Abstract
Thermal stability of isotactic polypropylene with or without calcium carbonate nanoparticles (nCaCO3) was investigated by chemiluminescence under isothermal regime at 190 °C. Two kinds of nCaCO3 particles, i.e., (neat and stearic acid-coated ones) were used. The contents of nCaCO3 within the iPP/nCaCO3 nanocomposites were 5, 10, 15, 20, and 25% w/w. Several parameters, i.e., oxidation induction time, oxidation half-time, maximum oxidation time, and oxidation rate were used to quantify the thermal stability of both the neat and the nCaCO3-filled iPP systems. The contribution of nanoparticles on the progress of oxidation is discussed. It has been found that the concentration of nCaCO3 increases the stability of systems when nanoparticles were covered, while the filler consisted of unmodified particles, the decrease in thermal strength with the increase in filler concentration was noticed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The favorable features of polymeric nanocomposites are determined by the great separation surfaces that are characterized the ultrafine filler particles homogenously distributed throughout the mass of matrix. These surfaces would act as obstacles that retired the diffusion of various molecules. This characteristic influences physical and chemical stability of nanostructured materials which, in turn, controls the material lifetime. General considerations have been reported earlier in connection with the obvious applications of such materials [1–9]. In spite of the large number of studies, some doubts that concern the post-processing behavior of intercalated composites still exist [10, 11]. The nature of filler particle surface, the processing temperature range and the environmental stresses play important role in the actual applicability of these materials.
Fundamentally, the thermal and mechanical properties of nanocomposites are strongly influenced either by type and composition of the matrix and the fillers or by the preparation conditions [11–15]. In fact, morphological development of composite materials relates closely to both the preparation procedures and the crystallization/solidification schemes that are used [16, 17]. An interesting aspect of nanoscaled composites is the effect of filler of the nanoscopic fillers on thermal conduction. This property should relate to the large total surface area of the fillers, which, when being present in a large enough quantity, could form a three-dimensional connection. The polar functional groups that are present in polar samples [18] or that are formed during thermal degradation [19] play a decisive role in enhancing the thermal conductivity of the resulting composites. However, nanocomposites based on non-polar polyolefins and inorganic nanoscopic fillers are interesting for their potential in the manufacture of flame retarding materials [20, 21].
In the present contribution, isotactic polypropylene (i-PP)/nanoparticles (nCaCO3) composites were studied for their thermal degradation behavior in order to emphasize the contribution of the filler in different surface treatments and contents to ensuing behavior of these composites.
Experimental
Composites of isotactic polypropylene (i-PP, Moplen CS-42 HEXP, HMC Polymers Co., Ltd, Thailand) and two grades of CaCO3 nanoparticles (nCaCO3, NPCC-111 = uncoated grade and NPCC-201 = stearic acid-coated grade) were prepared The contents of nCaCO3 in these formulations were 5, 10, 15, 20, and 25% (w/w). The characteristics of i-PP resin were presented elsewhere [14]. The average diameter of nCaCO3 primary particles was about 40 nm. It should be noticed that the stearic coating layer thickness was estimated to be about 0.5 nm, based on the following input data: density of CaCO3 = 2.71 g.cm−3, density of stearic acid = 0.847 g cm−3, and the content of stearic acid = 2.5% w/w).
The chemiluminescence investigation on i-PP/nCaCO3 composites were performed on circular disc specimens, cut from melt-pressed films of about 100 μm thick under constant temperature (190 °C) using a home-made device (Oxyluminograph OL-94). The description of this equipment and the evaluation procedure of kinetic parameters depicting thermal oxidation were previously reported [22].
Results and discussion
The durability of polymer materials depends on the susceptivity of the materials to oxidation, which, in turn, is the result of the diffusion of oxygen into the materials. The incorporation of inorganic filler induces the modification in the oxygen diffusivity. The extension of such modification depends on the type, size, distribution (i.e., total surface area), and degree of mix (in terms of both dispersion and distribution) of the filler particles within the matrix. The latter replies mostly on the type of processing aids used to modify the surface of the filler. Dangtungee et al. [14] showed that the addition of uncoated nCaCO3 caused significant increase in the shear viscosity of the prepared i-PP/CaCO3 nanocomposites in their melt state. Surface coverage on the nanofillers with stearic acid decreased the shear viscosity of the nanocomposite melts to match that of the neat i-PP [14]. These findings have indicated the enhancement in the degree of mix relative to the stearic acid-coated nCaCO3 within i-PP matrix.
In Fig. 1a and b, the dependencies of chemiluminescence intensity on the oxidation time for various i-PP/CaCO3 nanoparticles are presented. The opposite aspects that define the contribution of the two grades of calcium carbonate nanofiller to the thermal strength of host polypropylene are the contrary orders in the increase of oxidation stability. The samples consisting of isotactic polypropylene and uncoated carbonate particles (type NPCC-111) becomes more and more unstable, when the concentration of nanofiller increases. On the contrary, the carbonate particles covered with stearic acid layer contribute to the improvement in the thermal stability of polymer material. This outcome is supposed to be originated in the adsorption/desorption equilibrium on the surface of nanoparticles. The hydrocarbon radicals which initiate degradation as peroxyl intermediates, which feed the propagation step of oxidation, can be scavenged on the outer surface of carbonate nanoparticles. In the presence of stearic acid, the desorption is remarkably delayed due to the complete solubility of hydrocarbon chains in coating organic bed. The augmentation in the intensity of chemiluminescence signals during the early stage of oxidation (Fig. 1a) can be noticed. When the content of nanofiller becomes higher, the increase in CL emission is attributed to the reaction of molecular oxygen, which would be retained on the particle surface during sample preparation.
Dependencies of chemiluminescence intensity on time for iPP/CaCO3 nanoparticles. a Uncoated particles; b particles covered with stearic acid sheet. Filled star neat iPP; filled and open squares iPP + 5% CaCO3; filled and open circles iPP + 10% CaCO3; filled and open triangles iPP + 15% CaCO3; filled and open diamonds iPP + 20% CaCO3; filled and open inverted triangles iPP + 25% CaCO3
The contribution of initial amount of oxygen embedded in neat polypropylene appears for the low concentrations of nanofiler (5 and 10%) only on the first 5 min elapsed from the start of measurements. The augmentation in the nanophase concentration causes intensified emission of photons (CL emission), which confirms the reaction of free radicals with “dissolved” oxygen on the first 20 min. The former reactivity measured by initial chemiluminescence intensity in the iPP/uncovered CaCO3 nanoparticles is pointed out by the growing in the initial CL emission (Fig. 2). The continuation in the oxidation of iPP modified with CaCO3 nanoparticles is assisted by the diffused oxygen, as it is usually occurred in pristine polypropylene, according to the scheme reported by Bolland and Gee [23].
On the opposite site, the degradation of isotactic polypropylene in the presence of coated calcium carbonate (NPCC-201—protected by stearic acid layer) is obviously retarded. The gain brought about by the surface modification of nanoparticles leads to the prevention of oxygen “feed” during degradation on the early oxidation period. The increasing thermal stability of iPP/nCaCO3 samples involving coated nanoparticles brings about the long-term usage of polypropylene doped with higher amount of calcium carbonate. This feature may be taken into account when a certain manufacturer intends to decrease the loss of energy by the diminishing polypropylene melting point.
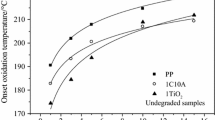
The temporal parameters that depict the development in the oxidation process occurred in polypropylene modified with two grades of CaCO3 nanoparticles follow straight line dependences on filler concentration. It demonstrates the effect of interphase surface that would be essential characteristic in the propagation stage of oxidation (Fig. 3a, b). The decrease noticed in oxidation induction time (OIT), demioxidation time (DOT) and maximum oxidation time (MOT) of thermal oxidation occurred in polypropylene substrate as the concentration of filler increases are the consequences of the delay of oxidation. This slowing down peculiarity is provided by the trapping of free radicals and by the superficial adsorption of peroxyl radicals in motionless positions. However, these later reactive positions may be permanently in contact with polypropylene molecules and the propagation chain would continue at a certain rate. When coated particles are present in polypropylene, the stearic acid layer “dissolves” degradation intermediates, they being abstracted from the system. The relevant increase in all temporary kinetic parameters found for the samples containing coated carbonate nanoparticles sustains the effect of inactivation of oxidation intermediates even when the concentration of filler reaches 25%.
The barrier properties of stearic acid coating are explained by the possibility of breaking the oxidation chain, which is defined by the opposite directions in oxidation rates. Undoubtedly, the samples consisting of iPP and uncoated CaCO3 nanoparticles have not revealed any improvement in their oxidation rate along the propagation stage of thermal degradation. Thus, the presence of stearic acid layer brought about an extension in the delay of oxidation. The dependence of oxidation rate on the concentration of nanofiller in iPP matrix (Fig. 4) emphasizes the contrary manners in which the two kinds of CaCO3 nanoparticles act during thermal degradation of polymer.
The experimental results have suggested the evolution scheme for oxidation occurred in the presence of the two kinds of CaCO3 nanoparticles (Fig. 5).
From Fig. 1a, it is obvious that the increase in the filler content causes the rise in initial CL emission that may be correlated with the increase in inorganic phase content. The presence of visible shoulder on the curves drawn for higher filler concentrations on the first stage of oxidation illustrates the existence of two types of radicals, one kind being depleted faster than the other type, which is trapped or blocked through an ester bond. This bond is possible because the oxygen atom belonging to C=O group has a partial charge due to its electronegativity and free radical presents a deficit of charge. Figure 1b displays a normal increase in the propagation stage, which suggests the existence of monotype radicals. Figure 1a exhibits insignificant differences in the values of initial CL intensities within the first 15 min. This aspect indicates the inert feature of stearic acid layer, in spite of the growth in its amount, which occurs simultaneously with increasing filler fraction. The progress of oxidation is sustained by the radicals, which are really free or trapped by organic substrate.
Conclusion
This study performed on the thermal behavior of isotactic polypropylene modified with uncoated or protected nanoparticles of calcium carbonate provides valuable information on the contribution of the superficial layer to the enhancement of thermal polymer stability. The barrier property of stearic acid covering nanofiller is relevant a proof for the increase in the kinetic characteristics of degradation for this substrate. The advantage in the addition of coated particles is the improvement in the durability of modified polypropylene that works under hard conditions promoting material alteration. On the other hand, the presence of uncoated calcium carbonate nanoparticles assists oxidation. The main kinetic parameters become harmful for all formulations iPP/CaCO3 uncoated nanoparticles. The chemiluminescence investigation on the thermal degradation of modified polypropylene has demonstrated the difference in the contribution of nanofiller (CaCO3) on the functional features of basic polymer material.
References
Andrievski RA (2003) Review: stability of nanostructured materials. J Mater Sci 38:1367
Yu YH, Liu CY, Yeh JM, Lin WH (2003) Preparation and properties of poly(vinyl alcohol)–clay nanocomposite materials. Polymer 44:3553
Ellis TS, D’Angelo JS (2003) Thermal and mechanical properties of a polypropylene nanocomposite. J Appl Polym Sci 90:1639
Yoon PJ, Hunter DL, Paul DR (2003) Polycarbonate nanocomposites: Part 2. Degradation and color formation. Polymer 44:534
Yoo YJ, Kim SS, Won JC, Choi KY, Lee JH (2004) Enhancement of the thermal stability, mechanical properties and morphologies of recycled PVC/clay nanocomposites. Polym Bull 52:373
Chow WS, Abu Bakar A, Mohd Ishak ZA (2005) Water absorption and hygrothermal aging study on organomontmorillonite reinforced polyamide 6/polypropylene nanocomposites. J Appl Polym Sci 98:780
Yang LW, Kim ES, Kin HS, Yoon JS (2005) Preparation and characterization of polypropylene/clay nanocomposites with polypropylene-graft-maleic anhydride. J Appl Polym Sci 98:1229
Zaharescu T, Kaci M, Setnescu R, Jipa S, Touti M (2006) Thermal stability evaluation of polypropylene protected with grafted amine. Polym Bull 56:405
Bertini F, Canetti M, Audisio G, Costa G, Falqui L (2006) Characterization and thermal degradation of polypropyleneemontmorillonite nanocomposites. Polym Degrad Stab 91:600
Hasegawa N, Okamoto H, Kato H, Usuki A (2000) Preparation and mechanical properties of polypropylene–clay hybrids based on modified polypropylene and organophilic clay. J Appl Polym Sci 78:1918
Wang D, Wilkie CA (2003) Thermal and flame properties of polyethylene and polypropylene nanocomposites based on an oligomerically-modified clay. Polym Degrad Stab 80:171
Tidjani A, Wald O, Pohl MM, Hentschel MP, Schartel B (2003) Polypropylene–graft–maleic anhydride-nanocomposites: I—Characterization and thermal stability of nanocomposites produced under nitrogen and in air. Polym Degrad Stab 82:133
Svoboda P, Zeng C, Wang H, Lee LJ, Tomasko L (2002) Morphology and mechanical properties of polypropylene/organoclay nanocomposites. J Appl Polym Sci 85:1562
Dangtungee R, Yun J, Supaphol P (2005) Melt rheology and extrudate swell of calcium carbonate nanoparticle-filled isotactic polypropylene. Polym Test 24:2
Zhang L, Chen XH, Li CZ (2005) Mechanical properties of PVC/nano-CaCO3 composites. J Mater Sci 40:2097
Jiasheng Q, Pingsheng H (2003) Non-isothermal crystallization of HDPE/nano-SiO2 composite. J Mater Sci 38:2299
Jie W, Yubao C, Yi Z (2003) A study on nano-composite of hydroxyapatite and polyamide. J Mater Sci 38:3303
Park DP, Sung JH, Lim ST, Choi HJ, Jhon HS (2003) Synthesis and characterization of soluble polypyrrole and polypyrrole/organoclay nanocomposites. J Mater Sci Lett 22:1299
Ciuprina F, Zaharescu T, Supaphol P (2006) Radiation effects on electrical resistivity of polypropylene nanocomposites. In: Proceedings of POLYCHAR conference, Nara, Japan, 17–22 April 2006, paper P20-65
Diagne M, Girèye M, Vidal L, Tidjani A (2005) Thermal stability and fire retardant performance of photo-oxidized nanocomposites of polypropylene-graft-maleic anhydride/clay. Polym Degrad Stab 89:418
Zhang J, Jiang DD, Wilkie CA (2006) Thermal and flame properties of polyethylene and polypropylene nanocomposites based on an oligomerically-modified clay. Polym Degrad Stab 91:298
Jipa S, Zaharescu T, Setnescu R, Gorghiu LM, Dumitrescu C, Oros C (2006) Chemiluminescence study on HALS antioxidant activity in LDPE. Polym Bull 57:545
Bolland JL, Gee G (1946) Kinetic studies in the chemistry of rubber and related materials. II. The kinetics of oxidation of unconjugated olefins. Trans Faraday Soc 42:236
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jipa, S., Zaharescu, T. & Supaphol, P. Thermal stability of isotactic polypropylene modified with calcium carbonate nanoparticles. Polym. Bull. 64, 783–790 (2010). https://doi.org/10.1007/s00289-009-0213-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-009-0213-1