Abstract
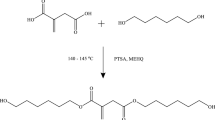
A new halo-ester diol has been synthesized by esterification reaction of trichloroacetic acid and pentaerythritol by using p-Toluenesulfonic acid as a catalyst. The synthesized diol was reacted with isophorone diisocyanates, polyester polyol, di-methylol propionic acid to form polyurethane dispersion. The antimicrobial properties of polyurethane dispersions were evaluated against Gram-negative E. coli and Gram-positive bacteria L. plantarum by Kirby–Bauer test. The structure of the diol was examined using Fourier transform infrared spectroscopy, 1H nuclear magnetic resonance and mass spectroscopy. The physical, chemical and thermal properties such as viscosity, particle size, chemical resistance and DSC, XRD, SEM and GPC of polyurethane dispersion were evaluated. The significant effect of diol and its chain length, particle size and zeta potential properties of resulting polyurethane dispersion were investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyurethane dispersions (PUDs) have been extensively utilized for commercial applications such as wood, biomedical, glass fiber sizing, adhesives, automotive parts finishes, gloves and other coating applications [1–3]. The development of these PUDs has been motivated mainly by environmental considerations as these materials reduce toxicity and avoid the release of volatile organic compounds into the atmosphere. However, the limitation of PUDs is that they do not exhibit antimicrobial activity [4, 5]. More newly the development of polyurethane dispersion made with functional materials on new ideas on decorative and antimicrobial coatings [6–8]. Jose Miguel have been synthesized water-borne polyurethane dispersions to improve hydrolysis resistance, aging, oil resistance, weathering and fungi resistance of the coating [9–11]. The anionic polyurethane dispersion is more widespread in industries than cationic polyurethane dispersion. In general, the anionic polyurethane dispersion is synthesized by using diol, polyol, and dimethylol propionic acid into the polymer backbone [12–23]. The polyurethane is then neutralized with tertiary amine and dispersion in water. However, conventional polyurethane dispersions synthesised from polyester polyol, DMPA and isocyanates do not exhibit antimicrobial properties. The antimicrobial properties of anionic polyurethane might be improved by incorporating antimicrobial agents into the polymer backbone. The antimicrobial properties will result from the incorporation of diester halides diol groups covalently bonded to the polymer backbone. The expected features of these polymers are relatively high stability and good antimicrobial properties.
In recent years, several antimicrobial agents have been introduced into the polyurethane backbone. The most common antimicrobial agents used in polymer films are triclosan, chlorhexidine and cyclodextrin. These antimicrobial agents were added to stabilize at high processing temperature [24]. Pittman has synthesized copolymers of pentachlorophenyl acrylate with ethyl acrylate as polymeric biocides and found that copolymer exhibited excellent antibacterial activity against pseudomonas species [25]. However, the halogen o-hydroxydiphenyl ether derivatives have been used for protection of organic materials, like synthetic resins, printing thickeners, lacquers, and paints because of significant biocidal activities [26]. The chlorinated bactericidal monomer 2,4,4-trichloro-2-acryloxydiphenylether was synthesized by Oh sang [27].
The main objective of this work was to enhance the antimicrobial activity of the polyurethane dispersion. This article focused on the synthesis of chlorinated diol for development waterborne polyurethane, which is suitable for improving antimicrobial activity. The zone-of-inhibition test was used to evaluate the antimicrobial properties of polyurethane dispersion. Whereas the zone of inhibition was found to be increased when the chlorine-based diester diol was incorporated into the polymer structure.
Experimental
Materials
Polyester polyol (Oxymer M112) was procured from Perstorp Chemicals Pvt. Ltd. Mumbai (India). Isophorone diisocyanate (IPDI) was procured from Bayer Materials Science Pvt. Ltd. Mumbai (India). Dimethylol propionic acid (DMPA, 98% Acros Organic India) was dried under vacuum at 75 °C, Triethylamine (TEA, 99%, SD Fine Chem. Ltd., Mumbai, India.), Pentaerythritol (99% Aldrich India), Dibutyl tin dilaurate (DBTDL, 99% Aldrich India). Para-toluene sulphonic acid (PTSA), Acetone and trichloroacetic acid were purchased from S. D. Fine Chemicals Mumbai (India) (L.R. grade). Nutrient broth (M002) and sabouraud dextrose broth (M033) were obtained from Hi-Media Laboratories, India. The bacterial strains of E. coli (ATCC-25922) and L. plantarum (ATCC-8014) were obtained from the pharmaceutical department of ICT, Mumbai. Millipore water was used for the preparation of nutrient broth media.
Methods
Synthesis of antimicrobial agent using esterification of trichloroacetic acid and pentaerythritol
Trichloroacetic acid (5 g 0.03 mol), pentaerythritol (8.33 g 0.06 mol) and xylene were poured in a 100 ml three-necked dry reactor equipped with a dean and stark with a condenser and an overhead stirrer under an inert atmosphere. In the reaction catalyst, p-Toluenesulfonic acid was added to the reaction mixture on the basis of 2% of pentaerythritol moles. The reaction mixture was heated at 160 °C and stirred for 3 h. The progress of the reaction was observed by collecting moles of water through dean stark and also by TLC using hexane and ethyl acetate (70:30) solvent system. The unreacted trichloroacetic acid was removed by saturated NaHCO3 solution and water. The organic layer was collected and dried with Na2SO4. The solvent was removed under reduced pressure and the desired product was obtained as a viscous liquid. Scheme 1 depicts the synthetic route for diester diol.
Synthesis of polyurethane dispersion
Synthesis of NCO terminated prepolymer
Polyurethane dispersions were synthesized in a 100 ml glass kettle with fitted accessories like mechanical stirrer, thermometer, reflux condenser and a gas inlet/outlet with temperature monitoring probe. The reaction was carried out at constant temperature water bath and in a nitrogen atmosphere. Calculated amounts of polyester polyol, dimethylol propionic acid, and diester diol were charged into the reactor together with the dibutyltin dilaurate. The reaction mixture was heated in the reactor to 60 °C maintaining the stirrer at 200 rpm. The temperature of the reactor was reduced to 50 °C as soon as the pre-mixture was in the molten state. To this molten mixture, IPDI was charged over the period of 30 min dropwise to the reaction mixture. The reaction was continued for 4 h. On reaching the expected NCO value in the range 4.6 the reaction was stopped. The reaction was continued until the amount of residual NCO groups reached the theoretical value. The theoretical value of the remaining NCO groups was determined by the standard dibutyl amine back-titration method [9]. The prepolymer was cooled and maintained at 35 °C. The formulation for polyurethane dispersion is shown in Table 1.
Neutralization and chain extension of NCO-terminated prepolymer
The NCO-terminated prepolymer was neutralized by a calculated amount of triethylamine, and it was carried out at 50 °C for 2 h for complete neutralization. The polyurethane anionomer was dispersed in distilled water and chain extension reaction proceeded with adding 1,6-hexamethylene diamine to the reaction between the unreacted NCO groups and water, because of the high reactivity of NCO groups with amines. In order to get uniform particle size of the dispersion, an addition was carried out dropwise at high speed of 800 rpm for 30 min and acetone was removed by a rotatory evaporator to get pure dispersion Scheme 2.
Characterizations
Physico-chemical analysis of diester diol: the hydroxyl value of the synthesized diol was evaluated by acetic anhydride-pyridine method ISO 4629-1978 (E).
Isocyanate value: A definite weight of the sample was taken in a dry Erlenmeyer flask and dissolved in dry toluene. Then, 25 ml of 0.1 N solution of n-dibutyl amine was added to the flask and allowed to stand for 30 min; 100 ml of isopropyl alcohol was then added to the solution in the flask. The contents of the flask were then titrated against 0.1 N hydrochloric acid. The indicator used to be bromophenol blue and the end point was noted by observing the disappearance of blue colour and the appearance of yellow colour. A blank titration was also performed simultaneously. Isocyanate value was then calculated using the formula as per reported method [9]. FTIR analysis was conducted on Shimadzu (8400 s, Japan) instrument using the ATR technique and the spectrum was obtained in the wavelength range 4000–600 cm−1. GC–MS analysis was recorded on Shimadzu (QP-2010 Japan) instrument by preparing 10 ppm solution in methanol. 1H NMR analysis was conducted at 400 MHz using Bruker Biospin (Avance AV500WB, Germany) in CDCl3 and tetramethylsilane (TMS) as an internal standard. Molecular weights of the PUD were obtained from gel permeation chromatography (Waters Co., USA) equipped with a series of Styragel columns (104, 105, 106 A pore sizes), HPLC pump (Waters 501), RI detector (Waters 410), and integrator at 40 °C. Polystyrene standards and universal calibration. Tetrahydrofuran (THF) was used as an eluent at 1.0 ml min−1 flow rate and 1.0 × 103 Pa was the pump pressure. The particle size of latex samples was carried out by dynamic light scattering (Malvern 3000HSA). The pH value of polyurethane aqueous dispersions was measured at 25 °C with a pH meter, using a silver reference electrode, model SV3T, Arvind Industries Ltd. (Mumbai). DSC experiments were carried out in a TA DSC Q100 V6.2 instrument (TA Instruments). Aluminium pans consisting 5 mg of samples were heated from −40 to 60 °C under nitrogen atmosphere. The heating rate was 10 °C/min. Antimicrobial activity of polyurethane dispersion was evaluated by using zone-of-inhibition test against Gram-positive and Gram-negative bacteria. The adhesion test was performed by the crosscut adhesion tester used to check the adhesion of the coated film to wood substrates as per ASTM D 3359. The impact resistance of a coating was carried out on steel panels as per ASTM D 2794. The flexibility of the coating was measured in terms of the “crack resistance” and the tests were carried out on a standard conical mandrel using coated mild steel panels as per ASTM D 522. Pencil hardness was checked as per ASTM D 3363. The scratch hardness was determined by the automatic scratch hardness tester as per ASTM D 7027. This test method covers hardness of organic coating on a substrate by increasing weight in an automatic scratch hardness tester. Solvent resistance of coating was determined by ASTM D 5402-93 using solvent rub technique for assessing the solvent resistance of an organic coating by rubbing the coating with a cloth saturated with the MEK. The solvent resistance rub test was carried out by using ASTM D 4752. The result reported was the number of double rubs at which the films were observed to fail or else 100, which was a maximum number of double rubs carried out. The gloss of coating was measured on a digital mini gloss meter (Rhopoint Instruments, ASTM D 523-99).
SEM (scanning electron microscope) micrographs for the PUDs were obtained on a JEOL JSM 6380LA instrument. Powder X-ray diffraction (XRD) patterns were obtained using a Bruker AXS diffractometer D8 Cu-K_ radiation (λ = 1.540562). Samples were step-scanned from 10.0° to 99.9° in a stepping time of 17.7 s.
Result and discussion
Physicochemical analysis: Hydroxyl value
Practical hydroxyl value was observed to be 249.12 mg of KOH/g of sample close to the theoretical value, viz. 263.2 mg of KOH/g of a sample. Units: milligrams of KOH per gram of sample.
Isocyanate value
The reaction was monitored by determining NCO values of polyurethane; it was continued until the amount of residual NCO groups reached the theoretical value.
The NCO value of polyurethane dispersion was in the range expected theoretical NCO values 4.6, then the reaction was stopped. The theoretical value of the remaining NCO groups was determined by the standard dibutyl amine back-titration method [27].
FTIR spectrum of diester diol
Figure 1 reveals the FTIR spectra of diester diol. The characteristic peak is at 3350 cm−1 due to OH stretching frequency. The peaks of 2935 and 2863 cm−1 correspond to CH2 symmetric and asymmetric stretching. The peak at 1761 cm−1 corresponds to –COO stretching frequency of ester group. The peak at 1050 cm−1 represents the C–O stretching frequency of primary alcohol. From the spectrum of FTIR it can be concluded that the esterification of pentaerythritol was confirmed.
Mass spectroscopy analysis
Figure 2 reveals the mass spectrum of diester diol. The molecular ion peak at M/Z = 427 indicates the formation of a diol having molecular weight 427. The peak maximum intensity is at M/Z = 427 and the other fragments having M/Z = 279 confirmed the formation of the diol. Thus, from mass spectral data, the structure of monomer was confirmed.
H1NMR analysis
Figure 3 shows the H1NMR spectrum of diester diol (a). The chemical shift value at δ 3.54 s assigns to –OH, (b) the peak at δ 4.05 s corresponds to (–O–CH2–), (c) the peak at δ 4.25 assigns to (–CH2–). Thus from 1H-NMR data, it can be concluded that the diol was successfully synthesized.
H1NMR analysis of polyurethane dispersions
The 1HNMR spectra of PUD are shown in Fig. 4 1HNMR (CDCI3):The peak at δ 0.96 s assign to (CH3–C–) (g), the peak at δ 1.05 s correspond to (–C–CH3) (e), the peak at δ 1.18 s (–CH2–CH2–CH2–) (f), the peak at δ 1.55 s assign to (C–CH2–C) (h), the peak at δ 2.88 s corresponds to (–C–CH2–C) (d), the peak at δ 5 s (–N–H) (c), the important characteristics peaks at δ 3.75 and δ 3.95(O–CH2–) (a, b) are observed in the spectrum indicating complete incorporation of diester diol in polyurethane dispersion. Thus from 1H-NMR data, it was concluded that the diester diol was completely incorporated in polyurethane dispersion (Fig. 4).
Fundamental properties of polyurethane dispersion
Figure 5 shows the viscosity of the polyurethane dispersion with constant solids content of 38%. For all these solids content, the viscosity remains constant at zero shear rate. As the shear rate increases its shows shear thinning behaviour at different solids content of dispersion. However, PUD with a higher solid content, the distance between dispersed particles becomes closer, resulting in strong interactions. When an external force is applied to the spherical particles, breaking or deformations may occur, followed immediately by recovery, leading to fluctuations of viscosity [28]. However, from the viscosity results it is concluded that there is no adverse effect on increases in viscosity by addition of diester diol in formulation.
The aqueous polyurethane dispersions have pH values between 8.2 and 8.5 (Table 2), which are within the stable pH range of commercial typical polyurethane dispersions. The viscosity and pH of the aqueous polyurethane dispersions increase as the diester diol increases.
FTIR of polyurethane dispersion
Figure 6 reveals the FTIR spectrum of prepolymer and polyurethane dispersion. In Fig. 6 spectrum, A shows the characteristic peak of NCO observed at 2263 cm−1. The peak at 2959 cm−1is due to CH2 stretching frequency. While reaction proceeded, NCO peak decreased and disappeared totally.
In spectrum B, the characteristic peaks at 3334 cm−1 and NH 1528 cm−1 are due to the formation of the urethane group which was confirmed. From spectrum, B it was concluded that all the residual NCO groups were consumed and disappeared after a chain extension.
Gel permeation chromatography
The molecular weight of polyurethane dispersions is shown in Table 3. Its shows that the molecular weights of PUDs were slightly affected by addition of diester diol. The molecular weights of PUDs were increased with concentration of diester diol. However, molecular weight formulated with 15% diester diol was found be more as compared to 3% diol were.
Particle size and zeta potential analysis of polyurethane dispersion
All synthesized PUDs were characterized by particle size and zeta potential and their results are shown in Table 4. It was observed that the increase in the diester diol percentage in polyurethane dispersion decreases particle size of dispersion [29]. The particle size depends on various factors such as DMPA, chain extender, and a neutralizing agent. In the synthesis of antimicrobial polyurethane dispersion, the DMPA content and ratio of NCO: OH were kept constant, only diester diol content varied. The particle size of polyurethane dispersion of 15% diol added in the formulation was obtained at 81.02 nm, which confirmed that dispersion has good stability. However, the particle size of the lowest loading on diol in polyurethane formulation was found to be 139.2 nm.
The zeta potential of 15 wt.% antimicrobial polyurethane dispersion is −61.09 mV which is on the higher side. It proves that the polyurethane dispersion is highly stable. It is because of high repulsion between positive charges, the chances of coalescence decrease and stability increases. The results of zeta potential are shown in Table 4. The zeta potential is important because it mainly represents the stability of the inner dispersion in an electric field. The zeta potential with large scale provides good stability against coagulation [30, 31].
Differential scanning calorimetry
Glass transition temperature (T g) of polyurethane dispersion was measured with Q-100 DSC using 2.5 mg of sample. The measurements were carried out at about −15 to 50 °C under the nitrogen atmosphere using sealed aluminium pans. The glass transition temperatures of polyurethane dispersion were observed from 15.30 to 18.21 °C shown in Fig. 7. However, from the data of glass transition temperature, it can be concluded that as a percentage of diol increases, the glass transition temperature of dispersion increases due to the percentage of hard segment increase.
Scanning electron microscopy
SEM images of fracture surface of PUD samples are shown in Fig. 8a–d. It can be seen that the surface of 3% diol (Fig. 8) is rough with some microspores and oddments due to its lower crosslinking density, whereas the surface of 15% diol is relatively homogeneous and smooth, suggesting dense structure of 15% diol chains can provide more uniform and ordered crosslinked structure.
X-ray diffraction(XRD)
WAXD pattern of polyurethane dispersion films with different concentration of diols is shown in Fig. 9 A broad diffraction peak observed at 2θ = of each sample, indicating the presence of orderly phase. However, characteristics peaks of PUDs areas at 18° to 22° concluded that amorphous nature with orderly arrangement is preserved in PUDs samples after crosslinking. Therefore, the average functionalities or crosslinking densities of PUDs samples slightly effect on their orderly phases [32]. The percentage crystanality of samples is shown in Table 5.
Thermogravimetric analysis
The thermogravimetric curves and data of PUD films are shown in Fig. 10 and Table 6. It can be seen that, PU films showed that the degradation of PU films starts at 278 °C and ended at 366 °C. The degradation at 500 °C corresponds to char formed. It shows that the first mass loss during thermal degradation of PUD is due to the degradation of the hard segment as a consequence of the relatively low thermal stability of the urethane ester terminal groups. Whereas, the second mass loss is associated with soft segment or polyester core decomposition [33].
Evaluation of antimicrobial activity
Polyurethanes films were screened for their antimicrobial activity against a bacterial strain of E. coli (ATCC 10536) and L. plantarum ATCC 6633). The antimicrobial activity of polyurethane film was determined by well-diffusion method and shown in Fig. 11. This method provides a quantitative measure of the influence of an antimicrobial agent against bacterial growth in culture media. In this test, all agar plates were prepared in 90 mm sterilized petri plates by pouring 20 ml of agar and allowed it to solidify giving the final depth of 5 mm. Then the holes 6 mm diameter was punched carefully by using sterile cork borer. The bacterial culture was incubated in nutrient broth media in aerobic condition at 37 °C for 24 h. In each petri plate, 100 µl of bacterial culture was poured carefully and spread over solid agar surface with the help of sterile glass spreader. The film was dissolved in DMSO at concentration 1 mg/ml wherein the antimicrobial activity of DMSO is nil. The 20 µl solution of polyurethane in DMSO was delivered into the holes. After incubation for 24 h at 37° C, all petri plates were examined for zone of growth inhibition, and the diameters of these zones were measured in millimetres.
The antimicrobial activity of polyurethane dispersion films was investigated against E. coli and L. plantarum strain in DMSO solvent as control. It was observed that as the concentration of diol increases, zone of inhibitions was found to increased. The zone of inhibition in the case of E. coli (gram-negative bacteria) was less as compared to L. plantarum (gram positive bacteria). It showed that the antimicrobial agents interact with bacterial cell walls, plasma membrane, bacterial DNA and protein, which results in disruption and disorganization membranes and thus preventing the growth of the microorganism.
In present study, prominent antimicrobial activity observed for E. coli compared to L. plantarum, this is because of specific structural feature of cell wall in both bacteria. The cell wall of gram negative bacteria (E. coli) is made up of thin layer of peptidoglycan which permits the penetration and adsorption of polymer into the inner parts of cell wall which inhibit the growth of bacteria (bactericidal effect) or killed them (bacteriostatic effect), where as in case of L. plantarum (gram positive bacteria) the cell wall is much denser and composed of thicker peptidoglycan layer consist of linear polysaccharides chains cross linked by short peptides units to form three dimensional compact structure which prevent the easy penetration of antimicrobial agent in inner parts of cell wall [34, 35].
Mechanical and optical properties
Mechanical properties of the PUDs coating are shown in Table 7. The coating showed good adhesion to wood which is inherent characteristic of polyurethane coatings due to strong hydrogen bonding of urethane linkages in backbone. In all PUDs coating formulations adhesion of coating was observed to slightly decreasing order 5B to 4B. However, the pencil hardness shows the increasing trend from HB to 3H, because the as the percentage of diol increases which increases the hardness of the coating. The flexibility of coatings shows good performance as the percentage of diester diol in polyurethane dispersion increases. The solvent xylene was used and double rubs were done. The result reported was the number of double rubs at which the films was observed to fail. The gloss of coating increased with the diol concentration because the hard segment in polyurethane increases which leads to improving in a gloss of coating.
Conclusion
Novel halide-based diester diol was successfully synthesized by coupling reaction of trichloroacetic acid with pentaerythritol. The synthesized diol was successfully incorporated in the polyurethane backbone in different concentrations (wt.%). It was concluded that as the weight fraction of diol in polyurethane dispersion increases, its inhibitory action towards growth of microorganism, i.e. bacteria was significantly found to be increased. However, the mechanical properties show that as the percentage of diol increased, the hardness and chemical resistance of coating also increased. The optical properties were also improved with the diol concentration in coating formulation.
References
Samuelson LA, Druy MA, Cao Y et al (2013) No title no title. Prog Org Coat 59:1689–1699. doi:10.1017/CBO9781107415324.004
Hapburn C (1991) Polyurethane elastomers. Second edi, New York
Szycher M (1999) Handbook of polyurethane. CRC Press, Boca Raton
Kim BK (1996) Aqueous polyurethane dispersions. Colloid Polym Sci 274:599–611. doi:10.1007/BF00653056
Kim BK, Lee JC (1996) Waterborne polyurethanes and their properties. J Polym Sci Part A 34:1095–1104. doi:10.1002/(SICI)1099-0518(19960430)34:6<1095:AID-POLA19>3.0.CO;2-2
Patel A, Patel C, Patel MG et al (2010) Fatty acid modified polyurethane dispersion for surface coatings: effect of fatty acid content and ionic content. Prog Org Coat 67:255–263. doi:10.1016/j.porgcoat.2009.11.006
Lu Y, Larock RC (2008) Soybean-oil-based waterborne polyurethane dispersions: effects of polyol functionality and hard segment content on properties. Biomacromolecules 9:3332–3340. doi:10.1021/bm801030g
Athawale VD, Nimbalkar RV (2011) Polyurethane dispersions based on sardine fish oil, soybean oil, and their interesterification products. J Dispersion Sci Technol 32:1014–1022. doi:10.1080/01932691.2010.497459
Lee D-K, Tsai H-B, Tsai R-S, Chen PH (2007) Preparation and properties of transparent thermoplastic segmented polyurethanes derived from different polyols. Polym Eng Sci 47:695–701. doi:10.1002/pen.20742
Lee D-K, Tsai H-B, Wang H-H, Tsai R-S (2004) Aqueous polyurethane dispersions derived from polycarbonatediols. J Appl Polym Sci 94:1723–1729. doi:10.1002/app.21090
Garrison TF, Zhang Z, Kim H-J et al (2014) Thermo-mechanical and antibacterial properties of soybean oil-based cationic polyurethane coatings: effects of amine ratio and degree of crosslinking. Macromol Mater Eng 299:1042–1051. doi:10.1002/mame.201300423
Noble K-L (1997) Waterborne polyurethanes. Prog Org Coat 32:131–136. doi:10.1177/152808378601600103
Brinkman E, Vandevoorde P (1998) Waterborne two-pack isocyanate-free systems for industrial coatings. Prog Org Coat 34:21–25. doi:10.1016/S0300-9440(98)00030-7
Jr ZWW, Wicks DA, Rosthauser JW (2002) Two package waterborne urethane systems. Prog Org Coat 44:161–183. doi:10.1016/S0300-9440(02)00002-4
Melchiors M, Sonntag M, Kobusch C, Jürgens E (2000) Recent developments in aqueous two-component polyurethane (2 K-PUR) coatings. Prog Org Coat 40:99–109. doi:10.1016/S0300-9440(00)00123-5
Delpech MC, Coutinho FMB (2000) Waterborne anionic polyurethanes and poly(urethane-urea)s: influence of the chain extender on mechanical and adhesive properties. Polym Testing 19:939–952. doi:10.1016/S0142-9418(99)00066-5
Athawale VD, Kulkarni MA (2010) Polyester polyols for waterborne polyurethanes and hybrid dispersions. Prog Org Coat 67:44–54. doi:10.1016/j.porgcoat.2009.09.015
Mohamed HA, Morsi SMM, Badran BM, Rabie AM (2016) Polyurethane/aromatic polyamide sulfone copolymer dispersions from transesterified castor oil. Polymer Bulletin 1–24. doi: 10.1007/s00289-016-1728-x
Seo WJ, Kim KB (2005) Preparations and properties of waterborne polyurethane/nanosilica composites. Polym Bull 54:123–128. doi:10.1007/s00289-005-0367-4
Du Y, Zhang J, Zhou C (2016) Synthesis and properties of waterborne polyurethane-based PTMG and PDMS as soft segment. Polym Bull 73:293–308. doi:10.1007/s00289-015-1487-0
Choi SH, Kim DH, Raghu AV et al (2012) Properties of graphene/waterborne polyurethane nanocomposites cast from colloidal dispersion mixtures. J Macromol Sci Part B 51:197–207. doi:10.1163/156855509X412054
Reddy KR, Raghu AV, Jeong HM (2008) Synthesis and characterization of novel polyurethanes based on 4,4′-{1,4-phenylenebis[methylylidenenitrilo]}diphenol. Polym Bull 60:609–616. doi:10.1007/s00289-008-0896-8
Reddy KR, Raghu AV, Jeong HM, Siddaramaiah (2009) Synthesis and characterization of pyridine-based polyurethanes. Des Monomers Polym 12:109–118. doi:10.1163/156855509X412054
Muñoz-Bonilla A, Fernández-García M (2012) Polymeric materials with antimicrobial activity. Prog Polym Sci 37:281–339. doi:10.1016/j.progpolymsci.2011.08.005
Pittman CU, Stahl GA (1981) Copolymerization of pentachlorophenyl acrylate with vinyl acetate and ethyl acrylate. Polymer-bound fungicides. J Appl Polym Sci 26:2403–2413. doi:10.1002/app.1981.070260726
Mahmoud H (2011) Anti-microbial polyurethane dispersion, method for the manufacture thereof, application method and articles provided with an anti-microbial coating. US20110177020
Oh TS, Ha CS, Cho WJ (1994) Synthesis and antimicrobial activity of acrylic polymer containing chloro-substitute diphenyl ether. Polymer(Korea) 18:309–315
Shay JS, English RJ, Khan SA (2000) Rheological behavior of a polymerically stabilized latex for use in water-borne coatings. Polym Eng Sci 40:1469–1479. doi:10.1002/pen.11276
Manvi GN, Jagtap RN (2010) Effect of DMPA content of polyurethane dispersion on coating properties. J Dispersion Sci Technol 31:1376–1382. doi:10.1080/01932690903269776
Sjöblom J (2001) Encyclopedic handbook of emulsion technology. chap 3, CRC Press, New York, pp 59–70. doi:10.1201/9781420029581
Hidalgo-Álvarez R, Martín A, Fernández A et al (1996) Electrokinetic properties, colloidal stability and aggregation kinetics of polymer colloids. Adv Colloid Interface Sci 67:1–118. doi:10.1016/0001-8686(96)00297-7
Yang C-H, Liu F-J, Liu Y-P, Liao W-T (2006) Hybrids of colloidal silica and waterborne polyurethane. J Colloid Interface Sci 302:123–132. doi:10.1016/j.jcis.2006.06.001
Ballistreri A, Foti S, Maravigna P et al (1980) Thermal degradation of polyurethanes investigated by direct pyrolysis in the mass spectrometer. Die Makromolekulare Chemie 181:2161–2173. doi:10.1002/macp.1980.021811013
Guo L, Yuan W, Lu Z, Ming C (2013) Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf A 439:69–83
Tamboli DP, Lee DS (2013) Mechanistic antimicrobial approach of extracellularly synthesized silver nanoparticles against gram positive and gram negative bacteria. J Hazard Mater 260:878–884. doi:10.1016/j.jhazmat.2013.06.003
Acknowledgements
The author immensely likes to thank UGC SAP-Green Tech Grant for providing financial support and Institute of Chemical Technology for conducting research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lokhande, G.P., Chambhare, S.U. & Jagtap, R.N. Anionic water-based polyurethane dispersions for antimicrobial coating application. Polym. Bull. 74, 4781–4798 (2017). https://doi.org/10.1007/s00289-017-1965-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-1965-7