK, Misra TR (2012) ASP flood Pilot in Jhalora (K-IV), India—a Case Study. In: Presented at the SPE oil and gas India conference and exhibition
Hernandez C et al (2001) ASP system design for an offshore application in the La Salina Field, Lake Maracaibo. In Presented at the SPE Latin American and Caribbean petroleum engineering conference
Manrique E, De Carvajal G, Anselmi L, Romero C, Chacon L (2000) Alkali/surfactant/polymer at VLA 6/9/21 field in Maracaibo Lake: experimental results and pilot project design. In: presented at the SPE/DOE improved oil recovery symposium
Sheng JJ, Leonhardt B, Azri N (2015) Status of polymer-flooding technology. J Can Pet Technol 54(2):116–126
Elraies KA, Tan IM (2010) Design and application of a new acid-alkali-surfactant flooding formulation for Malaysian reservoirs. In: Presented at the SPE Asia Pacific oil and gas conference and exhibition
Johnson CEJ (1976) Status of caustic and emulsion methods. J Pet Technol 28(1):85–92
Chai CF et al (2011) St Joseph Chemical EOR Pilot-A key de-risking step prior to offshore ASP full field implementation. In: Presented at the SPE enhanced oil recovery conference
Du K et al (2011) Evaluating chemical EOR potential of St Joseph oil field, offshore Malaysia. In: Presented at the SPE enhanced oil recovery conference
Guerra E et al (2007) Improved ASP design using organic compound-surfactant-polymer (OCSP) for La Salina Field, Maracaibo Lake. In: Presented at the Latin American and Caribbean petroleum engineering conference
Pope GA (2007) Overview of chemical EOR| enhanced oil recovery, surfactant. In: Presented at the Casper EOR workshop, Center for Petroleum and Geosystems Engineering, The University of Texas at Austin
Han D-K, Yang C-Z, Zhang Z-Q, Lou Z-H, Chang Y-I (1999) Recent development of enhanced oil recovery in China. J Pet Sci Eng 22(1–3):181–188
Darcy H (1856) Les fontaines publiques de la ville de Dijon. Exposition et application des principes à suivre et des formules à employer dans les questions de distribution d’eau: ouvrage terminé par un appendice relatif aux fournitures d’eau de plusieurs villes au filtrage des eaux et à la fabrication des tuyaux de fonte, de plomb, de tole et de bitume. Dalmont
Wever DAZ, Picchioni F, Broekhuis AA (2011) Polymers for enhanced oil recovery: a paradigm for structure–property relationship in aqueous solution. Prog Polym Sci 36(11):1558–1628
Zaitoun A (2006) Improved oil and gas recovery by polymer technology: EOR, water shutoff and sand control. In: Presented at the Society of petroleum engineers distinguished lecturer program
Sorbie KS (1991) Polymer-improved oil recovery. Blackie, London
Bock J, Pace SJ, Schulz DN (1987) Enhanced oil recovery with hydrophobically associating polymers containing N-vinyl-pyrrolidone functionality. US4709759 A
Chen H et al (2015) Effect of partially hydrolyzed polyacrylamide on emulsification stability of wastewater produced from polymer flooding. J Pet Sci Eng 133:431–439
Zhao X, Liu L, Wang Y, Dai H, Wang D, Cai H (2008) Influences of partially hydrolyzed polyacrylamide (HPAM) residue on the flocculation behavior of oily wastewater produced from polymer flooding. Sep Purif Technol 62(1):199–204
Seright RS, Campbell A, Mozley P, Han P (2010) Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE J 15(2):341–348
Maerker JM (1975) Shear Degradation of partially hydrolyzed polyacrylamide solutions. Soc Pet Eng J 15(4):311–322
Wang Z, Wu J, Zhu L, Zhan X (2016) Activation of glycerol metabolism in Xanthomonas campestris by adaptive evolution to produce a high-transparency and low-viscosity xanthan gum from glycerol. Bioresour Technol 211:390–397
Li P et al (2016) Biosynthesis of xanthan gum by Xanthomonas campestris LRELP-1 using kitchen waste as the sole substrate. Carbohydr Polym 151:684–691
Mihcakan IM, Van Kirk CW (1986) Blending alkaline and polymer solutions together into a single slug improves EOR. In: Presented at the SPE rocky mountain regional meeting
Sheng JJ (2011) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing, Houston
Fortenberry R et al (2015) Use of cosolvents to improve alkaline/polymer flooding. SPE J 20(2):255–266
Sheng JJ (2013) A comprehensive review of alkaline-surfactant-polymer (ASP) flooding. In: Presented at the SPE Western Regional and AAPG Pacific section meeting 2013 joint technical conference
Das SK, Choi SU, Yu W (2007) Wiley: Nanofluids: science and technology— Das SK, Choi SU, Yu W et al (2007). http://www.wiley.com/WileyCDA/WileyTitle/productCd-0470074736.html. Accessed 16 May 2017
Hendraningrat L, Li S, Torsæter O (2013) A coreflood investigation of nanofluid enhanced oil recovery. J Pet Sci Eng 111:128–138
Al-Anssari S, Barifcani A, Wang S, Maxim L, Iglauer S (2016) Wettability alteration of oil-wet carbonate by silica nanofluid. J Colloid Interface Sci 461:435–442
Dai C et al (2017) Spontaneous imbibition investigation of self-dispersing silica nanofluids for enhanced oil recovery in low-permeability cores. Energy Fuels 31(3):2663–2668
Son HA et al (2015) The potential applications in oil recovery with silica nanoparticle and polyvinyl alcohol stabilized emulsion. J Pet Sci Eng 126:152–161
Chauhan PD (2014) Data analysis and summary for surfactant-polymer flooding based on oil field projects and laboratory Data
Healy RN, Reed RL (1974) Physsicochemical aspects of microemulsion flooding. Soc Pet Eng J 14(5):491–501
Gogarty WB (1978) Micellar/polymer flooding an overview. J Pet Technol 30(8):1089–1101
Paul GW, Lake LW, Pope GA, Young GB (1982) A simplified predictive model for micellar-polymer flooding. In: Presented at the SPE California regional meeting
Esumi K, Miyazaki M, Arai T, Koide Y (1998) Mixed micellar properties of a cationic gemini surfactant and a nonionic surfactant. Colloids Surf Physicochem Eng Asp 135(1):117–122
Stephens RH, Himmelblau A, Donnelly RG (1978) Study to determine the technical and economic feasibility of reclaiming chemicals used in micellar polymer and low tension surfactant flooding; final report—University of Oklahoma Libraries. Department of Energy Washington US
Nagarajan R (1986) Micellization, mixed micellization and solubilization: the role of interfacial interactions. Adv Colloid Interface Sci 26:205–264
Reekmans S, Gehlen M, De Schryver FC, Boens N, Van der Auweraer M (1993) Micellar properties of aqueous solutions of hexadecyltrimethylammonium salts in the presence of nonionic polymer. Macromolecules 26(4):687–694
Samanta A, Bera A, Ojha K, Mandal A (2012) Comparative studies on enhanced oil recovery by alkali–surfactant and polymer flooding. J Pet Explor Prod Technol 2(2):67–74
Kon W, Pitts MJ, Surkalo H (2002) Mature waterfloods renew oil production by alkaline-surfactant-polymer flooding. In: Presented at the SPE Eastern regional meeting
Jun S et al (2000) Surfactant-alkaline-polymer flooding pilot project in non-acidic paraffin oil field in Daqing. In: Presented at the SPE Asia Pacific oil and gas conference and exhibition
Hirasaki G, Miller CA, Puerto M (2011) Recent advances in surfactant EOR. SPE J 16(4):889–907
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pogaku, R., Mohd Fuat, N.H., Sakar, S. et al. Polymer flooding and its combinations with other chemical injection methods in enhanced oil recovery. Polym. Bull. 75, 1753–1774 (2018). https://doi.org/10.1007/s00289-017-2106-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2106-z