Abstract
Modified poly(aspartic acid)s containing pendant allyl groups were synthesized by the reaction of poly(succinimide) with an allyl amine in dimethylformamide. The contents of the allyl groups in the poly(aspartic acid) ranged from 2 to 17.4% confirmed by 1H NMR. Hydrogels were prepared using modified poly(aspartic acid) by chemical crosslinking using redox radical initiators including ammonium persulfate and potassium peroxodisulfate. The morphologies of the poly(aspartic acid)-based hydrogels were investigated by scanning electron microscopy (SEM). The water-absorbent experiments were carried out, and revealed that lightly cross-linked hydrogels resulted in effective water-absorbent properties. These results suggested that allyl group-modified poly(aspartic acid)s are useful in providing biodegradable hydrogels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superabsorbent polymers present three-dimensionally crosslinking structures, and can incorporate water molecules in their networks greater than their own weight. The ability to absorb water leads to many applications, such as sanitary products, agricultural and horticultural water-holding material, and various other fields. Synthetic water-soluble polymers such as poly(acrylic acid) are widely used for such commercial applications. However, due to their structural stability, they are directly released into the earth’s environment after their disposal, resulting in potential issues that include the diffusion and accumulation of non-biodegradable polymers that can be harmful to the environment. The development of environmentally friendly water-absorbents requires the design and construction of three-dimensional networks from biodegradable natural polymers. Thus, biological polymers, such as starch [1], cellulose [2], chitosan [3], and poly(amino acid)s, have been extensively used for the production of biodegradable hydrogels. For example, poly(glutamic acid)s were crosslinked through esterification with saccharides to prepare bio-based hydrogels [4]. In addition, poly(aspartic acid)s end-capped with methylmethacrylates were polymerized with dimethylmethacrylate derivatives to produce hydrogels from biodegradable polymer backbones [5]. Moreover, crosslinking between poly(glutamic acid)s and poly(lysine)s was performed by γ-irradiation, resulting in poly(amino acid)-based three-dimensional networks [6]. Furthermore, Cao et al. [7, 8] recently reported on some drug delivery systems and Moon et al. [9] reported on thermo- and pH-responsive systems based on poly(aspartic acid) derivatives.
In previous studies, we have demonstrated the practical synthesis of poly(aspartic acid) from poly(succinimide) [10–15]. This system includes the convenient modification of poly(aspartic acid) by the reactions of poly(succinimide) with amine derivatives [16–18], and the resultant poly(aspartic acid)s showed biodegradability even with side chain modifications [15]. In addition, the gelation of poly(acrylamide) and polyesters was developed by crosslinking of the pendant allyl groups [19, 20]. Thus, the introduction of multiple allyl groups into poly(aspartic acid) allows us to construct biodegradable hydrogels through a reaction with radical initiators. In this article, we demonstrate the synthesis of allyl group-modified poly(aspartic acid)s, the preparation of hydrogels based on the biodegradable poly(aspartic acid) through crosslinking with radical initiators, and the water-absorbent abilities of the resultant hydrogels.
Experimental
Materials and measurements
Poly(succinimide) (PSI, M w = 64,300, M w /M n = 1.9) was obtained from the Mitsubishi Chemicals Corporation. N,N-Dimethylformamide (DMF), sodium hydroxide, allylamine, ammonium persulfate (APS), potassium peroxodisulfate (KPS), and N,N,N′N′-tetramethylenediamine (TMEDA) were purchased from the Wako Pure Chemical Co. and used without further purification. Nylon mesh bags (20 cm square) for water-absorption experiments were made from a nylon net (60 mesh) purchased from NRK Co., Ltd.
A Hitachi S-2600 was used for scanning electronic microscopy (SEM). NMR spectra were recorded using a JEOL JNM AL400. All mole percentages were calculated based on the moles of the monomeric unit in PSI and poly(aspartic acid)s.
Synthesis of allylamine-modified PSI
A typical procedure for the preparation of the allylamine-modified poly(succinimide) is as follows. A solution of PSI (1.00 g, 10.3 mmol) in dry DMF (10 mL) was prepared in a round bottom flask, and heated to 80 °C completely to dissolve the PSI under nitrogen. To this PSI solution, allylamine (0.588 g, 10.3 mmol) was added drop wise at 30 °C, and the mixture was stirred for 2.5 h at 45 °C. After cooling to room temperature, the reaction mixture was poured into MeOH (100 mL). After filtration, the precipitate was washed with MeOH (200 mL 2×), and dried at 60 °C for 6 h under reduced pressure to yield the allylamine-modified PSI (1.05 g, 95%).
Synthesis of poly(aspartic acid) with pendant allyl groups
A typical procedure for the preparation of poly(aspartic acid) with pendant allyl groups is as follows. To aqueous NaOH (0.55 g, 13.75 mmol) in deionized water (8.85 mL), allylamine-modified PSI (0.72 g, 7.4 mmol) was added with ice cooling. After stirring for 1 h, the reaction mixture was poured into MeOH (300 mL), and the precipitate was then filtered and dried at 40 °C in a vacuum oven for over 6 h (yield 80%).
Cross-linking of poly(aspartic acid) with pendant allyl groups
The hydrogels were prepared using the allyl group-modified poly(aspartic acid)s (allyl group contents ranged from 3.0 to 18 mol%) with the redox initiators, KPS or APS and TMEDA. A typical procedure for the preparation of cross-linked poly(aspartic acid) is as follows. 0.5 g of poly(aspartic acid) was dissolved in 10 mL water. 50 mol% (based on aspartic acid) of APS was then added at 40 °C. After complete dissolution of the polymer and initiator, 13 mol% of TMEDA was added at 40 °C and reacted for 2 h. The obtained hydrogel was milled, washed with methanol, and placed in deionized water overnight. Finally, the hydrogel was filtered, precipitated from methanol, and dried in a vacuum oven for over 6 h (yield 60%).
Water absorbency
The water absorbencies of the poly(aspartic acid) hydrogels were measured by the tea-bag method with reference to the Japanese Industrial Standard, JIS K 7223. The tea-bag containing sample was immersed in deionized water at 25 °C. After a 30 min immersion, the tea-bag was removed from the water, and the excess water was drained for 15 min. The same procedure was applied to an empty bag to calculate the blank absorption by the nylon mesh bags. The water absorbency (gram per gram) of the poly(aspartic acid) hydrogels was calculated according to the following equation:
where W p is the weight of the dry hydrogel sample, W t is the weight of the tea-bag and the swelled hydrogels, and W b is a blank tea-bag after water absorption.
Results and discussion
The synthesis and chemical crosslinking of the allyl group-modified poly(aspartic acid) are presented in Scheme 1. To produce the three-dimensionally crosslinked structures based on biodegradable polymers, poly(aspartic acid) susceptible to radical initiators was prepared as an intermediate product. Poly(succinimide) was treated with allyl amines, and subsequently hydrolyzed to poly(aspartic acid) using NaOH. The reaction of the poly(aspartic acid) containing pendant allyl groups with redox radical initiators provides biodegradable hydrogels.
Poly(succinimide) was quantitatively reacted with an allyl amine in dry DMF to produce hydrogels with a variety of crosslinking densities. The contents of the allyl groups ranged from 2.3 to 17.4% as confirmed by the 1H NMR spectra (Fig. 1 and Table 1). As shown in Fig. 1, the chemical shift at 5.6–5.8 ppm was due to the methine proton in the pendant allyl groups. The methine proton in the PSI backbones and methylene proton in the pendant allyl groups overlapped between 5.0 and 5.4 ppm. The allyl group contents in the resulting modified PSIs were obtained based on the quantitative analysis of these peaks. Water-soluble poly(aspartic acid)s with pendant allyl groups were quantitatively synthesized by alkaline hydrolysis of the modified poly(succinimide)s using NaOH, and obtained with high yields (over 80%).
The crosslinking was carried out in water at 40 °C using water-soluble radical initiators; i.e., APS and KPS. Tables 2 and 3 show the crosslinking results using KPS and APS, respectively. Regardless of the kind of redox radical initiators, the formation of hydrogels requires more than 50 mol% of the redox initiators relative to the pendant allyl groups. No hydrogel was obtained when the redox initiators ranged from 5 to 13 mol% (Tables 2 and 3). On the other hand, relatively small amounts of TMEDA were required for the formation of the hydrogels. The hydrogels were essentially produced using 28 and 13 mol% TMEDA.
Hydrogels based on the biodegradable poly(aspartic acid) were obtained with a variety of crosslinking densities (Table 4). The crosslinking of the modified poly(aspartic acid)s was carried out in water at 40 °C using 50 mol% APS and 13 mol% TMEDA. It was found that the formation of hydrogels required an allyl group content of greater than 5%. The modified poly(aspartic acid) with 2.3% pendant allyl groups resulted in no hydrogel formation (run 1 in Table 4). High allyl group contents in the polymers reflected the effective formation of the hydrogels. This is presumably due to the increased number of crosslinking points in comparison to the polymers with low allyl group contents. The modified poly(aspartic acid)s with relatively low allyl group contents (runs 2–4) provided hydrogels in 58.1–59.5% yields, whereas the modified poly(aspartic acid)s with high allyl group contents (runs 5–7) resulted in 67.2–71.9% yields.
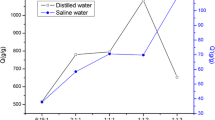
Water absorbency experiments were performed using dried hydrogels with variable crosslinking densities, and revealed that lightly crosslinked hydrogels possess an effective water-absorbing ability. Figure 2 shows the water absorption of hydrogels as a function of the allyl group contents. Hydrogels with low allyl group contents (5.3 and 5.6%) showed remarkable water-absorption characteristics (424 and 330 g/g, respectively). In contrast, hydrogels prepared from high allyl group contents (9.1–17.4%) showed relatively low water absorptions ranging from 38 to 100 g/g. This observation significantly indicates that the water-absorption ability depends on the three-dimensional network obtained through crosslinking, and light crosslinking is essential for the effective water-absorption ability.
Scanning electronic microscopy was carried out to investigate the morphologies of the hydrogels that determine the water-absorption property. As expected, the hydrogel from a low allyl group content (5.3%) showed a substantially more porous structure compared to the hydrogel from a high allyl group content (17.4%) (Fig. 3). This clearly demonstrates that extremely porous, lightly crosslinked structures can effectively incorporate water molecules in their three-dimensional network.
Conclusions
Superabsorbent hydrogels were successfully prepared by crosslinking of the allyl group-modified poly(aspartic acid) using redox radical initiators. As expected, the resultant hydrogels displayed water-absorbent properties depending on the density of the crosslinking. Light crosslinking was essential for effective water-absorption. The porous structures observed in lightly crosslinked hydrogels reflect their effective water-absorption ability. The biodegradability of the hydrogels is currently under investigation, and will be reported in due course.
References
Wu JH, Wei YL, Lin JM, Lin SB (2003) Polym Int 52:1909
Suo AL, Qian JM, Yao Y, Zhang WG (2007) J Appl Polym Sci 103:1382
Mahdavinia GR, Pourjavadi A, Hosseinzadeh H, Zohuriaan MJ (2004) Eur Polym J 40:1399
Murakami S, Aoki N (2006) Biomacromolecules 7:2122
Kakuchi T, Kusuno A, Shibata M, Nakato T (1999) Macromol Rapid Commun 20:410
Kunioka M (2004) Macromol Biosci 4:324
Cao H, Zhu J, Su H, Fang L, Tan T (2009) J Appl Polym Sci 113:327
Cao H, Ma X, Sun S, Su H, Tan T (2010) Polym Bull 64:623
Moon JR, Park YH, Kim J (2009) J Appl Polym Sci 111:998
Kakuchi T, Shibata M, Matsunami S, Nakato T, Tomida M (1997) J Polym Sci Polym Chem 35:285
Tomida M, Nakato T, Kuramochi M, Shibata M, Matsunami S, Kakuchi T (1996) Polymer 37:4435
Tomida M, Nakato T, Matsunami S, Kakuchi T (1997) Polymer 38:4733
Nakato T, Tomida M, Kusuno A, Shibata M, Kakuchi T (1998) Polym Bull 40:647
Nakato T, Yoshitake M, Matsubara K, Tomida M, Kakuchi T (1998) Macromolecules 31:2107
Nakato T, Tomida M, Suwa M, Morishima Y, Kusuno A, Kakuchi T (2000) Polym Bull 44:385
Fukuoka T, Uyama H, Kakuchi T, Kobayashi S (2002) Macromol Rapid Commun 23:698
Tachibana Y, Kurisawa M, Uyama H, Kakuchi T, Kobayashi S (2003) Chem Commun, p 106
Tachibana Y, Kurisawa M, Uyama H, Kakuchi T, Kobayashi S (2003) Chem Lett 32:374
Bicak N, Senkal BF, Gazi M (2004) Des Monomers Polym 7:261
Rokicki G, Szymanska E (1998) J Appl Polym Sci 70:2031
Acknowledgments
We thank Mr. Noriaki Kaneko (Micro Tech inc.) for SEM experiments. Mitsubishi Chemicals Corporation is deeply acknowledged for donation of poly(succinimide).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Umeda, S., Nakade, H. & Kakuchi, T. Preparation of superabsorbent hydrogels from poly(aspartic acid) by chemical crosslinking. Polym. Bull. 67, 1285–1292 (2011). https://doi.org/10.1007/s00289-011-0493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-011-0493-0