Abstract
Type IV pili (Tfp) are widely distributed adhesins of bacterial surfaces. In plant pathogenic bacteria, Tfp are involved in host colonization and pathogenesis. Xanthomonas citri subsp. citri (Xcc) is the phytopathogen responsible for citrus canker disease. In this work, three Tfp structural genes, fimA, fimA1, and pilA from Xcc were studied. A pilA mutant strain from Xcc (XccΔpilA) was constructed and differences in physiological features, such as motilities, adhesion, and biofilm formation, were observed. A structural study of the purified Tfp fractions from Xcc wild-type and Xcc∆pilA showed that pilins are glycosylated in both strains and that FimA and FimA1 are the main structural components of the pili. Furthermore, smaller lesion symptoms and reduced bacterial growth were produced by Xcc∆pilA in orange plants compared to the wild-type strain. These results indicate that the minor pilin-like gene, pilA, is involved in Tfp performance during the infection process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adhesion of pathogenic bacteria to eukaryotic cells is an essential step in the successful colonization of host tissue. The bacterial surface structures involved in adhesion include a broad group of fimbrial and non-fimbrial adhesins. Fimbrial adhesins, also known as pili, are hair-like appendages found on the surface of many bacteria. There are several types of pili, which differ in their mechanisms of assembly, structure, and function. Type IV pili (Tfp) are proteinaceous, flexible filaments with a diameter of 5–8 nm, which extend up to several micrometers in length and are generally located at one or both poles of a cell. These filaments are mainly composed of thousands copies of a small protein subunit named pilin, in most cases known as PilA [20]. Among fimbrial adhesins, Tfp are found in both gram-negative bacteria such as Neisseria spp., Pseudomonas aeruginosa, and enteropathogenic Escherichia coli, and in gram-positive bacteria such as Clostridium and Streptococcus species [20]. Tfp have been shown to be involved not only in bacterial adherence to eukaryotic cells but also in a variety of other bacterial functions such as natural transformation competence and a form of flagella-independent cell translocation known as twitching motility. In addition, twitching can be important for autoaggregation, biofilm formation, and multicellular development bodies. This motility is also required for host colonization and pathogenesis process, including the activation of host cell responses. These features make Tfp one of the most studied pili and an interesting target for pathogens inhibition [5].
The study of Tfp and its role in pathogenesis was focused mainly on animal pathogenic bacteria. However, the role of Tfp in the virulence process of plant pathogenic bacteria is poorly understood. In fact, contributions of this structure to virulence have been reported mainly in vascular plant pathogens. A substantial role of Tfp in virulence was demonstrated for the pathogen Ralstonia solanacearum [25]. Besides, evidences of Tfp contribution to host colonization were reported for Xylella fastidiosa [32] and for Pseudomonas syringae pv. tabaci [51].
Xanthomonas citri subsp. citri (Xcc) is the phytopathogen responsible for citrus canker, one of the most devastating citrus diseases [4, 23]. The complete genomes of some plant pathogenic Xanthomonads have been sequenced [9, 30, 37, 52] and several genes involved in the virulence process were detected. Previous studies determined the role of many structures and proteins of X. citri subsp. citri in the virulence process [14, 16, 22, 53]. In addition, a plant natriuretic peptide [21], a characteristic lipopolysaccharide [7, 41], and the presence of a higher number of photoreceptors [27, 28] only present in Xcc genome were also evaluated.
Tfp contribution to virulence was studied in some Xanthomonas species. As a result, an important role of Tfp during the pathogenesis process was found in several cases, including Xanthomonas campestris pv. campestris, Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, and X. citri subsp. citri [10, 15, 35, 54].
In this study, three genes related to the structural subunit of Tfp in Xcc genome, fimA, fimA1, and pilA were identified. In order to determine the importance of PilA in bacterial physiology and its involvement in plant colonization during citrus canker, an Xcc mutant strain defective in pilA gene (Xcc∆pilA) was constructed. Also, an analysis of the glycosylation profile and peptide composition of pilin proteins from the wild-type and the mutant strains was performed.
Materials and Methods
Bacterial Strains, Culture Conditions, and Media
X. citri subsp. citri (Hasse) strain used in this work was derivative of the strain Xcc 99–1330, which was kindly provided by Blanca I. Canteros (INTA Bella Vista, Argentina).
Xcc strains were grown aerobically at 28 °C with shaking at 200 rpm in Silva Buddenhagen (SB) medium [11] or in the minimal medium XVM2 [55] supplemented with the corresponding antibiotics. E. coli cells were aerobically cultivated at 37 °C in Luria–Bertani medium. Antibiotics were used at the following final concentrations: ampicillin, 100 μg/ml for E. coli and 25 μg/ml for Xcc; kanamycin, 40 μg/ml; and streptomycin, 50 μg/ml. For Xcc∆pilA(pBBad22K)-complemented strain growth, SB medium was supplemented with 1 % (w/v) L-arabinose.
Recombinant DNA and Microbiological Techniques
All DNA manipulations including plasmid purification, restriction enzyme digestion, DNA ligation, and agarose gel electrophoresis were performed with standard techniques [44] unless otherwise specified. Total bacterial genomic DNA was isolated by the cetyltrimethylammonium bromide procedure [38]. Plasmids for bacterial conjugations were transferred to Xcc by biparental mating from the broad host range-mobilizing E. coli strain S17-1 [47]. Bacterial mixtures were spotted onto Hybond-C membranes, placed on SB agar and incubated for 48 h at 28 °C. Membranes were then washed and bacteria transferred to a selective medium as previously described [14].
Construction of the XccΔpilA Mutant Strain
The XccΔpilA mutant was constructed by insertional inactivation of the pilA gene (XAC3805, accession number AAM38647) on the chromosome due to the introduction of a streptomycin/spectinomycin resistance cassette as previously described by Kraiselburd et al. [27]. Primers used in this work are listed in Online Resource 1. Mutants were verified by PCR using the specific primers pilAF/R located on the coding region of the gene fragment. For mutant complementation, a 405-bp DNA fragment containing the pilA-coding region was amplified using the primer pair pilAF3/pilAR. The amplified DNA fragment was then cloned into the broad host range L-arabinose-inducible expression vector pBBad22K [50] to generate the recombinant plasmid pBBad/pilA. This plasmid was transferred into the XccΔpilA mutant strain by conjugation, producing XccΔpilA(pBBad22 K) strain.
Quantification of Exopolysaccharide Production
Exopolysaccharide (EPS) production of Xcc strains was quantified as previously described by Petrocelli et al. [41].
Bacterial Motility Assays
Swimming and swarming assays were performed as previously described by Petrocelli et al. [41].
Twitching motility was analyzed as described by Kraiselburd et al. [46]. The zone of motility was visualized macroscopically and the morphology of colony edge was visualized microscopically by an optic Olympus BX40 microscope equipped with an Olympus camera system.
Bacterial Adhesion to Abiotic and Biotic Surfaces
For bacterial adherence to a plastic surface and to leaf surfaces, cultures of Xcc wild-type, XccΔpilA and XccΔpilA(pBBad22 K) were grown overnight in liquid XVM2 medium and the cell attachment was evaluated as previously described by Petrocelli et al. [41].
Biofilm Formation Assay
In order to study biofilm formation, 20 µl of each saturated bacterial culture (109 colony-forming units (CFU)/ml) was transferred to glass tubes containing 2 ml of fresh medium, and then statically incubated at 28 °C. Bacterial aggregates were visually examined after 3 and 6 days of incubation. At each time, the tubes were incubated at 60 °C during 20 min. After the medium removal, bacteria were washed with sterile water and stained with a solution of 0.1 % (w/v) crystal violet (CV) for 45 min. Finally, the CV dye was solubilized by the addition of 95 % (v/v) ethanol and quantified by measuring absorbance at 540 nm [18].
Pili Extraction from Xcc wt and XccΔpilA
Bacterial strains were streaked on freshly poured XVM2 plates and incubated at 28 °C for 24 h. Bacteria were washed off in a small volume of distilled water, and the cell suspension was forced through a 25-gage hypodermic needle five times. Bacteria were removed by centrifugation (twice for 30 min, 23,300 g). The pili were collected by ultracentrifugation (twice for 60 min, 136,000 g), and the two pellets were separately suspended in 50 µl of 1 × SDS loading buffer (10 % (v/v) glycerol, 0.02 % (w/v) bromophenol blue, 25 µM DTT, 5 µM EDTA, and 2 % (w/v) SDS) [25].
Glycoprotein Digestion
Samples obtained from pili extraction were applied onto a 12 % SDS-PAGE. The protein bands were reduced with 10 mM DTT, further washed with acetonitrile and alkylated with 55 mM IAA in 50 mM NH4HCO3. The gel slices were digested in 20 ng/µl trypsin (Sigma) at 37 °C overnight [40]. Peptides were extracted by sonication with 50 % (v/v) acetonitrile in 1 % (v/v) trifluoroacetic acid (TFA), and the supernatant was taken to dryness.
In Gel-Reductive β-Elimination
The gel band was treated with 0.05 M NaOH/1 M NaBH4 (0.5 ml) at 50 °C during 16 h. The solution was separated, acetic acid was added until pH 7 followed by repeated evaporation with methanol. The sample was dissolved in water, desalted in a Dowex 50 W (H+) (Fluka) column, and dried in speed vac.
Acid Hydrolysis and Analysis of Monosaccharide Composition by HPAEC-PAD
A sample of the trypsin-released material was hydrolyzed with 2 N TFA for 2 h at 100 °C and sugars were analyzed by HPAEC-PAD in a DX-500 Dionex BioLC system (Dionex Corp.) with a pulse amperometric detector. The following conditions were employed: (a) for monosaccharides a carbopack P-20 column equipped with a P-20 precolumn eluted with a 16 mM NaOH isocratic program and a flow rate of 0.5 ml/min. (b) for oligosaccharides, a carbopack P-200 column equipped with a P-200 precolumn using a gradient elution with 100 mM NaOH, 0–29 % sodium acetate for 29 min and a flow rate of 0.4 ml/min.
Mass Spectrometry Analysis
Matrix and calibrating chemicals were purchased from Sigma-Aldrich. Measurements were performed using an Ultraflex II TOF/TOF mass spectrometer equipped with a high-performance solid-state laser (λ 355 nm) and a reflector. The system is operated by the Flexcontrol version 2.4 software packages (Bruker Daltonics GmbH, Bremen, Germany). Samples were irradiated with a laser power of 25–50 % and measured in the linear and the reflectron modes, in positive and negative ion modes. For tandem time-of-flight LIFT mode, 8.0 kV as ion source voltage was used with a precursor ion mass window of 3 Da.
The glycopeptides mixtures obtained after trypsin digestion were purified according to Selman et al. [45]. The enriched glycopeptides were resuspended in 50 % (v/v) acetonitrile in 1 % (v/v) formic acid. The samples were loaded onto an AnchorChip target (Bruker Daltonics GmbH) with α-cyano-4-hydroxycinnamic acid as matrix.
External calibration reagents used were (commercial proteins bradykinin 1–7, Mr 757.399; angiotensin I, Mr 1296.685; renin substrate, Mr 1758.933; and insulin β chain, Mr 3494.6506), β-cyclodextrin (cycloheptaamylose, Mr 1135.0), and γ-cyclodextrin (cyclooctaamylose, Mr 1297.1) with α-cyano-4-hydroxycinnamic acid as matrix in the positive ion mode.
Plant Material and Plant Inoculations
Orange (Citrus sinensis cv. Valencia late) was used as host plant for Xcc. All plants were grown in a growth chamber in incandescent light at 25 °C with a photoperiod of 14 h. Overnight cultures of Xcc strains were diluted in 10 mM MgCl2 to a final concentration of 105 CFU/ml. Plant inoculation and in planta growth assays were performed as previously described by Petrocelli et al. [41].
Statistical Analysis
Data were subjected to a bifactorial ANOVA test for adhesion and biofilm formation and to a unifactorial ANOVA test for EPS production. Tukey’s multiple comparison tests with residual analysis and validation were also used in both cases. The analysis of growth curves in planta was performed using a mixed non-linear regression model with residual analysis and validation. Each experiment was repeated at least three times to ensure the reproducibility and consistency of the results.
Results
Construction of pilA Mutant from Xcc
Xcc genome sequence revealed the presence of three pilus-related genes: pilA (XAC3805) and fimA/A1 (XAC3240-XAC3241) that encode pilin-like proteins. A detailed in silico analysis of these genes and of the secondary structure of pilin proteins was performed and described in Online Resource 2. In addition, phylogenetic analysis of genes that encode pilin proteins of Xanthomonas, Xylella, and Stenotrophomonas genus revealed a clear distinction between pilA and fimA genes, clustering on different clades (Online Resource 3).
A pilA mutant strain from Xcc (Xcc∆pilA) was generated by insertional inactivation of the gene due to the introduction of a streptomycin/spectinomycin resistance cassette through a double crossover. Growth of liquid cultures in SB medium was spectrophotometrically monitored by optical density at 600 nm (OD600). No differences in growth curves were observed between Xcc wt and Xcc∆pilA after a 48 h period (Online Resource 4).
Xcc∆pilA Exhibits Different Motility Patterns Compared to Xcc wt
In this work, we evaluated the effect of the pilA mutation on bacterial flagella-dependent motilities by performing swimming and swarming assays on 0.3 and 0.7 % (w/v) SB agar plates, respectively. As shown in Fig. 1a, swimming motility capability was reduced for Xcc∆pilA mutant compared to Xcc wt, recovering the migration ability in the complemented strain Xcc∆pilA(pBBad22 K). When swarming was analyzed, higher migration diameters were observed for Xcc wt compared to Xcc∆pilA, showing that the colony border appearance presented some differences (Fig. 1b). Xcc∆pilA(pBBad22 K) partially recovered the migration capacity of the Xcc wt. We also observed that Xcc∆pilA colonies presented a significantly lower production of EPS (P < 0.05) than the wt and complemented strains (Table 1).
Bacterial motility assays. The different strains were centrally inoculated on SB plates supplemented with 0.3 % (w/v) agar for swimming (a) and 0.7 % (w/v) agar for swarming (b) assay and incubated 72 h at 28 °C. For twitching motility assay, Xcc strains were stab-inoculated with a sterile toothpick through SB 1 % (w/v) agar plates to the bottom of the Petri dish. c Macroscopic and d microscopic analysis of colony borders morphology after incubation at 28 °C for 24 h. An optic Olympus BX40 microscope equipped with an Olympus camera system was used in d
Although no differences in the migration capability were observed in 1 % (w/v) SB agar plates (which, in fact, are conditions commonly used for twitching motility evaluation), Xcc wt colony borders presented a particular pattern similar to the bacterial rafts described for some twitching-performing bacteria (Fig. 1c). The assessment of twitching motility by colony morphology is commonly used in plant pathogenic bacteria [2, 25]. On the other hand, XccΔpilA colony shapes were round and showed smooth borders without bacterial extensions radiating in these growth conditions (Fig. 1c). In addition, a bacterial disorganization on the edge of the twitching zones for Xcc wt was observed when a microscopic analysis was performed. In contrast, pilA mutant presented a smooth border and no displacement of bacteria from the main body of the colony (Fig. 1d).
Bacterial Adhesion and Biofilm Formation Decrease in the Xcc∆pilA
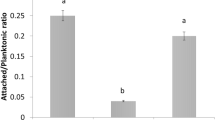
When the effect of pilA mutation on the attachment to abiotic and biotic surfaces was studied, the mutant strain presented a significantly reduced adhesion capacity to a plastic surface and to the abaxial face of orange leaves compared to Xcc wt and Xcc∆pilA(pBBad22 K) (P < 0.05) (Fig. 2a, b).
Bacterial adhesion to abiotic and biotic surfaces. a Bacterial adhesion on plastic (polystyrene microtiter plate) surface of Xcc wt (WT), Xcc∆pilA (ΔpilA) and Xcc∆pilA(pBBad22 K) (cΔpilA) strains grown in XVM2 medium. b Bacterial adhesion on abaxial orange leaf surfaces. Histograms represent spectrophotometric quantifications of CV attached to the different surfaces (Abs 540 nm). Data are expressed as means ± standard errors of three independent experiments. Statistically significant differences are identified by lowercase letters (P < 0.05). c Biofilm formation on glass tubes. Xcc strains were statically grown on glass tubes at 28 °C. The adhered cells to the glass surface after 3 and 6 days of incubation were stained with CV. The resulting CV bound to these cells was quantified by measuring absorbance at 540 nm and represented on the histogram. Data are expressed as means ± standard errors of three independent experiments. Statistically significant differences are identified by lowercase letters (P < 0.05) at each time
In order to evaluate the aggregation of bacterial cells of Xcc wt, Xcc∆pilA, and Xcc∆pilA(pBBad22 K), biofilm formation was assayed on glass tubes containing SB liquid medium. After 3 and 6 days of static incubation at 28 °C, Xcc∆pilA showed significantly lower cell aggregates and adhesion on the air–liquid interface than Xcc wt. An intermediate behavior was observed for Xcc∆pilA(pBBad22 K). These results were corroborated by CV staining and quantification (Fig. 2c).
Deletion of pilA Gene from Xcc Modifies the Levels of Pilin Glycosylation
The in silico analysis of the genes related to the structural subunit of Tfp from Xcc indicated that fimA/A1 and pilA genes present differences in their nucleotides composition, which is manifested in minor variations in the composition of their respective proteins in comparison to other bacteria from Xanthomonas genus. Although these differences are small, it allows separating the X. citri of X. oryzae and X. campestris clades with accuracy (Online resource 3).
To get some insight into the Xcc Tfp structure, partially purified Tfp fractions from the wt and the mutant strains were obtained. Interestingly, the SDS-PAGE profiles of these fractions showed that in both strains only one low migrating band was strongly stained with periodic acid–Schiff, indicating the glycoprotein nature of this component (Online Resource 5). In order to confirm this fact, the sugar components of the trypsin-released peptide mixture from Xcc wt and the mutant strains were hydrolyzed with 2 N TFA and subjected to HPAEC-PAD (Fig. 3a, b). Both samples presented glucose as the main component and mannose as the minor one but in different relationships. The peak with retention time 3.2 min may correspond to a substituted monosaccharide.
HPAEC-PAD analysis of the monosaccharide composition of Tfp from Xcc strains. Mixtures of the sugar components of the trypsin-released peptides from Xcc wt (a) and Xcc∆pilA (b) were totally hydrolyzed with 2 N trifluoroacetic acid and subjected to HPAEC-PAD under optimal conditions for neutral and amino sugars. c MALDI-TOF mass spectrum of the component migrating as oligosaccharides from figures (a) and (b). d HPAEC-PAD analysis of the oligosaccharides released by reductive β-elimination from the gel band corresponding to Tfp from Xcc wt
Although peptide hydrolysis was almost complete, a peak with a bigger retention time than the expected for a monosaccharide was detected. Therefore, it was collected and further analyzed by MALDI-TOF mass spectrometry in the positive reflectron mode (Fig. 3c). Signal at m/z 680.2 (calc. m/z 681.2448, C25H45O21) corresponded to a tetrasaccharide bearing a methyl substituent as [M + H]+. In accordance, ions at m/z 622.8 (calc. m/z621.8) and m/z 562.7 (calc. m/z 563.7) could be ascribed to 0,2A1 and 2,5X1 fragments, respectively [13]. Furthermore, ion at m/z 505.6 (calc. m/z 505.1763, C18H33O16) matched the same structure after the loss of a methylated hexose unit and signal at m/z 447.5 (calc. m/z 447.2) agrees with the corresponding 0,2A1fragment. To obtain more details about the oligosaccharide structure of both Tfp, gel bands corresponding to Xcc wt and XccΔpilA were subjected to a reductive β-elimination. The released oligosaccharides were analyzed by HPAEC-PAD. As expected, peaks with retention times similar to reduced maltose, maltotriose, and maltotetraose were detected in both strains indicating that oligosaccharides composed of 2–4 hexose units were present in both samples (Fig. 3d).
A MALDI-TOF mass spectrometry analysis of the trypsin-digested fractions was further performed in the positive reflectron mode (Fig. 4). No differences were observed between the spectra of the total peptide fraction (not shown) and the glycopeptide fraction obtained after an enrichment step using HILIC-SPE chromatography, indicating that Xcc wt and Xcc∆pilA pilins are highly glycosylated. Taking into account the known sequences of FimA, FimA1, and PilA, we were able to ascribe signals to different glycopeptides in the spectra of both samples (Fig. 4a, b). The highest ion detected in both strains corresponded to m/z 832.80 (calc. m/z 832.38) and to peptide 116–120 (calc. m/z 646.3671) substituted with a hexose unit as [M + Na]+. The fact that FimA1 and FimA share the same peptide (LTWAR or ITWAR) would account for the high intensity of this ion. Regarding FimA, signal at m/z 1475.57 (calc. m/z 1475.76) corresponded to peptide 39–52 (calc. m/z 1313.7060, 39SQVAAGLAEVSPGK52) substituted with a hexose unit and ion at m/z 1651.87 (calc. m/z 1651081) corresponded to the same peptide bearing another monomethyl hexose unit; ion at m/z 2282.23 (calc. m/z 2282.07) matched peptide 121–139 (m/z 2119.0124, 121DANGIWTCSTDLPLVDKDR139) substituted with a hexose unit and signal at m/z 3153.41 (calc. m/z 3153.52) was ascribed to peptide 55-82 (m/z 2828.4160, 55YEILVNDSEGSTLTSASAIGLTATATSR82) bearing two hexose units.
MALDI-TOF mass spectra obtained after trypsin digestion. The glycopeptide fractions obtained from Xcc wt (a) and Xcc∆pilA (b) were trypsin-digested and enriched using HILIC-SPE chromatography before the MALDI-TOF mass spectrometry analysis was performed. Insets correspond to expanded range from m/z 1400 to 2100 Da. c The highest ion detected in the MALDI-TOF mass spectrometry analysis for both strains (m/z 832.80) was selected as precursor ion and subjected to laser-induced LID-MS/MS analysis. Inset corresponds to expanded range from m/z 100 to 620 Da
On the other hand, other signals corresponding to FimA1 could be detected. Thus, ion at m/z 1519.48 (calc. m/z 1519.79) was ascribed to peptide 37–50 (m/z 1357.7322, 37SQVTAGLAELSPGK50) linked to a hexose unit; ion at m/z 1973.97 (calc. m/z 1973.85) matched peptide 121–137 (m/z 1797.7960, 121DTNGVWTCSTDIATADK137) bearing a monomethyl hexose; a signal at m/z 1996.97 corresponded to peptide 121–139 (m/z 1996.9280, 121DTNGVWTCSTDIATADKAK139) and a signal at m/z 2164.30 (calc. m/z 2164.06) corresponded to the sodium adduct of peptide 62–81 (m/z 1979.0040, 62GTTLTTADAVGLQATSTTNR81) bearing a hexose unit. In order to ensure the presence of the glycosidic substitution, ion at m/z 832.80 was selected as precursor ion and subjected to laser-induced LID-MS/MS analysis in the MALDI-TOF/TOF MS/MS instrument (Fig. 4c). The spectrum showed a main ion at m/z 646.1 (calc. m/z 646.37) corresponding to the deglycosylated peptide 116–120. In addition, ions at m/z 634.8 and m/z 618.1 correspond to b4 and b4-H2O fragments, respectively, bearing a hexose unit. In addition, ions at m/z 581.3 and 564.2 correspond to c3 and b3 fragments, respectively, linked to a hexose unit. Furthermore, m/z 490.2 corresponds to c4 and m/z 472.3 to b4 fragments.
Xcc∆pilA Develops Less Aggressive Disease Symptoms in Citrus Plants
In order to determine the effect of pilA mutation of Xcc on the development of citrus canker symptoms, orange leaves were infiltrated with 105 CFU/ml bacterial inoculums of Xcc wt, Xcc∆pilA, and Xcc∆pilA(pBBad22 K) strains and the phenotypes developed were analyzed at different days post-inoculation (dpi). The lesion symptoms were smaller and less humid after 7 days for Xcc∆pilA when compared to Xcc wt. After 10 days, the mutant produced a reduced number of cankers in comparison to the wt strain and this difference was maintained after 15 days of infection (Fig. 5a). The differences in leaf damage may be associated with the bacterial growths inside the host tissue. The bacterial number of Xcc∆pilA recovered from the infected leaves at 20 dpi was significantly lower compared to Xcc wt and the complemented strain (Fig. 5b), indicating an association between pilA and the infection process.
Effect of pilA disruption on pathogenicity of Xcc in host plants a Progression of symptoms from the infiltration of 105 CFU/ml inoculums of Xcc wt, Xcc∆pilA, and Xcc∆pilA(pBBad22 K) strains on abaxial faces of leaves is shown. The leaves were inoculated with 10 mM MgCl2 as control (mock). Representative leaves after 7, 10, and 15 dpi are shown. Scale bars, 1 cm. b Growth curves on plant tissues were performed inoculating the leaves with 105 CFU/ml of Xcc strains. Data are expressed as means ± standard errors of three independent experiments. Statistically significant differences are identified by lowercase letters (P < 0.05)
Discussion
At present, few studies have assessed Type IV pili participation in pathogenesis processes and host colonization in bacterial plant pathogens [3]. Type IV pili functionality from Xanthomonas citri subsp. citri was recently evaluated [15]. The in silico analysis of Xcc genome revealed the presence of pilA and fimA/A1 as structural components of the Tfp located in different loci in this bacterium. In addition, a great similarity was found between Tfp proteins from Xcc and from different species from the Xanthomonadaceae family, indicating that these proteins are important parts of the pili structure and/or biogenesis in this bacterium. A phylogenetic analysis of these genes revealed that pilA and fimA/A1 belong to two distinct groups in all the bacteria analyzed. The differences in the phylogeny observed for each Xanthomonas species could be associated with the particular process of bacterial colonization that characterized each phytopathogen during the interaction with its host plant [6].
In this work, we have constructed a mutant strain in the pilA gene from X. citri subsp. citri, named Xcc∆pilA, in order to get insight into its role during citrus canker. Several reports showed that bacterial motility capability is affected in Tfp mutants in different phytopathogens [2, 39, 51]. In our work, we observed that pilA mutant from Xcc presented reduced migration during swimming and swarming motilities. It has been previously demonstrated that Xcc swarming motility depends on EPS secretion [22]. Accordingly, in this work, a minor production of exopolysaccharide was observed in pilA mutant, indicating that the lack of this gene affects these features in Xcc. A similar result was observed for a Myxococcus xanthus pilA mutant, resulting in a decrease of exopolysaccharide production [57].
It is well known that, in many animal and plant pathogenic bacteria, Tfp are essential for the flagella-independent twitching motility [2, 25, 26, 48]. The difference in the morphology of the colony borders and the bacterial organization between Xcc wt and Xcc∆pilA in the twitching zones indicates a reduced twitching capability for the mutant strain. Likewise, twitching-negative mutants with smooth colony borders were reported for X. fastidiosa [32]. Additionally, it was recently shown that fimA1, pilB, pilZ, pilX, and fimX mutants from X. citri subsp. citri had reduced subsurface twitching motility when compared to the wild-type strain [15]. Kuchma and colleagues postulated that other components of Type IV pili, named minor pilins, are required for swarming motility in P. aeruginosa [29]. These pilin-like proteins have conserved N-terminal sequences and are thought to be essential to pilus assembly and twitching motility in this bacterium [19]. In this context, PilA from X. citri subsp. citri could be incorporated into the Tfp and the variations on surface motilities observed for the pilA mutant could be explained by a role of this protein in the assembly of Tfp.
In addition to Tfp-dependent twitching motility, bacterial adhesion and biofilm formation have been described as important features for plant colonization [22, 36, 39]. In particular, the attachment to host tissue by cell surface adhesins and Type IV pili is an essential early event during the virulence process of several plant pathogens [36]. Tfp from P. aeruginosa and P. syringae pv. tabaci have been shown to induce bacterial adhesion and aggregation as well as twitching motility, achieving mature biofilms [19, 51]. Biofilm formation in Xcc appears as a complex and regulated process that depends on adhesion, EPS production, flagella-dependent motility and Tfp, as described in several reports [31, 33, 37, 58]. In this work, we observed a reduced adhesion capacity, differences in biofilm formation and reduced twitching capability for the mutant strain confirming that PilA from Xcc participates in the modulation of these features.
Besides, HPAEC-PAD together with MALDI-TOF MS analysis showed that Xcc Tfp are highly glycosylated. Glucose and mannose are the main components of the glycosidic portions of Tfp present in both strains. The presence of similar monosaccharide residues was detected in other bacterial pathogens [20]. It is known that protein glycosylation in bacterial pathogens influences interactions with their host. In Neisseria spp., Tfp glycosylation [1] was involved in host cell invasion [24]. Pilins of both N. meningitides and N. gonorrhoeae are glycosylated at a Ser amino acid by the attachment of a short oligosaccharide of up to three sugar residues in length [1]. Moreover, it was found that a glycan moiety, composed of a trisaccharide (N-hydroxybutyryl-N-formyl-Pse-xylose-N-acetylglucosamine), attached to the C-terminal Ser residue of PilA protein from P. aeruginosa 1244 was probably implicated in twitching motility and mouse colonization [8, 12, 49]. Protein glycosylation systems are also present in plant pathogens, such as R. solanacearum. It was demonstrated that Type IV pilins from this bacterium are glycosylated with the oligosaccharide HexNAc-(Pen)-dHex3 that have the same structure of an O antigen subunit lipopolysaccharide. In R. solanacearum, the lack of glycosylation affected biofilm formation and virulence in tomato plant infections [17]. Further analysis performed in our work revealed the presence of a tetrasaccharide with a methyl substituent as the oligosaccharide present in Tfp from Xcc in contrast to the Tfp glycosidic portions found in the other bacteria analyzed. Despite these differences, the sugar substitution of pilin proteins from Xcc could be related to the physiology and the virulence of this bacterium.
Moreover, glycopeptides involving FimA and FimA1 sequences were detected. In the case of pilin subunits, this post-translational modification contributes to the structural complexity and functional diversification of Tfp [20]. In a recent report, Dunger et al. [15] suggested that fimA1 is the main pilin gene of Tfp from Xcc expressed in planta, whereas fimA and pilA genes did not change their expression levels. Nevertheless, the results obtained from the glycosylation analysis provide the first experimental evidence that both FimA and FimA1 proteins are structural components of Tfp.
Type IV pili are involved in the early step of host tissue colonization during bacterial pathogenesis; however, the specific role is still unclear [5, 34]. Different results were described in relation to the participation of this structure in the host infectivity. Tfp from Acidovorax avenae ssp. citrulli and P. syringae pv. tomato DC3000 promote the colonization of their respective host plants [2, 5, 42, 51]. In P. syringae pv. tabaci 6605, Tfp were implicated not only in the host invasion but also in the hypersensitive reaction in non-host Arabidopsis thaliana leaves [51]. Mutants of R. solanacearum in Tfp-related genes, deficient in twitching motility, presented reduced autoaggregation and biofilm formation, lack of attachment ability to tobacco cells and to tomato roots and, they also produced less severe symptoms in tomato when compared to the wild-type strain [25]. Besides, P. syringae pv. phaseolicola wild-type and a pilA mutant strains inoculated into the host plant caused disease symptoms. However, only the wild-type strain produced halo blight disease in bean when the less invasive spraying method was used [43].
Although Xanthomonas genus includes several phytopathogen species, the contribution of Tfp to virulence has been studied only in few cases. Tfp mutants of the vascular pathogen X. oryzae pv. oryzae, unable to move by twitching, were deficient in biofilm formation and presented reduced virulence [10]. In addition to a reduced virulence, mutants in the FimX and PilZ homologue of this bacterium presented a diminished hypersensitive response in non-host plants [56]. Furthermore, mutants defective in pilA gene, coding for the structural unit of Tfp from X. campestris pv. campestris showed an impaired surface motility and virulence [35].
A significant contribution of Tfp to virulence was shown for the non-vascular pathogen X. oryzae pv. oryzicola [54] and recently, a possible role of Tfp from Xcc in the pathogenesis process has been reported [15]. In our work, Xcc pilA mutant presented reduced plant colonization ability compared to the wild-type strain, which agrees with the results obtained for other Tfp mutants in different plant pathogens, supporting its role during citrus canker disease.
The contribution of PilA to surface motilities, adhesion, biofilm formation, and virulence indicates a functional role of this protein in Tfp from Xcc, corroborating the involvement of this structure in the infection process during citrus canker.
References
Aas FE, Vik A, Vedde J, Koomey M, Egge-Jacobsen W (2007) Neisseria gonorrhoeae O-linked pilin glycosylation: functional analyses define both the biosynthetic pathway and glycan structure. Mol Microbiol 65:607–624
Bahar O, Goffer T, Burdman S (2009) Type IV Pili are required for virulence, twitching motility, and biofilm formation of Acidovorax avenae subsp. citrulli. Mol Plant Microbe Interact 22:909–920
Berry JL, Pelicic V (2015) Exceptionally widespread nanomachines composed of type IV pilins: the prokaryotic Swiss Army knives. FEMS Microbiol Rev 39:1–21
Brunings AM, Gabriel DW (2003) Xanthomonas citri: breaking de surface. Mol Plant Pathol 4:141–157
Burdman S, Bahar O, Parker JK, De La Fuente L (2011) Involvement of Type IV pili in pathogenicity of plant pathogenic bacteria. Genes 2:706–735
Buttner D, Bonas U (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol Rev 34:107–133
Casabuono A, Petrocelli S, Ottado J, Orellano EG, Couto AS (2011) Structural analysis and involvement in plant innate immunity of Xanthomonas axonopodis pv. citri lipopolysaccharide. J Biol Chem 286:25628–25643
Castric P, Cassels FJ, Carlson RW (2001) Structural characterization of the Pseudomonas aeruginosa 1244 pilin glycan. J Biol Chem 276:26479–26485
Da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, Quaggio RB, Monteiro-Vitorello CB, Van Sluys MA, Almeida NF, Alves LM et al (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459–463
Das A, Rangaraj N, Sonti RV (2009) Multiple adhesin-like functions of Xanthomonas oryzae pv. oryzae are involved in promoting leaf attachment, entry, and virulence on rice. Mol Plant Microbe Interact 22:73–85
Daurelio LD, Romero MS, Petrocelli S, Merelo P, Cortadi AA, Talon M, Tadeo FR, Orellano EG (2013) Characterization of Citrus sinensis transcription factors closely associated with the non-host response to Xanthomonas campestris pv. vesicatoria. J Plant Physiol 170:934–942
DiGiandomenico A, Matewish MJ, Bisaillon A, Stehle JR, Lam JS, Castric P (2002) Glycosylation of Pseudomonas aeruginosa 1244 pilin: glycan substrate specificity. Mol Microbiol 46:519–530
Domon B, Costello CE (1988) A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj J 5:397–409
Dunger G, Arabolaza LN, Gottig N, Orellano EG, Ottado J (2005) Participation of Xanthomonas axonopodis pv. citri hrp cluster in citrus canker and in non-host plants responses. Plant Pathol 54:781–788
Dunger G, Guzzo CR, Andrade MO, Jones JB, Farah CS (2014) Xanthomonas citri subsp. citri type IV pilus is required for twitching motility, biofilm development, and adherence. Mol Plant Microbe Interact 27:1132–1147
Dunger G, Relling VM, Tondo ML, Barreras M, Ielpi L, Orellano EG, Ottado J (2007) Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Arch Microbiol 188:127–135
Elhenawy W, Scott NE, Tondo ML, Orellano EG, Foster LJ, Feldman MF (2016) Protein O-linked glycosylation in the plant pathogen Ralstonia solanacearum. Glycobiology 26:301–311
Friedman L, Kolter R (2004) Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690
Giltner CL, Habash M, Burrows LL (2010) Pseudomonas aeruginosa minor pilins are incorporated into type IV pili. J Mol Biol 398:444–461
Giltner CL, Nguyen Y, Burrows LL (2012) Type IV pilin proteins: versatile molecular modules. Microbiol Mol Biol Rev 76:740–772
Gottig N, Garavaglia BS, Daurelio LD, Valentine A, Gehring C, Orellano EG, Ottado J (2008) Xanthomonas axonopodis pv. citri uses a plant natriuretic peptide-like protein to modify host homeostasis. Proc Natl Acad Sci USA 105:18631–18636
Gottig N, Garavaglia BS, Garofalo CG, Orellano EG, Ottado J (2009) A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for citrus canker, is involved in bacterial virulence. PLoS One 4:e4358
Graham JH, Gottwald TR, Cubero J, Achor DS (2004) Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol Plant Pathol 5:1–15
Jennings MP, Jen FE, Roddam LF, Apicella MA, Edwards JL (2011) Neisseria gonorrhoeae pilin glycan contributes to CR3 activation during challenge of primary cervical epithelial cells. Cell Microbiol 13:885–896
Kang Y, Liu H, Genin S, Schell MA, Denny TP (2002) Ralstonia solanacearum requires type 4 pili to adhere to multiple surfaces and for natural transformation and virulence. Mol Microbiol 46:427–437
Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T (2003) Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol 48:1511–1524
Kraiselburd I, Alet AI, Tondo ML, Petrocelli S, Daurelio LD, Monzon J, Ruiz OA, Losi A, Orellano EG (2012) A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization. PLoS One 7:e38226
Kraiselburd I, Daurelio LD, Tondo ML, Merelo P, Cortadi AA, Talon M, Tadeo FR, Orellano EG (2013) The LOV protein of Xanthomonas citri subsp. citri plays a significant role in the counteraction of plant immune responses during citrus canker. PLoS One 8:e80930
Kuchma SL, Griffin EF, O’Toole GA (2012) Minor pilins of the type IV pilus system participate in the negative regulation of swarming motility. J Bacteriol 194:5388–5403
Lee BM, Park YJ, Park DS, Kang HW, Kim JG, Song ES, Park IC, Yoon UH, Hahn JH, Koo BS et al (2005) The genome sequence of Xanthomonas oryzae pathovar oryzae KACC10331, the bacterial blight pathogen of rice. Nucleic Acids Res 33:577–586
Li J, Wang N (2011) Genome-wide mutagenesis of Xanthomonas axonopodis pv. citri reveals novel genetic determinants and regulation mechanisms of biofilm formation. PLoS One 6:e21804
Li Y, Hao G, Galvani CD, Meng Y, De La Fuente L, Hoch HC, Burr TJ (2007) Type I and type IV pili of Xylella fastidiosa affect twitching motility, biofilm formation and cell-cell aggregation. Microbiology 153:719–726
Malamud F, Homem RA, Conforte VP, Yaryura PM, Castagnaro AP, Marano MR, do Amaral AM, Vojnov AA (2013) Identification and characterization of biofilm formation-defective mutants of Xanthomonas citri subsp. citri. Microbiology 159:1911–1919
Mattick JS (2002) Type IV pili and twitching motility. Annu Rev Microbiol 56:289–314
McCarthy Y, Ryan RP, O’Donovan K, He YQ, Jiang BL, Feng JX, Tang JL, Dow JM (2008) The role of PilZ domain proteins in the virulence of Xanthomonas campestris pv. campestris. Mol Plant Pathol 9:819–824
Mhedbi-Hajri N, Darrasse A, Pigne S, Durand K, Fouteau S, Barbe V, Manceau C, Lemaire C, Jacques MA (2011) Sensing and adhesion are adaptive functions in the plant pathogenic xanthomonads. BMC Evol Biol 11:67
Moreira LM, Almeida NF Jr, Potnis N, Digiampietri LA, Adi SS, Bortolossi JC, Da Silva AC, da Silva AM, de Moraes FE, de Oliveira JC et al (2010) Novel insights into the genomic basis of citrus canker based on the genome sequences of two strains of Xanthomonas fuscans subsp. aurantifolii. BMC Genom 11:238
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Nguyen LC, Taguchi F, Tran QM, Naito K, Yamamoto M, Ohnishi-Kameyama M, Ono H, Yoshida M, Chiku K, Ishii T et al (2012) Type IV pilin is glycosylated in Pseudomonas syringae pv. tabaci 6605 and is required for surface motility and virulence. Mol Plant Pathol 13:764–774
Parente J, Casabuono A, Ferrari MC, Paggi RA, De Castro RE, Couto AS, Gimenez MI (2014) A rhomboid protease gene deletion affects a novel oligosaccharide N-linked to the S-layer glycoprotein of Haloferax volcanii. J Biol Chem 289:11304–11317
Petrocelli S, Tondo ML, Daurelio LD, Orellano EG (2012) Modifications of Xanthomonas axonopodis pv. citri lipopolysaccharide affect the basal response and the virulence process during citrus canker. PLoS One 7:e40051
Roine E, Raineri DM, Romantschuk M, Wilson M, Nunn DN (1998) Characterization of type IV pilus genes in Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact 11:1048–1056
Romantschuk M, Bamford DH (1986) The causal agent of halo blight in bean, Pseudomonas syringae pv., attaches to stomata via its pili. Microb Pathog 1:139–148
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Selman MHJ, Hemayatkar M, Deelder AM, Wuhrer M (2011) Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem 83:2492–2499
Semmler AB, Whitchurch CB, Mattick JS (1999) A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology 145:2863–2873
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat Biotechnol 1:784–791
Siri MI, Sanabria A, Boucher C, Pianzzola MJ (2014) New type IV pili-related genes involved in early stages of Ralstonia solanacearum potato infection. Mol Plant Microbe Interact 27:712–724
Smedley JG III, Jewell E, Roguskie J, Horzempa J, Syboldt A, Stolz DB, Castric P (2005) Influence of pilin glycosylation on Pseudomonas aeruginosa 1244 pilus function. Infect Immun 73:7922–7931
Sukchawalit R, Vattanaviboon P, Sallabhan R, Mongkolsuk S (1999) Construction and characterization of regulated L-arabinose-inducible broad host range expression vectors in Xanthomonas. FEMS Microbiol Lett 181:217–223
Taguchi F, Ichinose Y (2011) Role of type IV pili in virulence of Pseudomonas syringae pv. tabaci 6605: correlation of motility, multidrug resistance, and HR-inducing activity on a nonhost plant. Mol Plant Microbe Interact 24:1001–1011
Thieme F, Koebnik R, Bekel T, Berger C, Boch J, Buttner D, Caldana C, Gaigalat L, Goesmann A, Kay S et al (2005) Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol 187:7254–7266
Tondo ML, Petrocelli S, Ottado J, Orellano EG (2010) The monofunctional catalase KatE of Xanthomonas axonopodis pv. citri is required for full virulence in citrus plants. PLoS One 5:e10803
Wang L, Makino S, Subedee A, Bogdanove AJ (2007) Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational analysis. Appl Environ Microbiol 73:8023–8027
Wengelnik K, Bonas U (1996) HrpXv, an AraC-type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria. J Bacteriol 178:3462–3469
Yang F, Tian F, Li X, Fan S, Chen H, Wu M, Yang CH, He C (2014) The degenerate EAL-GGDEF domain protein Filp functions as a cyclic di-GMP receptor and specifically interacts with the PilZ-domain protein PXO_02715 to regulate virulence in Xanthomonas oryzae pv. oryzae. Mol Plant Microbe Interact 27:578–589
Yang Z, Lux R, Hu W, Hu C, Shi W (2010) PilA localization affects extracellular polysaccharide production and fruiting body formation in Myxococcus xanthus. Mol Microbiol 76:1500–1513
Zimaro T, Thomas L, Marondedze C, Garavaglia BS, Gehring C, Ottado J, Gottig N (2013) Insights into Xanthomonas axonopodis pv. citri biofilm through proteomics. BMC Microbiol 13:186
Acknowledgments
We thank Catalina Anderson (INTA Concordia, Argentina), Gastón Alanis and Rubén Díaz Vélez (Proyecto El Alambrado) for the citrus plants, Sebastián Graziati for plant material support, the English Department (Facultad de Ciencias Bioquímicas y Farmacéuticas, UNR) for their assistance in the language revision of the manuscript and Biochemists Juan José Ivancovich and Hebe Bottai from Statistics and Data Processing Area (FBIOyF, UNR) for their assistance with statistical analysis. This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica [ANPCyT PICT 2010-1762] to Elena G. Orellano and [ANPCyT PICT 2013-0736] to Alicia S. Couto.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Petrocelli, S., Arana, M.R., Cabrini, M.N. et al. Deletion of pilA, a Minor Pilin-Like Gene, from Xanthomonas citri subsp. citri Influences Bacterial Physiology and Pathogenesis. Curr Microbiol 73, 904–914 (2016). https://doi.org/10.1007/s00284-016-1138-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-016-1138-1