Abstract
Spherical bridged polysilsesquioxane (BPS) particles with sulfonic groups (BPS–SO3 −) were prepared by the subsequent reduction and oxidation of BPS with disulfide groups (BPS–S–S) after the hydrolysis and condensation reaction of silane monomers with disulfide groups under ammonia and alcoholic solutions. Spherical aggregates of Pd nanoparticles (Pd–polyhedral oligomeric silsesquioxanes, Pd–POSS) were produced by the mixing of POSS and palladium (II) acetate in methanol solution. The average size of BPS–SO3 − and Pd–POSS was about 200–400 and 30–50 nm, respectively. New BPS–SO3 −/Pd–POSS composites with the shape of BPS–SO3 − covered with Pd–POSS nanoparticles were fabricated by ionic interactions between negatively charged BPS–SO3 − and positively charged Pd–POSS. Pd–POSS nanoparticles were more effectively attached to BPS–SO3 − than BPS–S–S, which resulted from the difference of zeta potential between BPS–SO3 − and BPS–S–S. That is, ionic interactions in BPS–SO3 −/Pd–POSS composites were stronger than those in BPS–S–S/Pd–POSS composites. As the storage time was increased, the precipitation of BPS–SO3 −/Pd–POSS composites in methanol solution resulted from the strong complex between BPS–SO3 − and Pd–POSS unlike BPS–S–S/Pd–POSS composites. New particle composites were characterized by Fourier transform infrared spectra, scanning electron microscopy, transmission electron microscopy, and energy dispersive X-ray spectroscopy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bridged polysilsesquioxane (BPS) is hybrid organic–inorganic material prepared by sol–gel processing of silane monomers containing organic bridging group and bis-trialkoxysilyl groups. The structure of BPS have organic bridging groups and silicon-oxide network, which is totally different from that of materials obtained from silane monomers without organic bridging group such as tetramethoxysilane (TMOS). Various tunings of organic bridging group make it easier to control the properties of BPS, such as hydrophobicity, porosity, thermal stability, and mechanical properties [1–4]. Also, BPS easily forms gels in rapid gelation due to the six reactive alkoxide groups. Recently, several papers have been published regarding how to make nanoparticles of BPS via self-assembly, emulsion, or non-emulsion method [5–9].
Polyhedral oligomeric silsesquioxanes (POSS) have unique cage structure with silica-like core and organic corner groups. POSS as hybrid organic–inorganic building blocks have been used to prepare nano-reinforced hybrid materials and hybrid polymers incorporating POSS moiety and to assemble metal nanoparticles into spherical aggregates via self-organized spherical templates [10–12]. We have explored various hybrid nanocomposites using POSS through physical bonding interactions such as hydrogen bonding or ionic interactions [13–15]. Hybrids through physical bonding interactions between two different materials are much easier and simpler to prepare hybrid materials in comparison with hybrids through chemical bond.
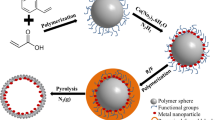
In this work, we report the synthesis of spherical BPS particles and aggregates of Pd nanoparticles capped with POSS, and subsequently two particles were employed to make new particle–particle composites via ionic interactions. To utilize ionic interactions, spherical BPS particles with negative charges were prepared by a simple sol–gel method without templates or surfactants. And POSS were used as a cubic linker to make aggregates of Pd nanoparticles (Pd–POSS) with positive charges [11, 15, 16]. BPS particles with sulfonic groups (BPS–SO3 −) were produced by the reduction and oxidation of BPS with disulfide groups (BPS–S–S) obtained after sol–gel polymerization through a non-emulsion method. Spherical Pd–POSS were composed of Pd nanoparticles stabilized by POSS–NH3 +. The particle size of BPS–SO3 − and Pd–POSS ranges from 200 to 400 and from 30 to 50 nm, respectively. The particle size of BPS is larger than that of Pd–POSS due to the long and bulky organic bridging groups in BPS. New BPS–SO3 −/Pd–POSS composites were successfully synthesized by ionic interactions between negatively charged BPS–SO3 − and positively charged Pd–POSS. Pd–POSS nanoparticles were randomly attached to the surface of BPS–SO3 −. The strength of ionic interactions between BPS–SO3 − and Pd–POSS was stronger than that of ionic interactions between BPS–S–S and Pd–POSS. The evidence of strong ionic interactions between BPS–SO3 − and Pd–POSS was confirmed by the easier precipitation of BPS–SO3 −/Pd–POSS composites than BPS–S–S/Pd–POSS composites in methanol. It is quite interesting to suggest a new hybrid combination to prepare particle–particle composites via ionic interactions. To our knowledge, few studies have been reported not only on particle–particle composites, but also on the use of ionic interactions between different particles.
Experimental section
Materials
Bis[3-(triethoxysilyl)propyl]disulfide was purchased from Gelest, Inc. Glacial acetic acid and ammonia solution were purchased from Junsei Chemical Co. Palladium(II) acetate and dithiothreitol (DTT) were purchased from Sigma-Aldrich. All were used as received unless otherwise specified.
Measurements
Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet IR 200 (Thermo) spectrometer. Zeta potential measurements were obtained on an ELS-Z, Otsuka Electronics Co. The morphologies and structures were observed by scanning electron microscopy (SEM) (JSM-6700, JEOL), field emission transmission electron microscopy (FE-TEM) (Tecnai F20, Philips), and energy dispersive spectrum (EDX) (EPMA-1600, Shimadzu). Photographic images of composites were measured by using the camera (D40, Nikon).
Material preparation
Spherical bridged polysilsesquioxane particles with disulfide groups (BPS–S–S) (2)
To a 40-mL vial equipped with hotplate were added H2O (3.4 mL, 0.189 mol), ammonia solution (18 mL), and 1-propanol (5.6 mL, 0.093 mol). To the solution was added bis[3-(triethoxysilyl)propyl]disulfide (1) (1 mL, 0.002 mol). The solution was vigorously stirred and heated at 60 °C for 24 h. After cooling to room temperature, H2O (1 L) was added to the solution and stirred for 12 h. The solution was filtered and dried under vacuum to obtain the white powders [1].
Spherical bridged polysilsesquioxane particles with sulfonic groups (BPS–SO3 −) (4)
BPS–S–S (2) was immersed in a 0.1 M solution of DTT and the mixture was stirred at room temperature for 12 h to break the disulfide bond into two thiol groups (–SH). Then, BPS–SH (3) was transferred to a 50:50 solution of hydrogen peroxide and glacial acetic acid and stirred at room temperature for 12 h to oxidize the –SH groups to SO3 − groups. Finally, BPS–SO3 − (4) was washed in a 10 mM NaOH aqueous solution for 1 day and then was immersed in water at 60 °C. BPS–SO3 − was removed from the solution and dried under vacuum for 1 day [17].
Spherical aggregates of Pd nanoparticles containing POSS (Pd–POSS)
Pd–POSS has been prepared by our group to fabricate various hybrid nanocomposites [11, 14–16]. The general synthesis route is as follows. When a methanol solution (50 mL) of octa(3-aminopropyl)octasilsesquioxane (POSS–NH3 +) (10 mg, 0.009 mmol) and palladium(II) acetate (20 mg, 0.089 mmol) was stirred at room temperature, the solution immediately became turbid. The turbid solution gradually turned from yellow to black with increasing reaction time, indicating the reduction of palladium ions.
Composites of BPS–SO3 − and Pd–POSS via ionic interactions (BPS–SO3 −/Pd–POSS)
BPS–SO3 − particles (4) (0.01 g) were added to the 20 mL methanol solution of Pd–POSS. The solution was vigorously stirred at room temperature for 3 h. After completion of the reaction, BPS–SO3 −/Pd–POSS composites began to precipitate on increasing the storage time.
Results and discussion
Spherical BPS particles were prepared by the hydrolysis and condensation of bis[3-(triethoxysilyl)propyl]disulfide in alcoholic solutions of ammonia and water. BPS–S–S (2) was reduced to BPS–SH (3) using DTT as a reducing agent (Scheme 1). BPS–SH was oxidized to final product, BPS–SO3 − (4), with hydrogen peroxide in acidic condition. Figure 1 shows FT-IR spectra of BPS–S–S and BPS–SO3 −. The broad absorption peak of Si–O–Si groups in both BPS particles was observed at 1,100–1,200 cm−1. The peak of aliphatic groups appeared at 2,700–3,000 cm−1. The presence of the sulfonic groups in BPS–SO3 − is evident by the characteristic adsorption peak at 1,196 and 1,036 cm−1. Pd–POSS was spherical aggregates of palladium nanoparticles with POSS as a cross-linker (Scheme 2). The unique structure and functionality of POSS–NH3 + resulted in the assembling of palladium nanoparticles into spherical aggregates as well as the positive charge of Pd–POSS.
Figure 2 presents SEM images of Pd–POSS, BPS–S–S, and BPS–SO3 −. The average size of Pd–POSS and BPS–SO3 − was around 30–50 and 200–400 nm, respectively. The different particle size between Pd–POSS and BPS–SO3 − might be attributed to the long and bulky bridging groups in BPS–SO3 −. Also, the size of BPS particles could be controlled by the monomer concentration and alcohol content. The data of zeta potential for Pd–POSS, BPS–S–S, and BPS–SO3 − explain that Pd–POSS can interact more strongly with BPS–SO3 − than with BPS–S–S because of different zeta potential value (Table 1).
We have explored various hybrid nanocomposites using positively charged Pd–POSS. The surface of carbon nanotube or graphene was modified to contain anionic functional groups for ionic interactions with Pd–POSS [14–16]. Using the same concept, new composites were fabricated by spherical BPS particles and Pd–POSS via ionic interactions.
SEM images of BPS–S–S/Pd–POSS and BPS–SO3 −/Pd–POSS composites are shown in Fig. 3. A large quantity of Pd–POSS was randomly and densely attached to the surface of BPS–SO3 − due to the stronger ionic interactions between BPS–SO3 − and Pd–POSS. This result is clearly confirmed by the TEM images.
As shown in Fig. 4, the average size of BPS–SO3 − and Pd–POSS is consistent with SEM images. In case of BPS–S–S/Pd–POSS composites, BPS–S–S particles were not covered well with Pd–POSS as compared with BPS–SO3 − particles which were densely covered with Pd–POSS. Uncovered parts of BPS–SO3 − particles with Pd–POSS were not observed. Figure 5 shows EDX images of BPS–SO3 − and BPS–SO3 −/Pd–POSS composites. There was no palladium peak in BPS–SO3 − particles. The new palladium peak derived from Pd–POSS appeared in BPS–SO3 −/Pd–POSS composites, which confirmed that particle–particle composites were successfully fabricated via ionic interactions.
The storage stability of each particle was observed in methanol. As shown in Fig. 6, each particle was homogeneously dispersed in methanol at first. As the storage time is increased up to 3 h, the other composites except BPS–SO3 −/Pd–POSS composites are still dispersed well in the solvent. Unlike BPS–S–S/Pd–POSS composites, BPS–SO3 −/Pd–POSS composites were precipitated because the strong ionic interactions were effectively exited between BPS–SO3 − and Pd–POSS.
Conclusions
In summary, spherical BPS particles with average size of 200–400 nm were synthesized by a non-emulsion method. To obtain particle–particle composites via ionic interactions, BPS particles were reduced and oxidized to make final BPS–SO3 − particles. The counterpart particles, Pd–POSS, were composed of spherical aggregates of palladium nanoparticles stabilized by POSS. Pd–POSS with the smaller size of 30–50 nm than BPS–SO3 − particles were densely deposited on the BPS–SO3 − particles via ionic interactions. That is, BPS–SO3 − particles were entirely covered with Pd–POSS unlike BPS–S–S particles. In case of BPS–S–S/Pd–POSS composites, weak ionic interactions between BPS–S–S and Pd–POSS resulted in the deposition of small quantity of Pd–POSS on the BPS–S–S particles as well as no precipitation in solvent. The strong complex between BPS–SO3 − and Pd–POSS shows the presence of precipitation in solvent. New BPS–SO3 −/Pd–POSS composites were successfully fabricated by ionic interactions between negatively charged BPS–SO3 − and positively charged Pd–POSS.
References
Shea KJ, Loy DA (2001) Bridged polysilsesquioxanes. Molecular-engineered hybrid organic–inorganic materials. Chem Mater 13(10):3306–3319
Loy DA, Shea KJ (1995) Bridged polysilsesquioxanes. Highly porous hybrid organic–inorganic materials. Chem Rev 95(5):1431–1442
Loy DA, Jamison GM, Baugher BM, Russick EM, Assink RA, Prabakar S, Shea KJ (1995) Alkylene-bridged polysilsesquioxane aerogels: highly porous hybrid organic–inorganic materials. J Non Cryst Solids 186:44–53
Zhao L, Vaupel M, Loy DA, Shea KJ (2008) Photoresponsive hybrid materials: synthesis and characterization of coumarin-dimer-bridged polysilsesquioxanes. Chem Mater 20:1870–1876
Zhao L, Loy DA, Shea KJ (2006) Photodeformable spherical hybrid nanoparticles. J Am Chem Soc 128:14250–14251
Khiterer M, Shea KJ (2007) Spherical, monodisperse, functional bridged polysilsesquioxane nanoparticles. Nano Lett 9:2684–2687
Hu LC, Khiterer M, Huang SJ, Chan JCC, Davey JR, Shea KJ (2010) Uniform, spherical bridged polysilsesquioxane nano and microparticles by a nonemulsion method. Chem Mater 22:5244–5250
Kapoor MP, Yang Q, Inagaki S (2002) Self-assembly of biphenylene-bridged hybrid mesoporous solid with molecular-scale periodicity in the pore walls. J Am Chem Soc 124:15176–15177
Hu LC, Yonamine Y, Lee SH, Veer WE, Shea KJ (2012) Light-triggered charge reversal of organic-silica hybrid nanoparticles. J Am Chem Soc 134:11072–11075
Letant SE, Maiti A, Jones TV, Herberg JL, Maxwell RS, Saab AP (2009) Polyhedral oligomeric silsesquioxane (POSS)-stabilized pd nanoparticles: factors governing crystallite morphology and secondary aggregate structure. J Phys Chem C 113:19424–19431
Naka K, Itoh H, Chujo Y (2002) Self-organization of spherical aggregates of palladium nanoparticles with a cubic silsesquioxane. Nano Lett 2(11):1183–1186
Luca CD, Soule ER, Zucchi IA, Hoppe CE, Fasce LA, Williams RJJ (2010) In-situ generation of a dispersion of POSS crystalline platelets in an epoxy matrix induced by polymerization. Macromolecules 42:9014–9021
Kim KM, Inakura T, Chujo Y (2001) Organic–inorganic polymer hybrids using octasilsesquioxanes with hydroxyl groups. Polym Bull 46(5):351–356
Jeon JH, Lim JH, Kim KM (2009) Hybrid nanocomposites of palladium nanoparticles having POSS and MWNTs via ionic interactions. Macromol Res 17(12):987–994
Jeon JH, Lim JH, Chujo Y, Kim KM (2009) Synthesis and characterization of hybrid nanocomposites containing POSS(Pd–POSS) and poly(acrylic acid) via ionic interaction. Polymer (Korea) 33(6):615–619
Lee JH, Nam JH, Lim JH, Moon SC, Kim KY, Kim KM (2012) Fabrication of hybrid nanocomposites of poly(acrylic acid)-grafted MWNTs and spherical aggregates of palladium nanoparticles with POSS. Compos Interfaces 19:583–592
Ruth DO, Carmen AL, Gregory S (2001) A new approach to design imprinted polymer gels without using a template. Macromolecules 34:4965–4971
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2012R1A1A2042629) and National Research Foundation of Korea (NRF) through the Human Resource Training Project for Regional Innovation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Park, DS., Ha, TS., Kim, KY. et al. New composites of spherical bridged polysilsesquioxanes and aggregates of Pd nanoparticles with POSS via ionic interactions. Polym. Bull. 71, 819–828 (2014). https://doi.org/10.1007/s00289-013-1094-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-013-1094-x