Abstract
Conducting polymer electrolytes based on poly(vinyl alcohol) (PVA) as a host polymer matrix and polyionic liquid (PIL) hydrophilic/hydrophobic were prepared by solution casting technique and characterized by FTIR, scanning electron microscope (SEM), mechanical, conductivity, dielectric spectroscopy and thermogravimetric analysis. It was found that the presence of PIL affected positively both mechanical and electrical properties of the polymer electrolytes. Polymer electrolytes with PIL-dispersion give the best tensile strength compared to those with PIL-solid. From the results of mechanical properties, it is concluded that PIL-dispersion can act as a plasticizer, whereas PIL-solid can act as traditional filler. The addition of PIL-dispersion significantly increased the permittivity ε′ and dielectric loss ε″ and improved the ionic conductivity of the polymer electrolytes compared to PIL-solid. The ionic conductivity of these polymer electrolytes is greatly increased with doping of PIL-dispersion owing to their strong plasticizing effect. The data of electric modulus M″ are analyzed in terms of Havriliak–Negami function through three relaxation mechanisms. In the temperature range from 50 to 150 °C, three distinct relaxation processes, namely α, αβ and β relaxations were detected in all samples. Polymer electrolytes based on hydrophilic PIL showing ionic conductivities in the range of 10−9–10−5 S/cm are good candidates in construction of electrochemical cells, whereas those based on hydrophobic PIL are suitable for use in liquid membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are organic salts that are being liquids at ambient temperatures. Ionic liquids have appeared as a new class of solvents for useful applications owing to their exceptional combination of chemical stability, low volatility, and high conductivity. Typically, an ionic liquid comprises a bulky asymmetric organic cation and a charge delocalized anion. They possess low melting point as a result of asymmetry, charge delocalization and weak electrostatic interactions. If the anions and the cations are small, they will be able to pack efficiently in the form of crystal structures with high melting temperatures such as sodium chloride (NaCl). The packing of them becomes less efficient when the size of the ions rises, consequently the melting temperature decreases (such as 1-butyl-3-methylimidazolium terafluoroborate).
The possibility to vary the molecular structure of anions and cations permits the design of millions of various ionic liquids with various properties. With small changes in their structure, it is conceivable to tune the physicochemical properties to make a specific material for a particular application. Accordingly, ionic liquids are considered as “designer solvents” [1].
Ionic liquids are also of interest from an applied point of view. New electrolytes have been developed which are able to impart high ionic conductivity together with chemical, thermal, and mechanical stability. Solutions based on ionic liquids may be suitable for the desired requirements for applications in batteries and fuel cells. To facilitate the application of ionic liquids in a battery or a fuel cell, it is an advantage to have a solid electrolyte and to control the mechanical properties [2–4]. However, the principle features of the poly(ionic liquid)s, or PILs are that they join all the exceptional properties related to ILs, while having the ability to form coatings, gels, films and membranes. PILs are polymer electrolytes whose potential uses are being investigated for a variety of technologies, such as batteries, membranes, solar cells and switchable surfaces [5].
Hydrophilic polymers, such as polyvinyl alcohol, polyethylene glycol, polymer electrolytes, and hydrogels, have attracted enormous attention because of their high hydrophilicity, flexibility, and biocompatibility. Polyvinyl alcohol (PVA) has virtues of being a host matrix because of its availability, good optical properties, cost, non-toxicity, hydrophilicity, good mechanical strength and high temperature resistance as well. Additional reasons for selecting polyvinyl alcohol as host matrix are higher extent of polar groups (hydroxyl group). The hydroxyl groups are capable of construction chain complexes with large variety of additives/dopants [6, 7]. Moreover, the dissolution of the salt would be easy in PVA owing to high chain flexibility and the presence of O–H groups [8].
In addition to mechanical properties studies of polymer electrolytes [9–11], the frequency dependence studies of the conductivity and of the dielectric response are useful techniques to obtain the detailed information of ionic conduction mechanism and differences in ion–polymer interactions in polymer electrolytes [12]. Dielectric relaxation study is an approach to investigate the dynamic and relaxation behavior of the electric dipoles in the polymer matrix by studying the electrode polarization effect onto the polymer electrolytes [13]. Conductivity relaxation refers as a phenomenon where the time is required to build up the charge carriers at the electrode–electrolyte interface before the electric field changes the direction [14].The resulting net cumulative moment will give rise to a relaxation. The buildup of the charge carriers are known as polarization. The polarization vanishes when the voltage is removed [15].
However, various methodologies such as blending, adding plasticizers, inorganic fillers to polymer electrolyte have been used to enhance the mechanical and electrical properties of the end product. Using solution casting technique, Baskaran et al. prepared polymer electrolytes blend composed of polyvinyl acetate (PVAc), polymethyl methacrylate (PMMA) as a host matrix and LiClO4 as a dopant [16]. The highest dc conductivity value obtained for the polymer complex PVAc/PMMA/20 wt.% LiClO4 is found to be 1.76 × 10−3 S cm−1 at 303 K.
Moreover, a proton battery is constructed with the highest conducting sample of PVA-PAN doped with ammonium thiocyanate, and the voltage of its open circuit is measured to be 1.2 V [17]. In addition, a novel microporous polymer electrolyte based on polyvinylidene fluoride (PVDF) and polyethylene oxide (PEO) blends was synthesized by a simple phase inversion technique. When the weight ratio of PEO to PVDF is 50 wt%, a maximum conductivity of about 2 m S cm−1 can be obtained [18]. Furthermore, ionic conductivity of about (1.2 × 10−4 S/cm) is observed together with a broad electrochemical and thermal stability at room temperature for a blend-based composite polymer electrolytes (CPEs) and doped with (15 wt%) BaTiO3 nanoparticles [19].
In another work, Zhang et al. synthesized core–shell SiO2/Poly(3-aminophenylboronic acid) (PAPBA) composites using ultrasonic irradiation method. It is found that the thermal and crystallographic properties of SiO2/PAPBA nanocomposite prepared by ultrasonication method are different from that of SiO2/PAPBA nanocomposite prepared via conventional stirring method. Moreover, the conductivity of the nanocomposite prepared via ultrasonication was around 0.2 S/cm [20]. Sol–gel and in situ emulsion polymerization have been used for preparing composites from polyaniline (PANI) and/or polypyrrole (PPY) doped spinel ferrite NiFe2O4. The results revealed that NiFe2O4-doped PPY composite had a higher conductivity value of 3.76 S/cm than that of NiFe2O4-doped PANI [21]. Moreover, to improve the dyeability of polylactic acid (PLA) Tayfun et al. [22] added organoclay during melt spinning method. They found that the presence of organoclay did not influence the wash stability properties of pristine PLA fiber. In the literature, many other hybrid systems based polymers achieved promising electrical conductivities and performance [23–26].
We report for the first time on preparation of ecofriendly conductive polymer electrolytes based on poly(vinyl alcohol) and polyionic liquid PIL without environmental loads. PIL were used in this study, namely “PIL-dispersion” as Hydrophilic and/or “PIL-solid” as hydrophobic [27]. The preparation of conducting polymer electrolytes based on poly(vinyl alcohol) (PVA)/PIL was done by solution casting technique. A detailed study was done to highlight their impact on the mechanical and electrical properties of the polymer electrolytes.
Materials and methods
Materials
The cross-linked PIL nanoparticles employed in this study as stabilizer were prepared via dispersion polymerization of 3-N-tetradecyl-1-vinylimidazolium bromide in the presence of 10 mol% of 1,4-butanediyl-3,3′-bis-lvinylimidazolium dibromide, a dicationic vinylimidazolium crosslinker) [28, 29] as PIL-dispersion. PVA purchased from Aldrich Co., Germany.
Preparation of the conducting polymer electrolytes
Polyionic liquids based poly(vinyl alcohol) as conducting polymer electrolytes were prepared by solution casting technique. The PVA was dissolved in aqueous solution first and then heated in a water bath and stirred for 30 min. The solution was cast into a Petri dish and different concentrations from PIL-dispersion and PIL-solid with added to the PVA solution. The resulting solution was stirred thoroughly and heated at 70 °C for few hours until a homogenous colorless solution is obtained. The solution was eventually casted on glass Petri dish and dried in an oven at 60 °C to obtain a free-standing conducting polymer electrolyte film.
Techniques
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra of samples were recorded using a Jascow FTIR 430 (Japan) instrument.
Scanning electron microscope
Scanning electron microscopy (SEM) analysis was performed using a Quanta instrument (model FEG250, FEI, Hillsboro, Oregon, USA). The samples were gold coated, and the electron microscope was operated at 10 kV of excitation potential. SEM was performed by mounting the polymer blends sample on standard specimen’s tube, and then created with very thin layer of gold by deposition.
Mechanical properties
The mechanical properties were determined at room temperature on a tensile testing machine using Zwick tensile testing machine (model-1425) Germany and at a crosshead speed of 500 mm/min according to ASTM D 412-06a. The PVA/PIL blend films were cut with a dumbbell-shaped cutter. Cross-section areas of the dumbbell specimens were measured accurately with the aid of a thickness gauge.
Dielectric relaxation spectroscopy (DRS)
The dielectric and conductivity measurements were carried out by means of high-resolution broadband impedance analyzer (Solartron 1260- Schlumberger Ltd. UK) interfaced with a PC. In addition to an electrometer, amplifier and measuring cell equipped with it. The frequency range of the applied ac electric field was between 0.1 Hz and 1 MHz; good electromagnetic shielding was implemented to the whole sample holder to diminish noise problems that are common especially at low frequencies. The measurements were automated by interfacing the impedance analyzer with a personal computer through a GPIB cable IEE488. A commercial interfacing and automation software Lab VIEW was used for acquisition of data. Prior to the sample measurements, the calibration was performed to eliminate the effect of stray capacitance. The percent of error in permittivity ε′ and dielectric loss ε″ amounts to 1 and 3%, respectively. The temperature of the samples was controlled by a temperature regulator with Pt 100 sensor. The percent of error in temperature measurements amounts 0.5 °C. To avoid moisture, the samples were stored in desiccators in the presence of silica gel. Thereafter the sample was transferred to the measuring cell and left with P2O5 until the measurements were carried out.
Thermogravimetric analysis (TGA)
TGA was performed using PerkinElmer (Waltham, MA) USA. The initial sample mass was about 8–10 mg; aluminum pans were used. The samples were heated from 25 to 600 °C at a heating rate of 10 °C/min under nitrogen atmosphere.
Results and discussion
FTIR spectroscopy
The main goal for determining the molecular structure of the polymer electrolytes using FTIR spectroscopy is to correlate the structural variation owing to the presence of the ionic liquid to the improvement of the electrical properties of the electrolytes. However, an incessant loss of the hydrogen bonding O–H, formation of the complexation and chain alignment prompts as a result of cations/anions coupling mechanism.
FTIR spectrum of pure PVA and those of its blends are presented in Fig. 1. This figure reveals clearly the main peaks related to polyvinyl alcohol. In pristine PVA, intramolecular and intermolecular hydrogen bonds are expected to occur between hydroxyl groups attached to the main chains. The broad band observed between 3300 and 3680 cm−1 was ascribed to the stretching of hydroxyl groups (O–H). Moreover, it is observed that the band intensity increases and bandwidth narrowing with the presence of PIL (dispersion and solid) and shifts to higher frequencies. This could be caused by strong interaction of salt cations with the hydroxyl groups (O–H) of PVA based PIL electrolytes.
However, the IR-spectra of dried imidazolium-based ILs offer a well-known absorption band at 3058 cm−1, which is referred to the C–H stretching vibration for C–H···X−. The aromatic C–H stretching vibration and the C–H stretching vibration of the C–H···X− peaks shift to higher wave numbers, upon uptake of water or other proton solvents [30]. Coordination between the cations of the PIL and the hydroxyl group of the polymer was occurred. This means that PVA undergoes significant transformation in which most hydrogen bonds are replaced with cations/anions interaction and coordination. A small band at 1744 cm−1 was allocated to the carboxyl groups C=O and the intensity of this band is clearly increased with the addition of both PIL. The relative increase of the C=O band indicates that PIL groups did not completely react with O–H groups of PVA chain. The bands around 1475 cm−1 are related to bending of the amine group (N–H). Additionally, the bands at 1360 cm−1 are attributed to the stretching of C–O group which slightly shifted to a higher value of 1390 cm−1 for the PVA-based PIL electrolytes.
Morphological characterization
The SEM micrographs of the PVA and PVA doped with 5 wt% PIL are shown in Fig. 2. Smooth surface of pure PVA can be seen in Fig. 2a. In Fig. 2a, PIL-solid seemed to be entirely embedded in the polymer. Although it was difficult to distinguish between the doping and the PVA matrix, some small agglomerates appear on the surface. Whereas, in case of PVA/5 wt% PIL-dispersion (see Fig. 2c), some wrinkled and spongy structures can be obviously observed due to membrane formation. This means that the PIL and PVA can be dispersed homogeneously in PVA/PIL membranes. This result is beneficial for the conductivity and chemical stability of PVA/PIL membrane [20].
Mechanical properties
The mechanical properties of PVA and its electrolytes represented by tensile strength and the elongation at break are illustrated graphically in Figs. 3 and 4, respectively. From each sample, minimum of five samples were cut as a dumbbell shape (115 mm × 4 mm) and tested. The data in Figs. 3 and 4 are the average of these results.
Figure 3 indicates that the tensile strength values decreases with increasing PIL-solid content. This may be attributed to the aggregation of the PIL-solid inside the polymeric matrix whose size increased by increasing doping content. Doping of PIL-solid into PVA matrix seems to cause interruption in the alignment process of the chains. Also, as doping increases, a higher restriction to molecular motion of the macromolecules is expected.
In case of PIL-dispersion doping, the tensile strength value was slightly increased with increasing PIL-dispersion content. From Fig. 4, the highly elastic behavior of these mixtures suggests the formation of a network structure of PVA in the presence of PIL-dispersion [21]. Moreover this relative enhancement in the mechanical properties is believed to be due to good interface between PVA- and PIL-dispersion; thus transferring load from polymer to the doping element.
So it could be concluded that the presence of hydrophilic ionic liquids (PIL-dispersion) in the PVA films slightly enhance the mechanical properties, whereas it deteriorated with PIL-solid or hydrophobic PIL.
Dielectric properties of PVA/PIL-dispersion and PIL-solid
The complex permittivity or dielectric constant (ε *) of a material is defined as;
where ε′ is the real part of permittivity and represents the energy stored while its imaginary part ε″ represents the loss of energy in every cycle of the applied electric field. The permittivity ε′ and dielectric loss ε″ of PVA/PIL-dispersion, solid were measured over a frequency range from 0.1 Hz to 1 MHz. The measurements were carried out at various temperatures from (50 to 150 °C). An example of the obtained data at 50 °C is illustrated graphically in Fig. 5. Figure 5 shows the variation of the permittivity ε′ and dielectric loss ε″ with frequency of PVA/PIL-dispersion, solid polymer electrolyte. As shown in these figures, permittivity ε′ and dielectric loss ε″ increase tremendously at low frequency [16] due to electrode polarization and space charge effect at the electrode–electrolyte interface. It has further confirmed the non-Debye response where more than one relaxation distribution is expressed onto the polymer matrix when the electrical fields change the direction. For this behavior, the space charge region with respect to the frequency is clarified in terms of ion diffusion. At low frequency, the free charge carriers build up at the electrode–electrolyte interface and then associate with electrode polarization when the electrical field is applied across the sample. The addition of PIL may result in more localization of charge carriers along with mobile ions causing higher ionic conductivity [13].
It is worth to notice that the values of ε′ and ε″ of PVA/PIL-liquid (Fig. 5a) are higher than those of PVA/PIL-solid (Fig. 5b). It was reported that the addition of salt to PVA causes a decrease in decomposition temperature since –OH groups in PVA form complex with salt cation [31]. In this study, the significantly reduced ε′ and ε″ values of solid polymer electrolyte can be attributed to the strong interaction of salt cation with the –OH groups of PVA. Additionally, PIL-solid increases the viscosity by forming entanglements within the polymer chain. As a result, free space for ion moving is reduced. Thus, the ionic conductivity is decreased [32]. Mostly, the conductivity of ILs depends on the mobility of their cations because in general the diffusion coefficients of PIL cations are higher than anions. The large cation size of hydrophobic PIL reduces the salt character and reduces conductivity [33–35].
In all samples, a sharp frequency dispersion of ε′ is noticed in the low frequency region and decreases with increasing frequency of applied alternating electric field till them attaining almost constant value at the high frequency region ≥104 Hz. The higher values of ε′ at the low frequency region can be described on the origin of electrode polarization effects. The electrode polarization superimposed with further relaxation processes at lower frequencies. Alternatively, the superior values of ε″ at low frequency are attributable to the motion of the free charge carriers within the electrolyte. For all samples under investigations it was found that conductivity effect is so considerable that it masks the lower frequency relaxations. Therefore, to diminish the effect of the conductivity, the formalism of “electric modulus” is introduced [36]. The electric modulus formalism had been presumed as it suppresses the polarization effects at the electrode/electrolyte interface. Hence, the complex electric modulus M * mirrors the dynamic properties alone. The electric modulus M * is defined by Eq. (2);
where M * is the complex electric modulus, \(M^{\prime}\) is the real and \(M^{\prime\prime}\) is the imaginary part of electric modulus. Figure 6 represents the imaginary part of electric modulus \(M^{\prime\prime}\) versus frequency of PVA/PIL-dispersion/solid polymer electrolyte at 50 °C. Two noticeable relaxations can be considered. The first of them is related to αβ-relaxation which is ascribed to a crankshaft motion of the main polymeric chain [36–38], whereas the other one in the higher frequencies is appointed to β-relaxation. αβ-relaxation is shifted to higher frequencies with increasing the content of PIL. Moreover, the increase of the molecular mobility is reflected by an increase of free charge mobility and the shift of the peak towards the higher frequency side with a simultaneous decrease of its magnitude.
For particular frequency (100 Hz) and at 150 °C, the values of ε′ and ε″ are found to increase by the increase of PIL content (Fig. 7) and temperature. The intermolecular forces between polymer chains are broken when the temperature increased. This in turn improves thermal disturbance. Moreover, the polar group will be liberated to orient permitting it to keep up with altering the electric field. The higher values of ε′ and ε″ are due to the increase in the degree of polarization and conductivity [38]. However, for PVA/PIL-solid (Fig. 7b), temperature has a less pronounced effect on chain mobility. The inflexibility of the structure and the lower density of dipoles (crosslinking hydroxyl groups) generate lower ε′ and ε″.
Figure 8 show the complex electric modulus plane plots (Cole–Cole plots) drawn as M″ vs. M′ (imaginary part of electric modulus vs. real part of electric modulus) for all PIL content at 150 °C. The advantage of M″ vs. M′ is that it offers better resolution than ε″ vs. ε′ plots. M″ vs. M′ demonstrate two arcs. The first one corresponds to lower frequency, i.e., left side semicircle, is a result of α-relaxation due to micro-Brownian cooperative reorientation of the polymeric segments “α-relaxation mechanism” and is associated with the glass transition, comes into frequency windows at ~80 °C and merges very fast with the particularly strong αβ at higher temperatures. The second strong relaxation mechanism is referred to αβ-relaxation which is attributed to a crankshaft type of motion of the main polymeric chain in which a few segments are involved [39]. The semicircle nature reduced at higher PIL content and becomes more skewed. The coincidence of the start of semicircles with the origin of the graph is an unambiguous sign that no additional relaxation process is present, at lower frequencies in all studied systems. Moreover, the change of the semicircles radius indicates that the relaxation mechanisms are affected by the PIL concentration.
The Cole–Cole plots Fig. 8 were aimed to analyze the contribution of the relaxation mechanisms, which are present at lower and elevated temperatures. The curves of M″ versus f are fitted using a multi-peak fitting procedure. The M″ data are analyzed using a computer program, based on Havriliak–Negami function [40, 41] which has been widely used to explain the dipole relaxation behavior of real polymeric systems. Examples of the analysis of 10 wt% PIL-dispersion, PIL-solid are shown graphically in Fig. 9. The analysis of \(M^{\prime\prime}\) of all samples shows the presence of three dielectric relaxations, α, αβ and β relaxations as discussed before. It seemed that the segmental mobility of the PVA molecules decreased after the addition of solid PIL. The shift of the peaks to lower frequencies is mainly due to the increased dipole relaxation time (or dielectric relaxation time, τ) see Fig. 10. PVA/PIL-solid chains are mostly bounded to each other inducing a longer time to return to their original equilibrium configuration. The rate of increase of relaxation time of α-relaxation is higher as it approaches glassy state compared to αβ- and β-relaxations, as the former relates to the segmental chain motion compared to the latter one. The alternating current (ac) conductivity (σ of all samples has been calculated from the dielectric losses according to the following equation;
where ω is the angular frequency and ε o is the vacuum permittivity 8.85 × 10−12 F/m; \(\omega = 2\pi f\). The conductivity as a function of frequency at 150 °C is illustrated graphically in Fig. 11. From this figure, it is clear that the conductivity rises with increasing PIL content. The gradual change from dc plateau to (ac) dispersive region denotes the distribution of relaxation times. The frequency dependence of electrical conductivity can be divided into distinctive regions implying the existence of various dissipated effects [42].
In the high frequency region conductivity increases with increasing frequency following the universal dielectric response law, \(\sigma \left( \omega \right) = A\omega^{n}\) where n was found to be very close to unity. In the low frequency region for PIL content ≥7 wt a leveling off of the electrical conductivity is observed. This corresponds to the dc conductivity of the samples, which increases significantly with increasing PIL content. In the intermediate region, dipolar relaxation processes are present, the intensity of which is dependent upon PIL content. On the other hand, the conductivity was significantly influenced by PIL concentration. The conductivity varied from 5 × 10−9 to 5 × 10−5 S/cm for liquid PIL and it varied from 3 × 10−10 to 2 × 10−6 S/cm for PIL-solid, respectively. The conductivity of an ionic liquid mainly depends on the mobility of its cations. However, PIL showing ionic conductivities in the range of 10−5–10−7 S/cm are useful in construction of electrochemical cells [34, 43] and for high temperature fuel cells [44]. The electrochemically mainly stable materials having analogous small conductivities like N-butyl-N-methylpyrrolidinium. It is also found that hydrophobic ionic liquids are suitable for use in liquid membrane [35].
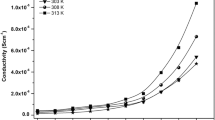
However, for all samples under investigations the conductivity increases with temperature as can be seen in Fig. 12. Figure 12 represents the frequency dependence of ac conductivity σ for PVA with 10 wt PIL-dispersion at different temperatures. The increase of conductivity is ascribed to the expansion of polymer matrix. The polymer matrix expands with increase in temperature and then weakens the interaction within the polymer matrix, promoting the decoupling of PIL cations from the polymer complex. This assists the mobility of ions. The polymeric chain also gains faster internal vibrational modes with increasing the temperature, which in turn to increase in mobility of polymer segments by initiating the bond rotation within the polymer matrix. Therefore, it favors the ionic transportation and hence increases the ionic conductivity [14, 43].
In Fig. 13, for both systems (PIL-dispersion, solid) strong correlation exhibits between ionic conductivity and its activation energy, where the activation energies are inversely proportional to ionic conductivity. That is, higher conductivity is correlated with lower activation energies and vice versa. This is mainly attributed to the amorphous region in the polymer matrix, which is the prerequisite of the higher ionic conductivity [35]. The presence of amorphous phase leads to the unruly arrangement of molecules in the polymer matrix which results in a more flexible polymer chain. Therefore, the attractive bonds between the macromolecules become weaker and demanding less energy to break the bond for reorganization and reformation of coordination with PIL. This enhances the PIL diffusivity in the polymer matrix [32, 45, 46]. Derived from the above discussion it can be concluded that the hopping process is favorable with decreasing the activation energy.
Thermogravimetric analysis (TGA)
Figure 14 represents the thermogravimetric analysis of PVA and its ionic liquid (Soild/dispersion PIL)-based polymer electrolytes. Thermogravimetric data for PVA and its electrolytes are summarized in Table 1.
From the collected data it is found that pure PVA shows three major weight losses, as reported before [47]. However, four and/or three thermal degradation steps are found for PVA and/or ionic liquid doped polymer electrolytes. The initial thermal degradation in the region of 25 to 125 °C is due to the dehydration of water or moisture. The insignificant weight loss in this stage is also attributable to the elimination of impurities in the polymer electrolytes. The weight loss of all the samples remains the same 175–240 °C. After that a rapid decrease in weight is observed for all the samples.
Among all the samples, PVA based PIL-dispersion samples depict the highest mass loss from 250 to 337 °C. However, the weight loss of PIL-dispersion based polymer electrolytes is found to be higher than PIL-solid until decomposition temperature (337 °C). After that, PIL-dispersion began to overcome decomposition with a step wise decrease. A consecutive enormous loss of PIL-solid was noticed until reaching its final thermal decomposition temperature T F (458 °C for 5 wt% PIL doping and 460 °C for 10 wt% PIL doping). In this stage, the thermal degradation arises from chemical decomposition processes comprise the breakdown of ether cross-linkages and random chain scissoring between C=C bond.
Moreover, PIL-dispersion illustrates the highest decomposition temperature (499 °C for 5 wt% PIL doping and with residual weight 6.58%). On the other hand, higher PIL-dispersion doping (10 wt%) into the backbone of PVA also led to increased thermal stability (T F ~ 592 °C with the highest residual weight 12.7%).
Finally, it can be concluded that PIL-dispersion based PVA electrolytes are stable below 337 °C, which may be due to their highly cross-linked structure. Therefore, they can be used in some catalytic reactions at high temperatures.
Conclusion
In this study, polymer electrolytes based on PVA were papered and characterized. Hydrophobic and hydrophilic PIL were added to PVA host matrix in different ratios to get a versatile polymer electrolyte which possesses many functions for preparation of advanced materials and polymeric products. The studied mechanical properties indicated that PIL-dispersion can be used as plasticizer for PVA.
FTIR results revealed that the band intensity increases and bandwidth narrowing with the presence of PIL (dispersion and solid) and shifts to higher frequencies. This could be due to strong interaction of salt cations with the hydroxyl groups (O–H) of PVA-based PIL electrolytes. This means coordination between the cations of the PIL and the hydroxyl group of the polymer was occurred. This means that PVA undergoes significant transformation in which most hydrogen bonds are replaced with cations/anions interaction and coordination.
Some small agglomerates appear on the surface of PVA/5 wt PIL-solid as detected by SEM, whereas some wrinkled and spongy structures in case of PVA/5 wt% PIL-dispersion can be obviously observed due to membrane formation.
The dielectric properties and the conductivity measurements of PVA/PIL-dispersion or PIL-solid have been studied in the frequency range of 0.1 Hz–1 MHz and temperatures from 50 to 150 °C. The addition of PIL was found to increase the permittivity ε′ and dielectric loss ε″ and improved the ionic conductivity of PVA/PIL-dispersion in comparable with PVA/PIL-solid. The data of electric modulus M′ and M″ were analyzed in terms of Havriliak–Negami function through three relaxation mechanisms, namely α, αβ and β relaxations. These dielectric processes are attributed to main chain and local motions resulting from reorientations of polar side groups and small segments of the polymer chain. The ionic conductivity of hydrophilic electrolytes was found to be in the order of 10−9–10−5 S/cm, are considered to be good candidates in construction of electrochemical cells while those based on hydrophobic electrolytes are suitable for use in liquid membranes. The ionic conductivity of electrolytes based PIL-dispersion greatly increased by increasing doping due to their plasticized effect. However, the higher conductivity is associated with lower activation energy and vice versa. Moreover, TGA results show that PIL-dispersion based PVA electrolytes exhibit high thermal stability, which may be due to their highly cross-linked structure. Therefore, they can be used in some catalytic reactions at high temperatures.
References
Freemantle M (2009) Introduction to ionic liquids, 1st edn. Royal Society of Chemistry (RSC) Publishing, Cambridge
Matsumoto H, Sakaebe H, Tatsumi K, Kikuta M, Ishiko E, Kono M (2006) Fast cycling of Li/LiCoO2 cell with low-viscosity ionic liquids based on bis (fluorosulfonyl) imide [FSI]. J Power Sources 160:1308–1313
Gorlov M, Kloo L (2008) Ionic liquid electrolytes for dye-sensitized solar cells. Dalton Trans 20:2655–2666
Endres F, El Abedin SZ (2006) Air and water stable ionic liquids in physical chemistry. Phys Chem Phys Chem 8:2101–2116
Yuan J, Antonietti M (2011) Poly(ionic liquid)s: polymers expanding classical property profiles. Polymer 52:1469–1482
Jitendra T, Khushbu K, Balasubramanian KR (2016) The dielectric properties and charge transport mechanism of p-conjugated segments decorated with intrinsic conducting polymer. RSC Adv 6:69733–69742
Sengwa RJ, Sankhla SJ (2007) Dielectric dispersion study of poly(vinyl pyrrolidone)–polar solvent solutions in the frequency range 20 Hz–1 MHz. Macromol Sci Part B Phys 46:717–747
Liew C, Ramesh S, Arof AK (2014) Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties. Int J Hydrogen Energy 39:2953–2963
Lizhen F, Zhimin D, Ce-Wen N, Ming L (2002) Thermal, electrical and mechanical properties of plasticized polymer electrolytes based on PEO/P(VDF-HFP) blends. Electrochim Acta 48:205–220
Zhenglin T, Li Q, Guitian G (2008) Dynamic mechanical properties of gel polymer electrolytes containing ionic liquid. Solid State Ionics 179:1880–1884
Angulakshmi N, Thomas S, Nahm KS, Manuel Stephan A, Nimma Elizabeth R (2011) Electrochemical and mechanical properties of nanochitin-incorporated PVDF-HFP-based polymer electrolytes for lithium batteries. Ionics 17:407–414
Gray FM (1991) Fundamentals of technological applications. Wiley, London, pp 149–179
Rajendran S, Sivakumar M, Subadevi R (2004) Investigations on the effect of various plasticizers in PVA-PMMA solid polymer blend electrolytes. Mater Lett 58:641–649
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Ac impedance, DSC and FT-IR investigations on (x) PVAc-(1 − x) PVdF blends with LiClO4. Mater Chem Phys 98:55–61
West AR (1999) Electrical properties: basic solid state chemistry. Wiley, West Sussex, pp 321–361
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamwa J, Hattori T (2006) Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ion 177:2679–2682
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Sanjeeviraja C, Nithya H, Iwai Y, Kawamura J (2015) Proton- conducting polymer electrolyte based on PVA-PAN doped with ammonium thiocyanate. Ionics 21:1017–1029
Xi J, Li X, Tang X, Zhu W, Chen L (2006) PVDF–PEO blends based microporous polymer electrolyte: effect of PEO on pore configurations and ionic conductivity. J Power Sources 157:501–506
Lee L, Park S, Kim S (2013) Effect of nano-sized barium titanate addition on PEO/PVDF blend-based composite polymer electrolytes. Solid State Ion 234:19–24
Zhang Y, Lee S, Reddy K, Gopalan A, Lee K (2007) Synthesis and characterization of core-shell SiO2 nanoparticles/poly(3-aminophenylboronic acid) composites. J Appl Polym Sci 104:2743–2750
Li Z, Ye M, Han A, Du H (2016) Preparation, characterization and microwave absorption properties of NiFe2O4 and its composites with conductive polymer. J Mater Sci Mater Electron 27:1031–1043
Tayfun U, Dogan M (2016) Improving the dyeability of poly(lactic acid) fiber using organoclay during melt spinning. Polym Bull 73:1581–1593
Xin H, Xue T, Xu C (2015) Conductive properties and mechanisms of different polymers doped by carbon nanotube/polypyrrole 1D hybrid nanotubes. RSC Adv 5:61383–61389
Reddy KR, Sin BC, Ryu KS, Kim JC, Chung H, Lee Y (2009) Conducting polymer functionalized multi-walled carbon nanotubes with noble metal nanoparticles: synthesis, morphological characteristics and electrical properties. Synth Met 159:595–603
Hassan M, Haque E, Faisal SN, Ghasemi S, Minett A, Gomes VG (2014) Hierarchical assembly of graphene/polyaniline nanostructures to synthesize free-standing supercapacitor electrode. Compos Sci Tech 98:1–8
Reddy KR, Lee KP, Gopalan AI (2007) Self-assembly directed synthesis of poly(orthotoluidine)- metal (gold and palladium) composite nanospheres. J Nanosci Nanotech 7:3117–3125
Yi Yang, Sun N, Sun P, Zheng L (2016) Effect of the bis-imidazolium-based poly(ionic liquid) on the Microstructure and the properties of AAEMs based on polyvinyl alcohol. RSC Adv 6:25311
Rozik N, Antonietti M, Yuan J, Tauer K (2013) Polymerized ionic liquid as stabilizer in aqueous emulsion polymerization enables a hydrophilic-hydrophobic transition during film formation. Macromol Rapid Commun 34:665–671
Rozik N, Abd El-Messieh S L (2017) Polyionic liquid incorporated PS/PANI-based polymer electrolytes: electrical and dielectric properties. Polym Bull. doi:10.1007/s00289-017-1904-7
Pimentel GC, McClellan AL (1960) The hydrogen bond. Freeman, San Francisco, pp 348–363
Çavuş S, Durgun E (2016) Poly(vinyl alcohol) based polymer gel electrolytes: investigation on their conductivity and characterization. Acta Physica Polonica A 129:621–624
Kumar R, Subramania A, Sundaram NTK, Kumar GV, Baskaran I (2007) Effect of MgO nanoparticles on ionic conductivity and electrochemical properties of nanocomposite polymer electrolyte. J Membr Sci 300:104–110
Saroj AI, Singh RK (2012) Thermal, dielectric and conductivity studies on PVA/Ionic liquid [EMIM][EtSO4] based polymer electrolytes. J Phys Chem Solids 73:162–168
Every H, Bishop AG, Forsyth M, MacFarlane DR (2000) Ion diffusion in molten salt mixtures. Electrochim Acta 45:1279–1284
Anderson JL, Armstrong DW, Wei G (2006) Ionic liquids in analytical chemistry. Anal Chem 78:2893–2902
Nassar MA, Ward AA, Abdel Baseer R (2013) Synthesis and characterization of polyaniline nanocomposites. KGK-kautschuk Gummi Kunststoffe 66:39–46
Bhargav PB, Mohan Sharma VM et al (2009) Investigations on electrical properties of (PVA:NaF) polymer electrolytes for electrochemical cell applications. Current Appl Phys 9:165–171
Kremer F, Arntt M (1997) Dielectric spectroscopy of polymeric materials. American chemical Society, Washington DC
El-Houssiny AS, Ward AAM, Mansour SH, Abd- El- Messieh SL (2012) Biodegradable blends based on polyvinyl pyrrolidone for insulation purposes. J Appl Polym Sci 124:3879–3891
Havriliak S, Negami S (1966) A complex plane analysis of α-dispersions in some polymer systems. J Polym Sci Part C Poly Symp 14:99–117
Mc Crum NG, Read BE, Williams G (1967) Anelastic and dielectric effects in polymeric solids. Wiley, London
von Hippel AR (1995) Dielectrics and waves. Artech House, Boston
Wilkes JS, Levisky JA, Wilson RA, Hurrey CL (1982) Dialkyimidazolium chloroaluminate melts: a new class of room- temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg Chem 21:1263–1264
Boroglu, Celik SU, Bozkurt A, Boz I (2103) Proton-conducting blend membranes of crosslinked poly(vinyl alcohol) sulfosuccinic acid ester and poly(1-vinyl-1,2,4-triazole) for high temperature fuel cells. Polym Eng Sci 53:153–158
Ayesh AI, Mohsin MA, Haik MY, Haik Y (2012) Investigations on electrical properties of poly(vinyl alcohol) doped with 1-methyl-3-n-decyl-imidazolium bromide ionic liquid. Curr Appl Phys 12:1223–1228
Thayumanasundaram S, Rangasamy VS, Greef ND, Seo JW, Locquet JP (2015) Hybrid polymer electrolytes based on a poly(vinyl alcohol)/poly(acrylic acid) blend and a pyrrolidinium-based ionic liquid for lithium-ion batteries. Eur J Inorg Chem 7:1290–1299
Yang JM, Chiang CY, Wang HZ, Yang CC (2009) Two step modification of poly(vinyl alcohol) by UV radiation with 2 -hydroxyl ethyl methacrylate and sol-gel process for the application of polymer electrolyte membrane. J Membr Sci 341:186–194
Acknowledgements
Nehad N. Rozik would like to thank Dr J. Y. Yuan for introducing the synthesis of PIL dispersion and Dr. K. Tauer for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rozik, N.N., Ward, A.A. A novel approach on poly(ionic liquid)-based poly(vinyl alcohol) as a hydrophilic/hydrophobic conductive polymer electrolytes. Polym. Bull. 75, 267–287 (2018). https://doi.org/10.1007/s00289-017-2027-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2027-x