Abstract
Ectoine is widely produced by various bacteria as a natural cell protectant against environment stress, e.g., osmotic and temperature stress. Its protective properties therefore exhibit high commercial value, especially in agriculture, medicine, cosmetics, and biotechnology. Here, we successfully constructed an engineered Escherichia coli for the heterologous production of ectoine. Firstly, the ectABC genes from Halomonas elongata were introduced into E. coli MG1655 to produce ectoine without high osmolarity. Subsequently, lysA gene was deleted to weaken the competitive l-lysine biosynthesis pathway and ectoine bioconversion was further optimized, leading to an increase of ectoine titer by 16.85-fold. Finally, at the low cell density of 5 OD600/mL in Erlenmeyer flask, the concentration of extracellular ectoine was increased to 3.05 mg/mL. At the high cell density of 15 OD600/mL, 12.7 g/L of ectoine was achieved in 24 h and the overall yield is 1.27 g/g glycerol and sodium aspartate. Our study herein provides a feasible and valuable biosynthesis pathway of ectoine with a potential for large-scale industrial production using simple and cheap feedstocks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
(S)-2-methyl-1,4,5,6-tetrahydropyrimidine-4-carboxylic acid, commonly known as ectoine (Fig. 1), is a cyclic amino acid derivative of aspartate [1]. It is widely produced by various bacteria as a protective extremolyte against environment stress, e.g., extreme temperature, high osmolarity, and dryness. The commercial value of ectoine is manifold, including skin protection against aging and cell damage, skin preservation [2], and disease treatment such as Alzheimer's Disease [3], atopic dermatitis [4], colitis [5], allergic rhinitis [6], and lung inflammation [7]. Its current trading price is approximately $1300/kg, making ectoine a high-priced commodity in the world market [8].
Ectoine was first discovered in the halophilic phototrophic bacterium Ectothiorhodospira halochloris [9]. Subsequently, many halophilic and halotolerant bacteria from saline environments also produced ectoine and therefore might serve as a source for its production. However, the requirement for high salt during culturing complicated ectoine extraction and purification. A bioprocess technique called “bacterial milking” was developed using the halophilic bacterium Halomonas elongata, enabling ectoine production up to 7.4 g/L by H. elongata DSM 142 under salt stress [10]. Ectoine production by Halomonas salina DSM 5928 using fermentative strategies reached 6.9 g/L [11]. However, their fermentation periods were often too long, high salt medium accelerated equipment corrosion, as well as ectoine titer was relatively low. To avoid the disadvantages, heterologous expression of the ectoine biosynthetic cluster has been pursued [5, 12,13,14,15].
In this study, we designed a microbial cell factory by recruiting the ectoine biosynthesis gene ectABC from H. elongate. The ectABC from H. elongata were successfully expressed in E. coli MG1655. We further optimized ectoine biosynthesis by deleting the competitive l-lysine metabolic pathway and optimizing its whole-cell bioconversion. Finally, the engineered stain ECT2 accumulated 12.7 g/L of ectoine in 24 h.
Materials and Methods
Biological Materials, Bacterial Strains, and Media
Isopropyl-β-d-thiogalactoside (IPTG) and ampicillin were purchased from TIANGEN (China). The restriction QuickCut enzymes BamHI, EcoRI, DNA Ligation Kit, and PrimerSTAR Max polymerase were purchased from Takara (Takara Bio Co., Ltd., China). Acetonitrile (HPLC grade) and ectoine of 95% purity were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals were commercially obtained as analytical grade. The strain H. elongata (Marine Culture Collection of China, 1A01717) was grown in Luria–Bertani (LB) consisting of 1.0% (w/v) tryptone, 0.5% (w/v) yeast extract, and 1% (w/v) NaCl. E. coli MG1655 was purchased from BeiNuo (BeiNuo life science Co., Ltd., China), and plasmid pTrc99a was purchased from Wuhan Miaoling Bioscience and Technology Co. (China) with an ampicillin coding sequence for use as a cloning and expression vector.
Construction of Recombinant Expression Plasmids
The published genome sequence of H. elongata provided the ectoine biosynthetic cluster used for this study [16]. The individual ectABC gene cluster was amplified by PCR using PrimerSTAR Max DNA polymerase from genomic DNA of H. elongata as the template and custom synthesized DNA primers containing restriction sites (Table 1). The reaction was performed in a 50 μL reaction volume containing 1 μL of genomic DNA, 1 μL of primer F, 1 μL of primer R, 25 μL of PrimerSTAR Max Premix, and 22 μL of ddH2O using the following conditions: denaturation at 98 °C for 10 s, annealing at 55 °C for 15 s, and extension at 72 °C for 30 s followed by 30 cycles. The PCR products of the ectABC and pTrc99a vector were digested using a QuickCut EcoRI enzyme and QuickCut BamHI enzyme at 37 °C for 5 min. The products were then ligated into the plasmid pTrc99a by DNA Ligation enzyme at 16 °C for 4 h yielding pTrc99a-ectABC. The recombinant plasmids were chemically transformed into E. coli MG1655 at 37 °C on LB medium with ampicillin (100 μg/mL) for selection of positive recombinants. The recombinants were confirmed by restriction enzyme digestion at 37 °C for 30 min and subsequently sequenced (Sangon Biotech Co. Ltd., China).
Deletion of lysA Gene
Deletion of lysA gene was performed using the λ-Red recombination system according to the previously described method with slight modification [12]. The primers used for gene manipulation are shown in Table 1. For lysA gene deletions, the linear knockout chloramphenicol resistance gene was prepared by PCR using plasmid pKD3 as template. The reaction was performed in a 50 μL reaction volume containing 1 μL of plasmid pKD3, 1 μL of primer lysA-F, 1 μL of primer lysA-R, 25 μL of PrimerSTAR Max Premix, and 22 μL of ddH2O using the following conditions: denaturation at 98 °C for 10 s, annealing at 52 °C for 30 s, and extension at 72 °C for 30 s followed by 30 cycles. The linear gene knockout was electroporated into the E. coli MG1655 harboring pkD46. Cells were selected on chloramphenicol (30 μg/mL) plates at 37 °C for 24 h. The positive gene replacement with the antibiotic marker was selected by PCR verification.
Protein Expression of Ectoine Enzymes
The recombinant strain E. coli MG1655 was grown at 37 °C in LB broth with ampicillin (100 μg/mL). When the optical density of the culture at 600 nm reached 0.8, IPTG was added at a final concentration of 0.8 mM and incubated at 37 °C for 8 h. The cells were harvested by centrifugation (10,000×g, 3 min) and resuspended in a 50 mM of potassium phosphate solution. The supernatant was subjected to SDS-PAGE analysis [17].
Preparation of E. coli for Biocatalysis
The recombinant strain was grown on LB medium plates overnight at 37 °C. The recombinant strain was cultured in 50 mL LB medium in 250 mL Erlenmeyer flasks at 37 °C on a rotary shaker (200 rpm) for 12 h. The seed medium was inoculated into 1 L Erlenmeyer flasks containing 400 mL LB medium and grown at 37 °C until reaching an OD600 of 0.6–0.8. IPTG was added to a final concentration of 0.1 mM, and the culture was further incubated at 30 °C for 12 h. After fermentation, the fermentation broth was centrifuged (4500 rpm, 10 min) and the cells were washed twice with 0.9% NaCl solution and resuspended in PBS buffer as the whole-cell biocatalysts.
Optimization of Ectoine Bioconversion
The whole-cell transformation was carried out in a 250-mL flask with 30 mL of reaction PBS buffer containing resting cells (OD600 = 5). The reaction PBS buffer (pH 6.5) included 10 g/L sodium aspartate, 10 g/L KCl, and 10 g/L glycerol. The reaction was performed at 40 °C and on a rotary shaker (200 rpm) for 24 h. The reaction broth was then centrifuged at 12,000 rpm for 1 min and the liquid fraction was passed through a 0.45 μm filter. HPLC was used to analyze and identify the purity of ectoine. To enhance ectoine yield, various concentrations of sodium aspartate, KCl, and glycerol, as well as reaction temperature, time, and pH were further optimized and investigated the effect on ectoine production.
Ectoine bioconversion was also performed under high-density cells. The cells were washed twice with 0.9% NaCl solution and resuspended in PBS buffer (OD600 = 15) as the whole-cell biocatalysts. Sodium aspartate, KCl, and glycerol were then added into the cells until 24 h and the pH was maintained at 6.5 by NH4OH (2 M) or HCl (2 M) [15].
Ectoine Analysis
Ectoine production was measured according to a previously described method with slight modification [18]. Briefly, the cells were centrifuged at 4500 rpm for 10 min and then subjected to ultrasonic extraction in 8% lysozyme for 6 min. The resulting extracellular of ectoine solutions was analyzed and purified by HPLC (Agilent Technologies, 1260, USA) on Ultimate Polar RP-C18 column (250 × 4.6 mm, 5 μm particle size, Welch, China). Chromatograhy was carried out isocratically at a flow rate of 1 mL/min with 100% purified water. NMR experiments were conducted using a Bruker Ultrashield 500 NMR spectrometer with D2O as the solvent (referenced to residual D2O at δH 4.80) at 25 °C. LC–MS was acquired using a Waters 2695 HPLC coupled to a Thermo LCQ TM Deca XP plus ion trap mass spectrometer in the negative ionization.
Results and Discussion
Heterologous Expression of Ectoine Biosynthesis Genes in E. coli MG1655
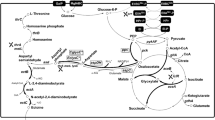
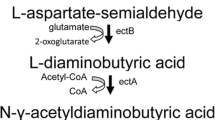
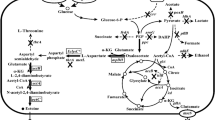
The biosynthesis of ectoine proceeds in three steps. Initially, precursor l-aspartate-β-semialdehyde (ASA) is converted to the diamine l-2,4-diaminobutyrate (DABA) by l-2,4-diaminobutyrate transaminase (EctB). DABA is next acetylated by catalysis with 2,4-diaminobutyrate acetyltransferase (EctA) to generate N-acetyl-2,4-diaminobutyrate (ADABA). Finally, ectoine synthase (EctC) catalyzes the final ring-closing reaction [12].
In this study, the ectABC genes from H. elongata were amplified from genomic DNA and cloned into the vector pTrc99a. Subsequently, the recombinant plasmids pTrc99a-ectABC were successfully constructed in E. coli MG1655 to obtain engineered strain E. coli ECT1. The transformants harboring recombinant plasmids were induced with IPTG, and SDS-PAGE electrophoresis of lysates of induced cells revealed proteins with molecular masses closely corresponding to the predicted sizes of EctA, EctB, and EctC (Fig. 2). Molecular masses of EctA, EctB, and EctC were predicted to be 21.2 kDa, 41.8 kDa, and 15.2 kDa [16].
Elimination of l-lysine Synthesis Pathway
In E. coli, l-aspartate-β-semialdehyde is the precursor for both l-lysine synthesis and ectoine synthesis [12]. Thus, an adequate supply of l-aspartate-β-semialdehyde precursor for ectoine biosynthesis was expectedly obtained following the elimination of l-lysine synthesis pathway [1]. Here, we knocked out lysA of E. coli ECT1 to increase the supply of l-aspartate-β-semialdehyde for ectoine biosynthesis, establishing the engineered strain E. coli ECT2 (△lysA). The strains E. coli ECT1 and E. coli ECT2 were cultured in LB medium for 12 h individually. Finally, the extracellular ectoine titer of strain ECT1 was 178 mg/L, while the ectoine titer of strain ECT2 was 380 mg/L. Compared with ECT1, extracellular ectoine titer of ECT2 (△lysA) increased by 134%.
Optimization of Ectoine Transformation Conditions
To determine the optimal conditions of ectoine biosynthesis by E. coli ECT2, we further investigated some key factors affecting ectoine biosynthesis. Sodium aspartate is a key starting substrate for synthesis of l-aspartate-β-semialdehyde, with glycerol providing both energy and substrate for the production of an acetyl group. KCl has previously been reported to improve the activity and stability of ectB [15]. Thus, Sodium aspartate, glycerol, and KCl as important nutrient factors are firstly investigated for ectoine production in whole-cell biocatalytic experiment. Furthermore, temperature, pH, and fermentation time are also assessed. The effects of sodium aspartate, KCl, and glycerol on ectoine production are shown in Fig. 3a–c. The optimal concentration of sodium aspartate is 10 g/L and 2.3 mg/mL of ectoine is detected at this concentration. Under higher concentrations of sodium aspartate (20–80 g/L), ectoine titer shows a dose-dependent reduction, with the minimum of 0.41 mg/mL. The maximum ectoine production reaches 2.6 mg/mL with the addition of KCl under the concentration of 10 g/L. The maximal ectoine titer of 2.4 mg/mL is obtained under 10 g/L of glycerol. A pH = 6.5 was determined to be optimal, with 2.4 mg/mL of ectoine production (Fig. 3d). As for culture time, we observed an optimal 2.4 mg/mL of ectoine at 24 h (Fig. 3e). The effect of temperature is investigated between 25 and 45 °C (Fig. 3f). The results show that the highest titer is achieved at 40 °C. In conclusion, the optimal ectoine transformation conditions in Erlenmeyer flask are determined as follows: 10 g/L sodium aspartate, 10 g/L KCl, and 10 g/L glycerol, pH 6.5, 24 h, 40 °C.
Effect of ectoine bioconversion conditions on ectoine production in E. coli ECT2. The bar charts show the effects of varying. a Effects of sodium aspartate on the biosynthesis of ectoine. b Effects of KCl on the biosynthesis of ectoine. c Effects of glycerol on the biosynthesis of ectoine. d Effects of pH on the biosynthesis of ectoine. e Effects of time on the biosynthesis of ectoine. f Effects of temperature on the biosynthesis of ectoine. An initial cell density of OD600 = 5 was used for each experiment
Production of Ectoine with High-Density Cells from the Engineered Strain E. coli ECT2
Ectoine bioconversion is also investigated with higher-density cells (OD600 = 15). When the reaction proceeds for 20 h, the sodium asparate and glycerol are all consumed. After a reaction time of 24 h, the ectoine titer reaches 12.7 g/L in the medium (Fig. 4). Compared with flask reactions with low-density cells (OD600 = 5), the ectoine bioconversion is obviously improved in the high-density cells (OD600 = 15).
Identification of Ectoine from the Engineered Strain E. coli ECT2
The ectoine was determined by HPLC, MS, and NMR. HPLC is used to identify the retention time of ectoine (tR = 3.7 min, Fig. 5). The molecular formula\ is determined to C6H10N2O2 using HRESIMS (m/z [141.0674]−) (Fig. 6). 1H NMR (500 MHz, D2O) δH: 4.05 (t, J = 5.5 Hz, 1H), 3.43 (dt, J = 13.6, 5.3 Hz, 1H), 3.30–3.23 (m, 1H), 2.21 (s, 3H), and 2.17–2.04 (m, 2H) (Fig. 7).
Conclusions
Whole-cell biocatalysis has numerous advantages for the production of commercially used organic compounds, including being environmentally friendly, low production costs, and time efficiency. A good example is l-methionine, which has attracted attention for its nutritional, pharmaceutical, and clinical applications. While chemical synthesis of methionine requires more hazardous and volatile chemicals and produces a racemate [19], the engineered strain E. coli is constructed and successfully used to produce l-methionine [20]. Ectoine can be also chemically synthesized, but stereoselectivity is a great obstacle and the process lacks an efficient biocatalysis method [21, 22]. Hence, heterologous expression represents a desirable strategy for ectoine production. A report established that 25.1 g/L of ectoine is synthesized by E. coli with glucose as a carbon source after 30 h fermentation [12]; the overall titer is 0.11 g/g of glucose. Chen et al. [23] showed that ectoine biosynthesis by H. salina BCRC17875 under salt stress can reach 13.96 g/L with yeast extract and NH4SO4 as carbon and nitrogen sources at 44 h of cultivation; the overall yield is 0.17 g/g of yeast extract. In our experiment, transgenic E. coli can efficiently synthesize and excrete 12.7 g/L of ectoine into the extracellular medium with glycerol and sodium aspartate as carbon sources at 24 h; the overall yield is 1.27 g/g of glycerol and sodium aspartate. The results demonstrated that our engineered strain exhibited more productivity or less cost using glycerol and sodium aspartate as bioconversion feedstocks. This study further demonstrates the promise and utility of whole-cell biocatalysis for commercial production of organic compounds.
In agreement with previous study of Ning et al. [12], we find that eliminating the L-lysine synthesis pathway is a key step in maximizing ectoine production. This single modification raised production in our strain E. coli ECT2 by 134%. This result suggests that modification of other metabolic branches might also benefit ectoine synthesis, but this remains to be investigated. The system presented here represents a simple method for the efficient production of high-purity ectoine using whole-cell biocatalysis. Strain ECT2 produced 12.7 g/L of ectoine by whole-cell biocatalysis production system, which is sufficient for commercial production. Further large fermentor experiments using high cell densities (OD600 = 50) are warranted to test the true commercial potential of this whole-cell biocatalysis production system.
References
Becker J, Schäfer R, Kohlstedt M, Harder BJ, Borchert NS, Stöveken N et al (2013) Systems metabolic engineering of Corynebacterium glutamicum for production of the chemical chaperone ectoine. Microb Cell Fact 12:110–126
Mamalis A, Nguyen DH, Brody N, Jagdeo J (2013) The active natural anti-oxidant properties of chamomile, milk thistle, and halophilic bacterial components in human skin in vitro. J Drugs Dermatol 12(7):780–784
Kanapathipillai M, Ku SH, Girigoswami K, Park CB (2008) Small stress molecules inhibit aggregation and neurotoxicity of prion peptide 106–126. Biochem Biophys Res Commun 365:808–813
Marini A, Reinelt K, Krutmann J, Bilstein A (2014) Ectoine-containing cream in the treatment of mild to moderate atopic dermatitis: a randomised, comparator-controlled, intra-individual double-blind, multi-center trial. Skin Pharmacol Physiol 27:57–65
Chen W, Zhang S, Jiang P, Yao J, He Y, Chen L et al (2015) Design of an ectoine-responsive AraC mutant and its application in metabolic engineering of ectoine biosynthesis. Metab Eng 30:149–155
Werkhäuser N, Bilstein A, Sonnemann U (2014) Treatment of allergic rhinitis with ectoine containing nasal spray and eye drops in comparison with azelastine containing nasal spray and eye drops or with cromoglycic acid containing nasal spray. J Allergy 2014:176597
Unfried K, Krämer U, Sydlik U, Autengruber A, Bilstein A, Stolz S et al (2016) Reduction of neutrophilic lung inflammation by inhalation of the compatible solute ectoine: a randomized trial with elderly individuals. Int J Chronic Obstruct Pulm Dis 11:2573–2583
Strong PJ, Xie S, Clarke WP (2015) Methane as a resource: can the methanotrophs add value? Environ Sci Technol 49:4001–4018
Galinski EA, Pfeiffer HP, Trueper HG (1985) 1, 4, 5, 6- tetrahydro-2-methyl-4-pyrimidinecarboxylic-acid a novel cyclic amino-acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem 149:135–140
Sauer T, Galinski EA (1998) Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol Bioeng 57(3):306–313
Zhang L, Lang YJ, Nagata S (2009) Efficient production of ectoine using ectoine-excreting strain. Extremophiles 13(4):717–724
Ning YK, Wu XJ, Zhang CL, Xu QY, Chen N, Xie XX (2016) Pathway construction and metabolic engineering for fermentative production of ectoine in Escherichia coli. Metab Eng 36:10–18
Bestvater T, Louis P, Galinski EA (2008) Heterologous ectoine production in Escherichia coli: by-passing the metabolic bottle-neck. Saline Syst 4:12–28
Tanimura K, Matsumoto T, Nakayama H, Tanaka T, Kondo A (2016) Improvement of ectoine productivity by using sugar transporter-overexpressing Halomonas elongata. Enzyme Microb Technol 89:63–68
He YZ, Gong J, Yu HY, Tao Y, Zhang S, Dong ZY (2015) High production of ectoine from aspartate and glycerol by use of whole-cell biocatalysis in recombinant Escherichia coli. Microb Cell Fact 14:55–65
Grammann K, Volke A, Kunte HJ (2581T) New type of osmoregulated solute transporter identified in halophilic members of the Bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581T. J Bacteriol 184:3078–3085
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:153–162
Chen J, Chen JW, Wang SJ, Zhou GM, Chen DQ, Zhang HW et al (2019) Development and validation of polar RP-HPLC method for screening for ectoine high-yield strains in marine bacteria with green chemistry. Nat Prod Res 33(8):1122–1126
Willke T (2014) Methionine production-a critical review. Appl Microbiol Biotechnol 98:9893–9914
Huang JF, Liu ZQ, Jin LQ, Tang XL, Shen ZY, Yin HH et al (2016) Metabolic engineering of Escherichia coli for microbial prdoduction of L-methionine. Biotechnol Bioeng 114:843–851
Himdikabbab S, Lavrador K, Bazureau JP, Hamelin J (1995) Synthesis of 1,4,5,6-tetrahydro 2-methyl 4-pyrimidine carboxylic-acid-osmoprotector amino-acid. Synth Commun 25:2223–2227
Pastor JM, Salvador M, Argandona M, Bernal V, Reina-bueno M, Csonka LN et al (2010) Ectoine in cell stress protection: uses and biotechnological production. Biotechnol Adv 28:782–801
Chen WC, Hsu CC, Lan CW, Chang YK, Wei YH (2018) Production and characterization of ectoine using a moderately halophilic strain Halomonas salina BCRC17875. J Biosci Bioeng 125(5):578–584
Acknowledgements
This research work was supported by National Natural Science Foundation of China (Nos. 81773628, and 41776139), National Key R & D Program of China (Nos. 2017YFE0103100 and 2018YFC0311003), and 111-National Overseas Expertise Introduction Center for Green Pharmaceutical Discipline Innovation (No. D17012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Liu, P., Chu, X. et al. Metabolic Pathway Construction and Optimization of Escherichia coli for High-Level Ectoine Production. Curr Microbiol 77, 1412–1418 (2020). https://doi.org/10.1007/s00284-020-01888-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-01888-6