Abstract
Antimicrobials fight microorganisms, preventing and treating infectious diseases. However, antimicrobial resistance (AMR) is a growing concern due to the inappropriate and excessive use of these drugs. Several mechanisms can lead to resistance, including efflux pumps such as the NorA pump in Staphylococcus aureus, which reduces the effectiveness of fluoroquinolones. Thiadiazines are heterocyclic compounds whose chemical structure resembles that of cephalosporins. Therefore, these compounds and their derivatives have been studied for their potential in combating increased bacterial resistance. To analyze this hypothesis, direct activity assays, antibiotic action-modifying activity, fluorescence assays to evaluate the retention of ethidium bromide inside bacteria, and molecular docking were carried out. These experiments involved serial dilutions in microplates against Staphylococcus aureus strain 1199B under the influence of six thiadiazine derivatives (IJ10, IJ11, IJ21, IJ22, IJ23, and IJ25). The tests revealed that, despite not showing effective direct activity, some thiadiazine derivatives (IJ11, IJ21, and IJ22) inhibited the function of the bromide pump both in microdilution tests and in fluorescence and docking assays. Particularly, the IJ11 compound stood out for its activity similar to efflux inhibitors, as well as its inhibition of the norfloxacin pump of this bacterium. Among the results of this study, it deserves to be highlighted for anchoring future experiments, as it represents the first investigation of this group of thiadiazine derivatives against the NorA pump.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobials are therapeutic agents of natural or synthetic origin that act on different types of microorganisms, inhibiting their growth and preventing their dissemination, thus contributing to the prevention and treatment of a wide variety of infectious diseases. They can be used prophylactically or therapeutically, representing a pharmacological advancement of great importance to society [10].
Antimicrobial resistance (AMR) is one of the 10 causes of global public health and development threats. According to the WHO, one of the causes of the increase in this adaptive selectivity of resistant pathogens is the improper and excessive use of antimicrobials. AMR occurs when bacteria, viruses, and fungi no longer respond to certain drugs at certain concentrations previously sufficient to control them. Therefore, several antibiotics from different classes that are present on the market have become obsolete against different types of infections [6, 11].
Bacterial resistance is a complex and comprehensive phenomenon that affects a variety of pathogenic bacteria. One of the most notable is methicillin-resistant Staphylococcus aureus (MRSA); this strain has developed resistance to a wide range of beta-lactam antibiotics, including methicillin and other penicillins. This makes MRSA infections more difficult to treat and potentially more severe. This example highlights the importance of bacterial resistance and the need for comprehensive measures to prevent and control its development and spread. Proper use of antimicrobials, infection control, research into new therapeutic agents, and prevention strategies are crucial in the fight against bacterial resistance [15, 21, 26].
There are several mechanisms by which bacteria can develop resistance to antibiotics. Some of the main mechanisms of bacterial resistance include 1. Production of enzymes that inactivate the antibiotic; 2. Alteration of the antibiotic’s target; 3. Modification or reduction of bacterial membrane permeability; 4. Acquisition of resistance genes by horizontal transfer; 5. Formation of biofilms; and 6. Efflux pump [3, 7, 23].
Bacterial resistance through efflux pumps is an important mechanism by which bacteria can protect themselves from the effects of antibiotics. Efflux pumps are transport proteins present in the bacterial membrane that act as active transport pumps, removing antibiotics from inside the bacterial cell before they can exert their antimicrobial action [3, 14].
The NorA efflux pump is a resistance mechanism present in Staphylococcus aureus, specifically giving it resistance to fluoroquinolones, a class of antibiotics widely used in the treatment of bacterial infections. This efflux pump is encoded by a gene called norA, located on the bacterial chromosome of S. aureus. Its expression can be regulated by several factors, including previous exposure to fluoroquinolones. Through the proton motive force and the antiport mechanism, the pump facilitates the influx of H+ ions and efficiently transports fluoroquinolones out of the cell, reducing their antimicrobial action [1, 13, 27].
Among the compounds capable of exhibiting antimicrobial and pharmacological activities, thiadiazines are a promising class of organic compounds that have a heterocyclic ring containing two nitrogen atoms and one sulfur atom. These compounds are known for their chemical reactivity and for presenting interesting biologic and pharmacological activities, in addition to demonstrating good results against a multidrug-resistant Staphylococcus aureus strain in a previous study [8].
Methodology
Acquisition of the Isolated Substances
The substances used in the present study were synthesized according to Araújo et al. [8], and details of this procedure can be found in the article. The substances are being reused to continue research in this line of compounds, as they were previously tested against multi-resistant strains.
Bacterial Strains
An isolated strain of Staphylococcus aureus 1199B isolate was provided by the University of London. The strain was cultured on Brain Heart Infusion Agar (BHI) at 37 °C for 24 h before the assays. After this period, a portion of the culture was transferred from the solid medium to test tubes containing sterile saline solution, and the density was evaluated using a value of 0.5 on the McFarland scale, corresponding to 108 colony-forming units (CFU).
Dilution of Substances for Microdilution Assays
Initially, 10 mg of norfloxacin, chlorpromazine and each of the 6 thiadiazine derivatives were weighed and stored in microtubes, which were later diluted in 0.5 mL of DMSO. Then, the resulting solution from this first dilution was transferred to a 15-mL conical tube and an additional 9.265 mL of sterile distilled water was added, totaling 9.765 mL of solution with a concentration of 1024 µg/mL.
Ethidium bromide and carbonyl cyanide 3-chlorophenylhydrazone (CCCP) were diluted to achieve the same concentration as the previous substances. For the dilution of ethidium bromide, only distilled water was used. For CCCP, solution A was prepared using 5 mL of methanol and 5 mL of distilled water; then, 10 mg of CCCP was weighed; and 9.765 mL of solution A was used to finish this dilution. These substances were purchased from Sigma-Aldrich [25].
Preparation of Bacterial Inoculum for Microdilution Assays
The bacterial strains were cultured on Heart Infusion Agar (HIA) plates and incubated in an oven at 37 °C to allow growth for 24 h before the experiment. After this period, a sample of bacteria was diluted in triplicate in test tubes containing sterile saline solution. The turbidity of the solution was adjusted to match that of the control, which had a reading of 0.5 on the McFarland scale.
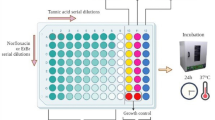
Determination of Minimum Inhibitory Concentration (MIC)
Microtubes were prepared in groups of three for each type of bacterium. Each microtube contained 900 μL of a 10% BHI liquid solution and 100 μL of inoculum, representing 10% of the total solution for the MIC experiment. Then, 100 μL of the final inoculum solution was added to each well of a 96-well plate. A serial microdilution was performed in columns, using a solution of 100 μL of the substances, with concentrations ranging from 512 μg/mL in the first well to 8 μg/mL in the last well; the last well is not microdiluted as it serves as the growth control. The plates were incubated at a temperature of 35 ± 2 °C for 24 h. After this period, an indicator solution with a concentration of 20 μg/mL was added to each well, and the plates were kept at room temperature for one hour. After this period, the test was read by observing the colors of the culture medium. Wells that did not show microbial growth were considered positive, retaining a blue color, while those that showed a red color were considered negative.
Modifying Activity of Antibiotic Action
The bacterial inoculum was prepared in BHI medium following the procedure described above. The thiadiazines were added at a concentration equivalent to MIC⁄8 value (eighth of the minimum inhibitory concentration) to evaluate their ability to modulate the induced efflux pump in the presence of other drugs [5, 25].
In a 96-well plate, the wells were filled with 100 μL of inoculum and only BHI in the control wells. In the test wells, the thiadiazine derivatives were added to the medium. Then, ethidium bromide or norfloxacin was added and microdiluted at concentrations ranging from 512 to 0.5 μg/mL. The positive control was performed using CCCP and CPZ, which were added to the wells in a similar manner to the products. The experimental controls and MIC values for each compound were set as described earlier.
Evaluation of Efflux Protein Inhibition Through Fluorescence Quantification
Staphylococcus aureus 1199B strain, previously cultured in Brain Heart Infusion Agar (BHI) medium for 24 h before the experiment, was used. Bacterial cultivation was maintained in a bacteriological incubator at a temperature of 37 ℃. The inoculum was prepared in phosphate-buffered saline (PBS) until reaching a count of 1.5 × 108 colony-forming units, according to the 0.5 McFarland scale.
IJ variants of thiadiazine analogs that showed the best results in the microdilution test were selected. A solution containing the inoculum and thiadiazine derivatives was prepared in microtubes, with a concentration of 50 µg/mL for the test. A CCCP solution at 50 µg/mL was used as a positive control. After the addition of the inoculum and the substance, PBS was added until reaching a final volume of 1 mL. The solutions were incubated and then ethidium bromide was added at a concentration of 100 µg/mL to all solutions except the growth control. After 1 h, the solutions were centrifuged at 10,000 rpm for 2 min and washed with PBS twice, with each wash followed by centrifugation. The reading was performed using a BioTek® Cytation 1 microplate fluorescence reader and Gen5™ Software, with excitation at 530 nm and emission at 590 nm. Readings were taken at 10-min intervals, totaling 1 h and 10 min after washing. The following groups were read: Growth control (without EtBr). Negative control composed of inoculum with EtBr. Inoculum with EtBr and CCCP as positive control. Test containing inoculum, EtBr, and thiadiazine derivatives at 50 µg/mL [2, 20].
Molecular Docking
The amino acid sequence of the NorA transporter from S. aureus (UniProt ID: P0A0J7) was inputted into SWISS-MODEL [28], which identified 7LO8 (Protein Data Bank ID) as the best template and generated the model used in the analyses. The thiadiazine analogous molecules were designed in MarvinSketch (Chemaxon©), and energy minimization was performed using Open Babel 2.4.1, employing the Merck Molecular Force Field (MMFF94).
Gasteiger partial charges and hydrogen atoms (non-polar mixing) were added to the NorA model and the ligands using AutoDock Tools software [19]. Flexibility was also added to the ligands and the 11 key residues of NorA reported by Brawley et al. [4]. The docking was conducted using the tools of AutoDock software version 4.2.6, employing the same parameters as the study by Martin et al. [17], with a 30 Å × 30 Å × 30 Å grid box centered on the macromolecule. The determination of the best conformation for each ligand was based on the lowest binding energies.
Statistical Analysis
Results were expressed as geometric mean ± standard deviation, statistically evaluated using one-way ANOVA followed by Bonferroni’s post-test using GraphPad Prism software version 9.0. Differences were considered significant when p < 0.05.
Results
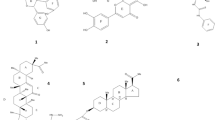
Five compounds were synthesized using the IJ25 structure as a model. To enhance bioactive performance, various ions were introduced into different regions of the base structure. The resulting compounds are depicted in Fig. 1. The corresponding chromatogram is provided in Supplementary Material 1.
In the MIC assays, the compounds were tested directly against the strain carrying the NorA efflux pump, resulting in a value of 1024 µg/mL, and for IJ 22 and IJ23, the values were equal to 128 µg/mL. These concentrations are not considered clinically relevant, as the concentration of substance required for this inhibition would be unfeasible for the volume of human blood [12].
Figure 2 presents the results obtained by the association of ethidium bromide, thiadiazines, and the control substances CCCP and CPZ. Among the thiadiazine derivatives tested, the compound IJ25 had no effect on inhibiting the efflux pump but somehow increased its activity, increasing bromide efflux through the NorA pump. The compound IJ23 had a similar action to isolated ethidium bromide, thus not altering the functioning of the pump. However, the other compounds managed to interfere with the efflux mechanism, thus reducing the amount of EtBr necessary for the bactericidal effect, mainly the compound IJ22, which had a similar action to CCCP, an efflux pump inhibitor, but toxic, different from the compound IJ22.
Tests associated with the antibiotic norfloxacin (Fig. 3) showed only 3 compounds that managed to reduce the concentration of the antibiotic necessary to inhibit bacterial growth; these were IJ11, IJ21 and IJ22. The compound IJ25 again potentiated the pump effect by increasing norfloxacin efflux, an effect also played by IJ23. The compound IJ10 acted similarly to CCCP and norfloxacin alone.
When evaluating fluorescence emission, it was observed that all thiadiazine derivatives, except compound IJ25, increased fluorescence compared to the negative control (inoculum and EtBr) (Fig. 4). A significant result was also observed for the pump inhibitor, CCCP, indicating reproducibility of the experiment.
Molecular docking analysis shows that compound IJ11 had the lowest binding energy of − 7.77 kcal/mol, inhibition constant of 2.03 µM, and ligand efficiency of − 0.55, followed by compounds IJ10, IJ22, IJ21, IJ23, and IJ25 (Table 1). The affinity for the NorA efflux pump, including the notion of effective compounds, corroborates the results of the previous sections, which associate thiadiazine derivatives with the inhibition of the efflux system. The values corresponding to CCCP are similar to those of thiadiazine derivatives. The results for norfloxacin and ethidium bromide are also presented.
With the lowest binding energy among the thiadiazine derivatives, the compound IJ11 established three main classes of interactions with the NorA model, namely hydrogen bonds, hydrophobic interactions, and Van der Waals forces, distributed as follows: Arg310, Asn340, and Glu222 residues (hydrogen bonds), Thr336 (π-sigma), Ile136 (alkyl), Phe140 (π-alkyl), Phe16 (π–π T-shaped and π-alkyl), and Asn137, Gln51, Leu218, Met109, and Ser337 (Van der Waals) (Fig. 5).
As shown in the results of the association with norfloxacin (Fig. 3), the compound IJ11 reduced the MIC of the antibiotic, unlike CCCP (the most effective inhibitor in other in vitro assays), which had no effect. From the analysis of norfloxacin interactions with NorA (Fig. 6A), also grouped into hydrogen bonds, hydrophobic interactions, and Van der Waals interactions, it can be observed that residues Arg310, Asn137, Asn340, Gln51, Glu222, Ile136, Met109, Phe16, Phe140, and Thr336 constitute the binding sites of the antibiotic and compound IJ11.
CCCP performed π-anion (Asp307 and Glu222), π-alkyl (Phe303 and Tyr225), and Van der Waals interactions (Asn137, Ile244 and Phe140) and hydrogen bonds (Arg310) with the transporter (Fig. 6B), having less commonalities with the location of norfloxacin compared to the best in silico thiadiazine derivative. Evaluating the level of similarity of inhibitor and substrate binding sites as an important factor for efflux pump inhibition, these differences observed in docking constitute the main explanation for the mentioned in vitro results.
Discussion
Ethidium bromide is a toxic and fluorescent substance that intercalates with DNA. In bacteria that carry efflux pump genes in their genome, bromide is expelled from the bacteria only by the pump and cannot be metabolized or attenuated. Microdilution assays using EtBr aim to evaluate the functionality of the bacterial pump. A lower value observed in the interaction between EtBr and thiadiazines indicates that these substances inhibited the efflux pump and ethidium bromide remained inside the bacterial cell, leading to cell death [28].
The compound IJ25 enhanced the efflux pump effect against both bromide and the antibiotic, showed no significant effect on ethidium bromide retention and had the lowest binding energy detected in the in silico assay. These results underscore the importance of the chemical variations introduced during synthesis, indicating that the ions added to the other substances improve the biologic activities of thiadiazine derivatives against the NorA pump [18].
The fluorescence control group is essential to show that the isolated bacteria do not emit fluorescence. Thus, all detected fluorescence is due to the accumulation of EtBr. This compound emits fluorescence when bound to genetic material. Inhibition of efflux pumps increases the intracellular concentration of EtBr, increasing the level of fluorescence detected during reading. The increase in fluorescence emission in groups treated with IJ derivatives indicates that NorA efflux pumps were possibly inhibited [17].
In the study by Waterhouse et al. [28], thiazole compounds were used against two strains carrying the norA gene, namely strains 1199 (wild type) and 1199B (mutant). Even carrying the gene, there is a difference in pump expression between the two strains, with the mutant strain overexpressing this efflux protein, developing even more resistance compared to strain 1199. Further tests were performed using 1,2,3-triazole and ciprofloxacin as efflux antibiotics, and the authors used NorA bacteria and found a probable inhibition of efflux [8]. The results evidenced by the authors corroborate those found in the present study, where most of the compounds used potentiated the efflux of antibiotics.
Martin et al. [17] conducted fluorescence assays with ethidium bromide against Staphylococcus aureus strain 1199B, where they found that the coumarins used in the experiments were able to decrease efflux pump expression, causing bromide to accumulate inside the bacteria, leading to cell death. Freitas et al. [9] used the synthetic compound limonene, an oxygenated monoterpene found in citrus plant essential oils, against another bacterium carrying a fluoroquinolone pump and managed to inhibit the pump, promoting EtBr accumulation.
Related to docking with thiadiazine derivatives, Stephen et al. [24] reported that residues identified in our study, such as Arg310, Asn340, Gln51, and Phe140, are consistent in silico analyses of compounds from different classes that showed inhibitory effects on bacterial transporters. Additionally, Majumder et al. [16] analyses indicated that acidic residues of another member of the Major Facilitator Superfamily (MFS) act both in substrate recognition and proton-coupled transport, which highlights the importance of the hydrogen bonding performed by IJ11 with the residue Glu222.
Based on the location in the NorA cavity, we hypothesized that the effect of compound IJ11 on norfloxacin efflux was due to the similarity of the potential inhibitor binding site to the antibiotic binding site. This preliminary conclusion is supported by Waterhouse et al. [28] and Siqueira et al. [22], whose molecular docking assays elucidated that compound with more significant in vitro activities against S. aureus 1199B interacted with NorA in regions similar to the substrates tested.
Conclusion
The results presented in this study demonstrate the inhibitory potential of thiadiazine derivatives against the NorA efflux pump carried by the bacteria Staphylococcus aureus. Although some of these compounds did not show inhibitory activity, others, such as compounds IJ11 and IJ22, demonstrated this potential in all tests performed. However, it is worth highlighting the novelty of using these compounds against this strain; therefore, more experiments need to be carried out to clarify how this mechanism is affected and to continue the use of these compounds as a future hope in combating bacterial resistance.
References
Aldred KJ, Kerns RJ, Osheroff N (2014) Mechanism of quinolone action and resistance. Biochemistry 53(10):1565–1574
Blair JMA, Piddock LJV (2016) How to measure export via bacterial multidrug resistance efflux pumps. MBio 7(4):e00840-e916
Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13(1):42–51
Brawley DN, Sauer DB, Li J, Zheng X, Koide A, Jedhe GS, Suwatthee T, Song J, Liu Z, Arora PS (2022) Structural basis for inhibition of the drug efflux pump NorA from Staphylococcus aureus. Nat Chem Biol 18(7):706–712
Coutinho HDM, Costa JGM, Lima EO, Falcão-Silva VS, Siqueira-Júnior JP (2008) Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 54(4):328–330
Dadgostar P (2019) Antimicrobial resistance: implications and costs. Infect Drug Resist. https://doi.org/10.2147/IDR.S234610
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433
de Araújo ACJ, Freitas PR, Araújo IM, Siqueira GM, de Oliveira Borges JA, Alves DS, Miranda GM, dos Santos Nascimento IJ, de Araújo-Júnior JX, da Silva-Júnior EF, de Aquino TM (2024) Potentiating-antibiotic activity and absorption, distribution, metabolism, excretion and toxicity properties (ADMET) analysis of synthetic thiadiazines against multi-drug resistant (MDR) strains. Fundam Clin Pharmacol 38(1):84–98
Freitas PR, Justino AC, de Araújo C, dos Santos R, Barbosa DF, Muniz RS, de Almeida I, Alencar R, de Menezes J, Martins G, da Costa F, Rodrigues FG, Rocha JE, Pereira-Junior FN (2022) Inhibition of the MepA efflux pump by limonene demonstrated by in vitro and in silico methods. Folia Microbiol 67(1):15–20
Furtado DMF, Silveira VSD, Carneiro ICDRS, Furtado DMF, Kilishek MP (2019) Consumo de Antimicrobianos e o Impacto Na Resistência Bacteriana Em Um Hospital Público Do Estado Do Pará, Brasil, de 2012 a 2016. Revista Pan-Amazônica de Saúde. https://doi.org/10.5123/s2176-6223201900041
Giono-Cerezo S, Santos-Preciado JI, RayoMorfín-Otero MD, Torres-López FJ, Alcántar-Curiel MD (2020) Antimicrobial resistance. Its importance and efforts to control it. Gac Med Mex 156(2):172–180
Houghton PJ, Howes MJ, Lee CC, Steventon G (2007) Uses and abuses of in vitro tests in ethnopharmacology: visualizing an elephant. J Ethnopharmacol 110(3):391–400. https://doi.org/10.1016/j.jep.2007.01.032
Kaatz GW, Seo SM, Ruble CA (1991) Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J Infect Dis 163(5):1080–1086
Li X-Z, Nikaido H (2009) Efflux-mediated drug resistance in bacteria: an update. Drugs 69:1555–1623
Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE (2011) Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52(3):e18-55
Majumder P, Khare S, Athreya A, Hussain N, Gulati A, Penmatsa A (2019) Dissection of protonation sites for antibacterial recognition and transport in QacA, a multi-drug efflux transporter. J Mol Biol 431(11):2163–2179
Martin ALA, Pereira RLS, Rocha JE, Farias PA, Freitas TS, de Lemos Caldas FR, Figueredo FG, Sampaio NFL, de Morais Oliveira-Tintino CD, Tintino SR, da Hora GCA (2024) Unlocking bacterial defense: exploring the potent inhibition of NorA efflux pump by coumarin derivatives in Staphylococcus aureus. Microb Pathog 190:106608
Metwally MA, Bondock S, El-Azap H, Kandeel E-E (2011) Thiosemicarbazides: synthesis and reactions. J Sulfur Chem 32(5):489–519
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Pal S, Misra A, Banerjee S, Dam B (2020) Adaptation of ethidium bromide fluorescence assay to monitor activity of efflux pumps in bacterial pure cultures or mixed population from environmental samples. J King Saud Univ-Sci 32(1):939–945
Ragab FA, Abdel-Aziz SA, Kamel M, Ouf AMA, Allam HA (2019) Design, synthesis and biological evaluation of some new 1, 3, 4-thiadiazine-thiourea derivatives as potential antitumor agents against non-small cell lung cancer cells. Bioorganic Chem 93:103323
Siqueira MMR, Freire PDTC, Cruz BG, de Freitas TS, Bandeira PN, Dos Santos HS, Nogueira CES, Teixeira AMR, Pereira RLS, da Cunha Xavier J, Campina FF (2021) Aminophenyl chalcones potentiating antibiotic activity and inhibiting bacterial efflux pump. Eur J Pharm Sci 158:105695
Spellberg B, Gilbert DN (2014) The future of antibiotics and resistance: a tribute to a career of leadership by John Bartlett. Clin Infect Dis 59:S71-75
Stephen J, Salam F, Lekshmi M, Kumar SH, Varela MF (2023) The major facilitator superfamily and antimicrobial resistance efflux pumps of the ESKAPEE pathogen Staphylococcus aureus. Antibiotics 12(2):343
Tintino SR, Oliveira-Tintino CDM, Campina FF, Limaverde PW, Pereira PS, Siqueira-Junior JP, Coutinho HDM, Quintans-Júnior LJ, da Silva TG, Leal-Balbino TC (2018) Vitamin K enhances the effect of antibiotics inhibiting the efflux pumps of Staphylococcus aureus strains. Med Chem Res 27:261–267
Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler Jr VG (2015) Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28(3):603–661
Truong-Bolduc QC, Hooper DC (2007) The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and β-lactams in Staphylococcus aureus. J Bacteriol 189(8):2996–3005
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296-303
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Araújo, A.C.J., Freitas, P.R., Araújo, I.M. et al. Assessment In vitro and In silico of the Activity of Thiadiazines as NorA Efflux Pump Inhibitors. Curr Microbiol 81, 325 (2024). https://doi.org/10.1007/s00284-024-03836-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03836-0