Abstract
The essential oil from Callistemon viminalis (EOC) is rich in monoterpenes, with a variety of biological properties: antibacterial, antifungal, insecticide, and antioxidant. Inclusion complexes (ICs) with cyclodextrins (CDs) is an alternative to prevent toxicity, improve the activity, and reduce the concentration to be used. Thus, the objective of this work was to prepare an IC (EOC/β-CD) and evaluate the antibacterial, antifungal and phospholipase activities, as well as the toxicity. Antimicrobial activity used the agar diffusion test and antifungal activity the disc diffusion test. Toxicity tests were carried out using Lactuca sativa L. The inhibition of phospholipase activity using the venom of Bothrops atrox as an inducer was performed. Antibacterial and antifungal tests demonstrated a decrease in the minimum inhibitory concentration (MIC) of the IC. It was most significantly observed for the bacterium Listeria monocytogenes, for which there was a decrease in the MIC from 250 µg mL−1 to 62.5 µg mL−1 after complexation, and for the fungus Aspergillus flavus, with a decrease in MIC from 125 µg mL−1 to 62.5 µg mL−1 after complexation. Toxicity tests with Lactuca sativa showed a decrease in toxicity after complexation in all parameters analyzed, with no statistical difference from the negative control. Inhibition of phospholipase activity induced by Bothrops atrox venom was more expressive in the highest proportion studied (1:10 m:m), exerting 23% inhibition. The assays demonstrated that the complexation between the EOC and β-CD is a promising alternative for use in different branches, especially in food industry, to fully exploit its application potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential oils (EO) belong to the class of secondary metabolites. They have various chemical structures and biological properties, being colorless or slightly yellow and volatile. They are present in low concentrations, and in certain groups of plants, they are very unstable, especially in the presence of air, light, heat, humidity, and metals. They play an important role in the adaptation of plants to their environments, and they also represent an important source of biologically active substances [1].
The use of EOs as medicine or in food has increased in recent years, but some of their characteristics might be considered undesirable, such as high volatility, strong odor, insolubility in water, low physicochemical stability and high toxicity when used in high concentrations, so they can be highly harmful to human health [2]. Thus, it is extremely important to perform studies of the toxicity of these natural products, as well as to evaluate their biological properties to obtain a reasonable margin for their safe use [3].
Assays of superior plants such as Lactuca sativa L. have been used to evaluate the toxicity of various compounds by associating the effects that a given substance can cause on plant germination, initial development and cell cycle. The advantages of using plant models in toxicity study environments are their low cost, easy handling and sensitivity, as well as their similarity to animal models, including human cells [4].
Snake venoms have also been widely used as laboratory tools in physiological studies for evaluating the toxicity of various naturally occurring compounds. The venoms consist mainly of proteins (phospholipases A2 and proteases) that have various biological activities such as enzymatic, myotoxic, cardiotoxic and cytotoxic activities. Phospholipases A2 (PLA2) and proteases present in Bothrops snake venoms can act directly on erythrocytes, myocytes, blood coagulation cascade factors, epithelial cells and vascular endothelium to cause physiological disorganization that result in intravascular coagulation or hemolysis [5].
As a means of circumventing undesirable characteristics and improving the applicability of EOs, several studies have been conducted to complex EOs with cyclodextrins (CDs) [6]. CDs are glucose polysaccharides, joined by α-1,4 glycosidic bonds. They have a conical trunk or hollow cylindric shape with a hydrophilic exterior and hydrophobic cavity. The widely used natural CDs are α-, β- and γ-CD, consisting of 6, 7 and 8 glucose units, respectively. CDs are capable of forming inclusion complexes (ICs) with a wide variety of molecules to modify their physicochemical properties, such as solubility, instability to light, heat and humidity, as well as enabling controlled release of the complexed compound [7].
This research focused on the study of the EO from the leaves from Callistemon viminalis, an ornamental plant belonging to the Myrtaceae family, popularly known as the bottle brush [5]. It is a medium-sized tree with persistent foliage found worldwide, being distributed mainly in tropical regions. Several authors report that the essential oil from C. viminalis (EOC) is rich in several chemical components. 1, 8-cineol (50–80%), α-pinene (10–20%) and α-terpineol (2–15%) are the most commonly found compounds, and they are the principal components [5, 8,9,10]. These components have insecticidal, fungicidal, antimicrobial, antifungal and allelopathic properties, and they have previously been reported in the literature by various authors [5, 11,12,13].
Thus, the IC between the EOC and β-CD was prepared, and the antibacterial, antifungal and phospholipase activities were evaluated. Toxicity tests using the Lactuca sativa L. model were performed before and after complexation of EOC with β-CD.
Experimental Section
Material
The leaves of C. viminalis (Myrtaceae) plant were collected at the Federal University of Lavras (UFLA) campus, located in Lavras, MG, Brazil. The species were identified in the Department of Biology of UFLA, with number 26,624 exsiccata. The cyclodextrin used was β-cyclodextrin from Sigma Aldrich (97%). All solutions were prepared using Milli-Q ultrapure water (Millipore®, Bedford, MA, USA) and 99.8% HPLC grade ethanol (Merck®) was used.
Antibacterial and antifungal activities were determined at the Laboratory of Mycotoxins and Food Mycology of the UFLA Department of Food Science. The bacteria evaluated were gram-positive: Staphylococcus aureus ATCC 13,565, Listeria monocytogenes ATCC 19,117 and gram-negative: Escherichia coli ATCC 11,229, Salmonella cholerasuis ATCC 6539. The fungi tested were: Aspergillus carbonarius (CCDCA 10,507), Aspergillus flavus (CCDCA 10,508) and Aspergillus ochraceus (CCDCA 10,506). They were all obtained from the microorganism culture collection of the UFLA Department of Food Science. Lactuca sativa L. seeds of the Butter variety obtained from the local commerce of Lavras were used for the toxicity test. Egg yolk, which is rich in phospholipids, was used to prepare the gel in which the treatments were incubated for the phospholipase assay.
Methods
Extraction of the Essential Oil from C. viminalis
300 g of C. viminalis plant material were weighed and distilled water was added until the leaves were completely covered (approximately 3 L). The process took pace in 3 h and the hydrodistillation technique using a modified Clevenger apparatus connected to a six-liter round bottom flask [14] was employed to extract the essential oil from C. viminalis. The essential oil was identified by gas chromatography using a mass spectrometer detector (GC/MS-Shimadzu, model GCMS-QP 2010), according to the conditions described by Martins [15].
Preparation of the Inclusion Complex
The kneading technique was used to prepare the inclusion complex (IC) according to the method described by Menezes et al. [16], with modifications. A 100 µL aliquot of the EOC was initially mixed with 1000 mg of β-CD, followed by the addition of 5 mL of a hydroalcoholic solution, and the mixture was homogenized manually in a mortar for 20 min until it formed a homogeneous paste, which was allowed to dry in a desiccator at 25 °C for 24 h. The resulting powder was stored in a microtube until its use.
Antibacterial Activity
The antibacterial activity was determined using the agar diffusion technique according to the method described by Camargo et al. [17]. The cultures were enriched with brain and heart infusion broth (BHI), incubated at 37 °C for 24 h and transferred to a tube containing 5 mL of tryptic soy broth (TSB), which was incubated again under the same conditions until it reached the turbidity of a standard McFarland solution of 0.5, which corresponds to a concentration of 108 CFU mL−1. Inoculum were then diluted to a concentration of 106 CFU mL−1 and incorporated into Mueller–Hinton (MH) agar. A thin layer of pure MH agar was poured into the Petri dishes, and sterile glass beads were placed on the gel. The agar on which the bacteria was inoculated was decanted onto the first layer. After solidification, the glass beads were removed, and 10 μL of EOC and IC at concentrations of 500; 250; 125; 62.5; 31.25; 15.62; 7.81; 3.90, 1.95 and 0 µg mL−1 in DMSO were added to each well. The experiment was performed in triplicate using chloramphenicol (CP) antibiotic as a positive standard and DMSO as the negative standard. Plates were maintained in BOD incubator chamber at 37 °C, and inhibition zone diameters were measured after 24 h. The minimum inhibitory concentration (MIC) was defined as the lowest concentration capable of inhibiting the growth of the bacteria under study.
Antifungal Activity
Analysis of the inhibitory effect on the growth of filamentous fungi was performed using the disc diffusion test accepted by the Food and Drug Administration (FDA) and established by the Clinical and Laboratory Standards Institute [18]. An inoculum was used at a concentration of 106 spores mL−1, counted with a Newbauer chamber. The inoculum was transferred to the plate containing malt agar extract (MAE) medium by the surface spreading technique. Sterile 5-mm-diameter discs of filter paper were soaked with 10 μL of EOC and IC diluted with DMSO to give final concentrations of 500; 250; 125; 62.5; 31.25, 15.62 and 7.81 µg mL−1 and placed on the culture medium, as described by Andrade et al. [19]. DMSO was used as the negative control and the synthetic fungicide fludioxonil (2 µg mL−1) was employed as the positive control. The plates were maintained in BOD incubator chamber at 25 °C for a period of 72 h. The test was performed in triplicate. The inhibition halo was measured from the circumference of the paper disk to the microorganism growing edge. Orthogonal measurements were obtained, each corresponding to the average of two diametrically opposite measurements. The minimum inhibitory concentration (MIC) was defined as the lowest concentration required for inhibition of fungal growth. The inhibition percentage (%I) was calculated using Eq. 1.
where dc is the control mycelial growth diameter (fludioxonil) and dt is the treatment mycelial growth diameter.
Bioassays Using the Lactuca sativa L Model
The protocol described by Aragão et al. [20] was followed for the macroscopic analysis: germination rate (%GR), germination speed index (GSI) and root growth (RG). EOC and ICs were tested at a concentration of 5 × 10–3 g L−1. The negative and positive controls were pure water and noncomplexed EOC, respectively. L. sativa seeds (Butter variety) were placed on filter paper in Petri dishes (9 cm diameter) with 3 mL of the treatment solutions. Each Petri dish contained 30 seeds, and there were 5 Petri dishes per treatment. The number of germinated seeds was counted every 8 h, for a total of 48 h of exposure, and the GSI was calculated according to Eq. 2.
where Ny: Number of germinated seeds in a given period, y: Total numbers of evaluation times (5-in this study).
The %GR was calculated according to Eq. 3, after 48 h of exposure. The root length was measured with a digital caliper (LEE Tools, China) after 120 h of exposure to furnish the RG.
where GS is the total number of germinated seeds and GT is the total number of seeds per treatment.
Phospholipase Activity
Phospholipase activity was evaluated as described by Gutiérrez et al. [21]. A gel was prepared (0.01 mol L−1 CaCl2; egg yolk diluted 1:3 v/v in PBS; 0.005% sodium azide with 1% agarose, pH 7.2). After solidification of the gel, holes of approximately 0.5 cm diameter were drilled. The final concentration of EOC and IC solutions was 5 µL mL−1 in a final volume of 30 µL. The inhibition of phospholipase activity induced by Bothrops atrox snake venom at a concentration of 10 μg mL−1 was evaluated. The samples were incubated in a water bath at 37 °C for 30 min. The ratios of venom/EOC or IC for determining the phospholipase activities were 1:0.1; 1:0.5; 1:1; 1:2.5; 1:5 and 1:10 (m:m). Assays were performed in triplicate. The gels containing the samples were kept in a cell culture chamber at 37 °C for 24 h, and the formation of a translucent halo around the orifice in the gel characterizing the phospholipase activity was quantified by measuring the diameter of the halo [22, 23].
Results and Discussion
Characterization of the EOC by Gas Chromatography
Identification of the EOC constituents employed a gas chromatograph coupled to a mass spectrometer. Twelve components were identified, with the major compounds being 1,8-cineole (67.70%), α-pinene (15.01%), and α-terpineol (7.72%). Other informations such as the peaks and retention times of the constituents are described in Martins et al. [24].
Antibacterial and Antifungal Activities
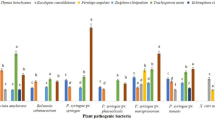
The minimum inhibitory concentrations (MIC) of EOC, β-CD and IC obtained for the bacteria and fungi under study are presented in Table 1, and the percentage inhibition (%I) for fungi is shown in Fig. 1.
The antibacterial activity of pure EOC was determined for gram-positive S. aureus and L. monocytogenes bacteria, obtaining a MIC of 250 µg mL−1. A MIC of 500 µg mL−1 was obtained for the gram-negative E. coli. Salem et al. [25] evaluated the antimicrobial activity of EOC against different gram-positive and gram-negative bacteria. They observed a high MIC value for gram-positive bacteria and a low value for gram-negative bacteria. A lower MIC of 62.5 µg mL−1 was observed for L. monocytogenes in the presence of the IC, followed by S. aureus, which was inhibited with a MIC of 125 µg mL−1. For E. coli, the same MIC was observed as with the pure EOC. The S. cholerasuis bacterium was not inhibited by either ICs or the pure 500 µg mL−1 EOC.
The microbiological results obtained for β-CD do not indicate the formation of inhibition halos. Hanci et al. [26] also observed no inhibition for Staphylococcus aureus ATCC 29,213, Enterococcus fecalis ATCC 29,212, Escherichia coli ATCC 25,922 or Pseudomonas aeruginosa ATCC 27,853 in their study with modified γ-CD (sugammadex) at any of the concentrations tested. When the MIC of the IC was compared with that of pure EOC, the antibacterial activity of the complex was equal to that of the free compound, but the minimum concentration required for inhibition was lower for the IC.
The antimicrobial activity of guava leaf oil complexed with hydroxypropyl-β-cyclodextrin against S. aureus and E. coli bacteria was evaluated by Rakmai et al. [27]. The oil contained 34% of 1,8-cineol. The MIC observed for S. aureus bacteria was higher (500 µg mL−1) for the pure oil than that observed with the IC (125 µg L−1). The MIC observed for E. coli with the pure oil was 500 µg mL−1, whereas that observed with the IC was 125 µg mL−1. Results similar to those found in this study for S. aureus bacteria were obtained, in which a lower MIC was observed. In the same research, the authors report that the IC produced with CDs can increase the aqueous solubility of apolar molecules that are encapsulated, thereby improving the antimicrobial efficiency of EOs. Lower concentrations can be used because of the better accessibility of the active compounds to cells.
Gram-positive bacteria were more sensitive than gram-negative bacteria when they were compared using pure EOC. The greater sensitivity of gram-positive bacteria is a feature reported in most studies performed with EOs, and it is explained by the differences in the cell wall of these two groups of bacteria [17]. In gram-positive bacteria, 90 to 95% of the cell wall is composed of peptide glycans, which facilitates the penetration of hydrophobic components. Gram-negative bacteria, on the other hand, have a double hydrophilic outer layer, which helps prevent the penetration of hydrophobic components, making them more resistant to EOs and other natural extracts [28]. Thus, according to Santos et al. [29], antibacterial activity against gram-positive bacteria was higher because the IC probably improves EOC access in the regions where it will act and facilitates their passage through the plasma membrane and entry into the cell cytoplasm. It promotes irreversible damage, leading to the death of the bacteria.
For all fungi studied, pure EOC showed a higher percentage of inhibition, being the highest for A. flavus, for which the inhibition was 73.3%. For the A. flavus fungus, the IC had a lower MIC of 62.5 µg mL−1 with 60% inhibition. For the fungus A. carbonarius, the same MIC of 500 µg mL−1 was observed for EOC and IC, but a difference in percent inhibition was observed. A 66.7% inhibition was observed in the presence of EOC, whereas that observed with IC was 40%. For the fungus A. ochraceus, the MIC for the IC was 15.62 µg mL−1 with 46.7% inhibition, whereas the MIC observed in the presence of the EOC was 62.5 µg mL−1, with 50% inhibition.
Sales et al. [5], evaluating the antifungal activity of the EO from C. viminalis leaves, observed that the growth of F. oxysporum and B. cinerea fungi was inhibited at concentrations of 250 µg mL−1 and 100 µg mL−1, respectively. The antifungal activity of the IC of eugenol with β-CD on the mycelial growth of the fungi present in litchi was evaluated by Gong et al. [30]. The authors observed that mycelial growth was 92.6% inhibited by IC after 30 days of storage.
The β-CD was evaluated separately at the same concentration used in the IC, and no growth inhibitory activity against the fungi was observed. Munhuwey et al. [31] studied the effect of β-CD complexation with EOs from cinnamon and oregano and observed no antifungal activity against the growth of Botrytis sp. This observation was attributed to the action of β-CD as a source of carbohydrates for the fungus. The chemical structure of CDs (hydrophilic nature and volume) does not allow them to permeate biological membranes. ICs can release the guest molecule slowly, so any observed effect could only be attributed to the activity of the guest molecule and not to the CD molecule [32].
The components present in the EOs act on the fungal mycelium hyphae. Consequently, they cause loss of rigidity and integrity of the cell wall and the consequent loss of cytoplasmic components that result in cell death. More hydrophobic compounds such as EOs have a greater ability to interact and accumulate in the fungal hydrophobic cell membrane, which ultimately induces membrane destabilization. Thus, the cell membrane can be considered to be the main site of action of these molecules [32].
The antibacterial and antifungal activities of EOC can be attributed to the presence of monoterpenes such as 1,8-cineol, α-pinene and α-terpineol [15]. The intensity of activity of an EO depends on its origin, the environmental conditions and its composition [33]. The antimicrobial and antifungal activities of these compounds result from a combination of processes that cause disorders in pathogenic cells and, ultimately, their lysis. A very important stage in the mechanism of activity is its incorporation into the membrane, which leads to changes in integrity and permeability and causes the loss of cellular material [34].
Bioassay with Lactuca Sativa L
The results of the germination rate (%GR), germination speed index (GSI) and root growth (RG) of Lactuca sativa L. seeds are presented in Table 2.
The %GR and GSI results for the IC were significant when compared with those of the EOC. There was a reduction in EOC toxicity after complexation because L. sativa seeds germinated in the presence of the IC, unlike the positive control (EOC) for which no germination was observed. The IC presented 91% GR and 10.04 GSI, and this treatment did not differ statistically from the negative control (distilled water) (Fig. 2). Thus, the toxicity of the IC was lower than that of the EOC, and it can be considered to be an alternative of lower toxicity.
The RG of the lettuce seeds was directly affected by the EOC, in contrast to that observed with the IC that was statistically equal to water. The RG parameter is more effective in indicating the toxicity of the tested compound as it is directly affected by late germination, which is reflected in a lower GSI [4]. Oliveira et al. [8] studied the toxicity of EOC obtained from flowers on L. sativa seeds and observed that the toxicity of this EO increased with increasing concentration. These same authors reported that the EOC toxicity was due to the presence of the principal components 1,8-cineol (66.93%), α-pinene (16.0%) and α-terpineol (10.04%) or the chemical synergism between all the compounds present. In the present study, the principal components identified by gas chromatography coupled with mass spectrometry (GC/MS) were 1,8-cineol (67.7%), α-pinene (15.01%) and α-terpineol (7.72%), results similar to those found by Oliveira et al. [8]. This fact corroborates the results obtained for the EOC in this paper [15].
Bali et al. [35] studied the phytotoxicity of the leaf extract from C. viminalis on the germination and initial growth of rice (Oryza sativa L.). The study concluded that the extract from C. viminalis retarded the germination and growth of rice-associated weeds under laboratory and greenhouse conditions. Venceslau et al. [4] evaluated changes in atrazine toxicity when it was complexed with silica-anchored CDs. They used L. sativa seeds as a plant model and observed that free atrazine-induced genotoxic effects in the roots. The use of the IC between atrazine and γ-CD/silica considerably reduced atrazine's genotoxic effects, making it a viable option for reducing the toxicity of this herbicide in non-target plants.
Phospholipase Activity
Phospholipase activity (%) induced by Bothrops atrox snake venom previously incubated with EOC is shown in Fig. 3. The EOC previously incubated with venom (EOC + P) in the largest proportions (1:2.5, 1:5 and 1:10 venom/EOC m:m) lead to a significant 4% to 13% potentiation of the action of the phospholipases A2 present in the venom when compared to the positive control (venom). Rezende et al. [36] reported that the phospholipids that make up the membranes can be degraded by natural compounds, and they can interact with different compounds, resulting in the destabilization of the membranes and changes in the flow of liquids and ions across the membranes.
A significant inhibition of approximately 12.5% was observed for the IC in the smallest proportions (1:0.1; 1:0.5; 1:1 and 1:2.5 venom/IC m:m) and 23% inhibition of B. atrox venom-induced phospholipase activity was observed in the highest proportion (1:10 m:m). The action of phospholipases A2 present in the venom result in breakdown of cell membrane components causing lysis of red blood cells. In addition, the breakdown of membrane phospholipids results in the generation of arachidonic acid, which is a precursor of eicosanoids, acting mainly on the inflammatory response and blood coagulation process. Thus, the inhibition of phospholipases A2 by natural compounds highlights their potential use as anti-inflammatory and anticoagulant agents, which is of great importance in the medical-scientific context [37]. The inhibition of phospholipase activity caused by the IC probably occurred because β-CD trapped the EOC in its cavity, leaving it partially unavailable to interact with the venom, so the toxicity of the IC was lower.
Conclusion
The complexation between EOC and β-CD was proven to be a promising alternative for its use in pharmaceutical, food or even agricultural products. Antibacterial and antifungal tests have shown the potential of this complex for reducing the minimum inhibitory concentration required to inhibit bacterial and fungal growth, thus being an advantage over pure essential oil. So, with less concentration, the activity increased. Further studies regarding the concentrations to be used should be performed to obtain a safe margin for use. The toxicity tests with L. sativa L. seeds and the phospholipase tests revealed that, after formation of the inclusion complex, the EOC had a lower toxicity, demonstrating that the use of complexed EOC in β-CD can improve its safety. Thus, the results of this work support the application of EOC complexed with β-CD for use in different systems, such as prevention of food spoilage and plant pathogenicity.
References
Rosa RCA, Ribeiro LR, Souza AMG, Fonseca TA (2016) Triagem Fitoquímica dos Extratos Aquosos de Bauhinia candicans, Foeniculum vulgare, Mentha pulegium e Morus nigra. Phytochemical screening of aqueous extracts of Bauhinia candicans, Foeniculum vulgare, Mentha pulegium and Morus nigra. Revista Conexão Ciência 11(1):44–51
Bors M, Sicińska P, Michałowicz J, Wieteska P, Gulewicz K, Bukowska B (2012) Evaluation of the effect of Uncaria tomentosa extracts on the size and shape of human erythrocytes (in vitro). Environ Toxicol Pharmacol 33(2):127–134. https://doi.org/10.1016/j.etap.2011.11.003
Benigni R (2005) Structure-activity relationship studies of chemical mutagens and carcinogens: mechanistic investigations and prediction approaches. Chem Rev 105(5):1767–1800. https://doi.org/10.1021/cr030049y
Venceslau AFA et al (2018) Cyclodextrins as effective tools to reduce the toxicity of atrazine. Energy Ecol Environ 3(2):81–86. https://doi.org/10.1007/s40974-017-0073-8
Sales TA et al (2017) Essential oils from the leaves and flowers of Callistemon viminalis: chemical characterization and evaluation of the insecticide and antifungal activities. Am J Plant Sci 08(10):2516–2529. https://doi.org/10.4236/ajps.2017.810171
Aguiar UN et al (2014) Preparação e caracterização do complexo de inclusão do óleo essencial de Croton zehntneri com β-ciclodextrina. Quím Nova 37(1):50–55. https://doi.org/10.1590/S0100-40422014000100010
Iacovino R et al (2016) Cyclodextrins as complexing agents: preparation and applications. Curr Org Chem 21(2):162–176. https://doi.org/10.2174/1385272820666160909111842
de Oliveira CM et al (2014) Chemical composition and allelopathic activity of the essential oil from Callistemon viminalis (Myrtaceae) Blossoms on Lettuce (Lactuca sativa L.) Seedlings. Am J Plant Sci 05(24):3551–3557. https://doi.org/10.4236/ajps.2014.524371
Badawy MEI, Abdelgaleil SAM (2014) Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind Crops Prod 52:776–782. https://doi.org/10.1016/j.indcrop.2013.12.003
Fall R, Ngom S, Sall D, Sembène M, Samb A (2017) Chemical characterization of essential oil from the leaves of Callistemon viminalis (D.R.) and Melaleuca leucadendron (Linn.). Asian Pac J Trop Biomed 7(4):347–351. https://doi.org/10.1016/j.apjtb.2017.01.004
Caldas GFR et al (2016) Repeated-doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in Wistar rats. Food Chem Toxicol 97:297–306. https://doi.org/10.1016/j.fct.2016.09.020
Pires HC et al (2013) Composição química e atividade antimicrobiana dos óleos essenciais das folhas e flores de Callistemon viminalis (sol. ex Gaertn.) G. Don ex Loudon (Myrtaceae). Rev Ciências Farm Básica e Apl 34(4):597–601
Yadav R et al (2014) Differential larval toxicity and oviposition altering activity of some indigenous plant extracts against dengue and chikungunya vector Aedes albopictus. J Arthropod Borne Dis 8(2):174–185
Mello DR (2010) “Farmacopeia Brasileira,” Farm. Bras. 5a edição 2 1–523 [Online] Available: https://www.anvisa.gov.br
Martins LNSB (2018) Complexo de inclusão entre óleo essencial de Callistemon viminalis e β-ciclodextrina: preparação, caracterização, atividade antibacteriana, antifúngica e testes de toxicidade. PhD thesis, Universidade Federal de Lavras, Brasil
Menezes PP et al (2012) Solid-state β-cyclodextrin complexes containing geraniol. Thermochim Acta 548:45–50. https://doi.org/10.1016/j.tca.2012.08.023
Camargo KC et al (2017) Antimicrobial activity of the essential oil from Hyptis carpinifolia Benth. Am J Plant Sci 08(11):2871–2877. https://doi.org/10.4236/ajps.2017.811195
Clinical and Laboratory Standards Institute (CLSI) (2008) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, approved standard. CLSI document M38-A2, Pennsylvania
Andrade MA et al (2015) Biological activity of the essential oils from Cinnamodendron dinisii and Siparuna guianensis. Brazilian J Microbiol 46(1):189–194. https://doi.org/10.1590/S1517-838246120130683
Aragão FB et al (2015) Phytotoxic and cytotoxic effects of eucalyptus essential oil on lettuce (Lactuca sativa L.). Allelopath J 35(2):259–272
Gutiérrez J, Avila C, Rojas E, Cerdas L (1988) An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 26(4):411–413. https://doi.org/10.1016/0041-0101(88)90010-4
Price MF, Wilkinson ID, Gentry LO (1982) Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 20(1):7–14. https://doi.org/10.1080/00362178285380031
Rangel M, Malpezzi ELA, Susini SMM, De Freitas J (1997) hemolytic activity in extracts of the diatom Nitzschia. Toxicon 35(2):305–309. https://doi.org/10.1016/S0041-0101(96)00148-1
Martins LNSB, Venceslau AFA, Carvalho LB, Jaime C, Cardoso MG, Pinto LMA (2020) Inclusion complex of Callistemon viminalis essential oil prepared by kneading. J Incl Phenom Macrocycl Chem 97:109–119. https://doi.org/10.1007/s10847-020-00989-w
Salem MZM, Ali HM, El-Shanhorey NA, Abdel-Megeed A (2013) Evaluation of extracts and essential oil from Callistemon viminalis leaves: antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac J Trop Med. https://doi.org/10.1016/S1995-7645(13)60139-X
Hanci V, Vural A, Hanci SY, Ali Kiraz H, Ömür D, Ünver A (2014) In vitro evaluation of antimicrobial features of sugammadex. Brazilian J Anesthesiol Engl Ed 64(2):105–108. https://doi.org/10.1016/j.bjane.2013.09.003
Rakmai J, Cheirsilp B, Mejuto JC, Simal-Gándara J, Torrado-Agrasar A (2018) Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind Crops Prod 111:219–225. https://doi.org/10.1016/j.indcrop.2017.10.027
Antunes MD et al (2017) Antimicrobial electrospun ultrafine fibers from zein containing eucalyptus essential oil/cyclodextrin inclusion complex. Int J Biol Macromol 104:874–882. https://doi.org/10.1016/j.ijbiomac.2017.06.095
Santos EH, Kamimura JA, Hill LE, Gomes CL (2015) Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT - Food Sci Technol 60(1):583–592. https://doi.org/10.1016/j.lwt.2014.08.046
Gong L et al (2016) An inclusion complex of eugenol into β-cyclodextrin: preparation, and physicochemical and antifungal characterization. Food Chem 196:324–330. https://doi.org/10.1016/j.foodchem.2015.09.052
Munhuweyi K, Caleb OJ, van Reenen AJ, Opara UL (2018) Physical and antifungal properties of β-cyclodextrin microcapsules and nanofibre films containing cinnamon and oregano essential oils. LWT 87:413–422. https://doi.org/10.1016/J.LWT.2017.09.012
Kfoury M et al (2016) Solubility, photostability and antifungal activity of phenylpropanoids encapsulated in cyclodextrins. Food Chem 196:518–525. https://doi.org/10.1016/j.foodchem.2015.09.078
Calo JR, Crandall PG, O’Bryan CA, Ricke SC (2015) Essential oils as antimicrobials in food systems-a review. Food Control 54:111–119. https://doi.org/10.1016/j.foodcont.2014.12.040
Hąc-Wydro K, Szydło K (2016) The influence of environmentally friendly pesticide–Eucalyptol-alone and in combination with terpinen-4-ol—on model bacterial membranes. Colloids Surf B 146:918–923. https://doi.org/10.1016/j.colsurfb.2016.07.044
Bali AS, Batish DR, Singh HP, Kaur S, Kohli RK (2017) Phytotoxicity and weed management potential of leaf extracts of Callistemon viminalis against the weeds of rice. Acta Physiol Plant 39(1):1–9. https://doi.org/10.1007/s11738-016-2313-5
Rezende DACS et al (2017) Essential Oils from Mentha piperita, Cymbopogon citratus, Rosmarinus officinalis, Peumus boldus and Foeniculum vulgare: inhibition of phospholipase A2 and cytotoxicity to human erythrocytes. Am J Plant Sci 08(09):2196–2207. https://doi.org/10.4236/ajps.2017.89147
Nirmal N, Om Praba G, Velmurugan D (2008) Modeling studies on phospholipase A2-inhibitor complexes. Indian J Biochem Biophys 45(4):256
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Centro de Análise e Prospecção Química (CAPQ), the Laboratório de Produtos Naturais e Sintéticos, the Laboratório de Micotoxinas e Micologia de Alimentos of the Departamento de Ciência dos Alimentos and the Universidade Federal de Lavras for their financial support for the execution of this research. We also acknowledge Dr. D. L. Nelson for the English review of the paper.
Author information
Authors and Affiliations
Contributions
LNSBM: Conceptualization, Methodology, Validation, Investigation, Writing—original draft, Writing—review & editing. AFAV: Methodology, Investigation, Formal analysis. RMB: Methodology, Investigation, Formal analysis. MAB: Methodology, Investigation, Formal analysis. LRB: Conceptualization, Resources, Formal analysis. MGC: Resources, Methodology. LMAP: Conceptualization, Resources, Writing—original draft, Writing—review & editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest, and that they all agree with the submission of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, L.N.S.B., Venceslau, A.F.A., Brandão, R.M. et al. Antibacterial and Antifungal Activities and Toxicity of the Essential Oil from Callistemon viminalis Complexed with β-Cyclodextrin. Curr Microbiol 78, 2251–2258 (2021). https://doi.org/10.1007/s00284-021-02480-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02480-2