Abstract
In Pseudomonas aeruginosa, quorum sensing (QS) autoinducer known as acyl homoserine lactone (AHL) acts as a key regulator in the expression of pathogenic characters. In this work, the efficiency of phenylacetic acid (PAA) in reducing the production of AHL-dependent factors in P. aeruginosa PAO1 was studied. PAA at a concentration of 200 μg ml−1 displayed significant reduction in QS-dependent pyocyanin, exopolysaccharide, and protease and elastase production in PAO1. In swimming inhibition assay, PAA-treated PAO1 cells exhibited poor motility in swimming agar plate. In in vivo analysis, PAO1-preinfected Caenorhabditis elegans showed enhanced survival when treated with PAA. PAA at the QS inhibitory concentration showed no growth inhibitory activity on PAO1. Results of the present study revealed the potential of PAA as antipathogenic compound to prevent QS-dependent pathogenicity of P. aeruginosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quorum sensing (QS) is the autoinducer-dependent cell to cell communication system which regulates the expression of phenotypic characters in a wide range of bacterial organisms [10, 11]. The Gram-positive bacteria utilize their small peptide molecules as autoinducers, while Gram-negative bacteria respond to acyl homoserine lactone (AHL) autoinducer molecules. Among these two autoinducer systems, the AHL-based autoinducer system is widely studied, and so far about 70 bacterial species are known to utilize this QS system for the regulation of their phenotypic expressions [3, 11, 29]. It is also well demonstrated that the AHL-based QS system is responsible for the expression of virulence factors production in many Gram-negative bacterial pathogens [3, 29].
Pseudomonas aeruginosa is a well-known opportunistic human pathogen known to cause nosocomial infections, urinary tract infections, bloodstream infections, pneumonia, and burn wound infections. It is also known for its association in chronic infections of the respiratory pathways including cystic fibrosis, diffuse panbronchiolitis, and bronchiectasia [3]. P. aeruginosa requires the production of lytic enzymes like protease and elastase, pyocyanin pigment, exopolysaccharide (EPS), and motility for its survival and pathogenicity [1, 3, 6, 15, 28]. It has been well stated that the above said factors in P. aeruginosa are under the control of AHL-based QS system. There are two different AHL molecules, such as N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), known to regulate the pathogenicity in P. aeruginosa. These two AHL molecules upon binding to their respective receptor known as LasR and RhlR trigger the expression of pathogenic phenotypes [3, 23]. Consequently, targeting these QS system by means of interference with AHLs will be a suitable alternative to abolish emerging P. aeruginosa infections.
Phenylacetic acid (PAA), also known as α-toluic acid or benzene acetic acid has various biological properties and has drawn the attention of pharmaceutical industry. PAA is released as a common by-product when Penicillin G acylase acts on the side chains of penicillin G, cephalosporin G, and related antibiotics [4]. PAA and its derivatives have been isolated from microbes like Streptomyces humidus [13] and S. malachitofuscus [25], and plants like Ilex aquifolium [19] and are known to possess antifungal [13, 25], antioxidant [19], and anti-inflammatory properties [2]. Though PAA is known to possess a range of therapeutic properties, the role of this compound in reducing the production of QS-dependent factors in bacterial pathogens has not yet been studied. Hence, in this present work, an effort was made to study the potential of PAA in inhibiting the production of QS-dependent factors in P. aeruginosa both in vitro and in vivo.
Materials and Methods
Compound Preparation
The test compound PAA was purchased from Central Drug House (CDH), India. The stock solution was maintained at the concentration of 10 mg of PAA in 1 ml of 96 % ethanol and stored in 4 °C. Further, from the stock solution, working solution (1 mg ml−1) was prepared in sterile MilliQ water.
Bacterial Strains and Growth Condition
Pseudomonas aeruginosa PAO1 was used as a target pathogen and cultured aerobically in Luria-Bertani (LB) broth (pH 7.2) under 150 rev min−1 agitation in a rotatory shaker at 37 °C for overnight. For experimental analysis, PAO1 was subcultured in the same medium to reach a final OD of 0.4 at 600 nm.
Minimum Inhibitory Concentration (MIC)
The MIC of PAA against PAO1 was determined as per Clinical and Laboratory Standards Institute guidelines [5]. The PAO1 at aforementioned cell density was added to 1 ml of LB broth supplemented with the twofold serially diluted test compound to yield final concentrations ranging from 50 to 800 μg ml−1 and incubated at 37 °C for 24 h. Further, the growth rate of PAO1 cultivated in the absence and the presence of PAA was observed for every in vitro assay by measuring the cell density spectrophotometrically at OD600 after 18 h incubation using UV–Visible spectrophotometer (HITACHI U-2800, Japan).
Pyocyanin Quantification Assay
The test compound PAA at 200 μg ml−1 concentration was added in 5 ml of LB broth containing 1 % (50 μl) of PAO1 culture and incubated at 37 °C for a minimum of 18 h. After incubation, the cell-free supernatants of PAO1 cultivated in the presence and the absence of PAA were extracted with 3 ml of chloroform and then re-extracted into 1 ml of 0.2 N HCl to get a pink to deep red solution [7]. The absorbance of the solution was measured spectrophotometrically at OD520.
Quantification of EPS
PAO1 cells were allowed to form biofilm in cover glass (1 × 1 cm) in the presence and the absence of PAA (200 μg ml−1) in 24-well micro titer plate (MTP) at 37 °C, and EPS quantification was carried out by total carbohydrate assay [8]. In brief, cover glasses were washed in 0.9 % NaCl (0.5 ml) and incubated in an equal volume of 0.5 ml of 5 % phenol and 5 volumes of concentrated H2SO4. The mixture was incubated for 1 h in dark and absorbance was measured at OD490.
Protease and Elastase Assay
For protease and elastase assay, the PAA at a final concentration of 200 μg ml−1 was added in 2 ml of LB broth inoculated with 1 % (20 μl) of PAO1 culture (0.4 OD at 600 nm). PAO1 cells without the treatment of PAA were maintained as control. The culture set-up was incubated at 37 °C for a minimum of 18 h. After incubation, the protease activity was determined by an azocasein assay [14]. In brief, 100 µl of cell-free supernatant of PAA-treated and -untreated PAO1 was separately mixed with 1,000 µl of 0.3 % azocasein substrate (Sigma, St. Louis, USA) in 0.05 M Tris–HCl and 0.5 mM CaCl2 (pH 7.5), and incubated at 37 °C for 15 min. Ten percentage (0.5 ml) of ice-cold trichloroacetic acid was added to stop the reaction. After centrifugation at 10,000 rpm for 10 min, the absorbance of clear supernatant was measured spectrophotometrically at OD400. The elastolytic activity was determined by following the method of Ohman et al. [21] using Elastin Congo Red (ECR) (Sigma, St. Louis, USA) as the substrate. In brief, 100 µl of treated and untreated PAO1 culture supernatant was added into 900 µl of ECR buffer (100 mM Tris and 1 mM CaCl2) (pH 7.5) containing 20 mg of ECR and incubated with shaking at 37 °C for 3 h. The reaction was stopped by adding 1,000 µl of 0.7 M sodium phosphate buffer (pH 6.0). The tubes were placed in an ice water bath for 15 min and centrifuged to remove insoluble ECR. The absorbance of the supernatant was measured at OD495.
Swimming Assay
The swimming motility was assessed as described previously [20]. Ten microliters of PAO1 was point inoculated at the center of the swimming agar medium containing 1 % (w/v) tryptone, 0.5 % NaCl, and 0.3 % agar along with PAA at a final concentration of 50 μg ml−1. Swim agar plate without the addition of PAA was maintained as control. The plates were incubated at 37 °C in upright position for the period of 16 h.
In Vivo Assessment with C. elegans
Young adult worms were maintained and obtained by the earlier method of Sivamaruthi et al. [26]. The young adult animals were infected with PAO1 for 12 h at 25 °C in the wells of 24-well MTP. After incubation, the worms from the wells were washed thrice with M9 buffer (KH2PO4—3 g, Na2HPO4—6 g, NaCl—5 g, 1 M MgSO4—1 ml, and Distilled water—1,000 ml) to remove surface-bound bacterial cells. Around ten infected worms were transferred to the wells of MTP containing 10 % of LB broth in M9 buffer along with Escherichia coli OP50 and incubated without or with PAA at 200 μg ml−1 concentration. Each assay was carried out in triplicate; the plate was incubated at 25 °C and scored for live and dead worms in every 12 h for 4 days. A control set consisting of uninfected C. elegans with PAA alone was maintained to assess the toxicity of test compounds on C. elegans, if any. The survival of C. elegans was scored by following the previous methods [16, 26].
Statistical Analysis
All the experiments were performed in triplicates to validate reproducibility and the p values were calculated statistically by Student’s t test.
Results
Effect on Pyocyanin Production
The MIC of PAA was found to be 800 μg ml−1; thus, the test compound at its sub-MIC concentration (200 μg ml−1) was selected for the assessment of anti-quorum sensing (anti-QS) activity. In order to analyze the efficiency of the PAA to reduce QS-dependent pyocyanin production, the PAO1 cells were cultivated in the presence and the absence of test compound. A significant decrease in pyocyanin production of PAO1 was observed to the level of 87 % after treatment with PAA (Fig. 1).
Effect of PAA on pyocyanin, EPS, and protease and elastase production in P. aeruginosa PAO1. The data are represented in terms of percentage inhibition. Mean values of triplicate independent experiments and SD are shown. *Significance at p < 0.05, **significance at p < 0.005, ***significance at p < 0.0005
Effect on EPS Production
In light of the promising results attained from pyocyanin quantification assay, the test compound PAA was further tested for its efficiency in reducing EPS production in PAO1. When compared to the control, PAA at the tested concentration showed significant reduction in EPS production to the level of 54 % (Fig. 1).
Inhibition of Protease and Elastase Activity
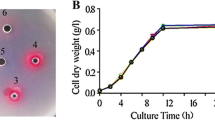
The ability of PAA in reducing QS-dependent azocasein-degrading protease and elastin-degrading elastase activity was assessed. As shown in Fig. 1, a decrease in protease and elastase activity was observed in the supernatant of PAA-treated PAO1, with that of untreated PAO1 supernatant. Moreover, the test compound showed no considerable growth inhibitory activity against PAO1 at the tested concentration (Fig. 2).
Effect on Motility
The motility behavior of PAO1 in the presence and the absence of PAA was assessed through swimming assay. PAA-treated PAO1 cells exhibited poor flagellum-driven motility on swim agar plates, when compared to the PAA-untreated PAO1 (Fig. 3).
C. elegans Survival Assay
The anti-infective potential of PAA was studied using PAO1-preinfected C. elegans as a host model. A complete mortality of PAO1-preinfected C. elegans was observed in 72 h. However, PAO1-preinfected C. elegans, further exposed to PAA displayed enhanced survival rate to the level of 53 % (Fig. 4). Further, PAA alone showed no considerable lethal effect on C. elegans.
Discussion
The AHL plays a vital role in the regulation of pathogenesis of P. aeruginosa and interference of AHL activity might possibly inhibit the pathogenicity of this pathogen. Thus, in this study, the efficiency of PAA was assessed for its ability to reduce AHL-dependent factors production in PAO1. The AHL system of PAO1 regulates the production of various virulence factors which are very much essential to onset the infection of this pathogen and also helps in the development of resistance to host immune attack. One such AHL-dependent factor pyocyanin, along with its precursor molecule phenazine-1-carboxylic acid, inhibits the beating of human respiratory cilia and alters the immune modulatory proteins expression in cystic fibrosis patients [9]. Likewise, protease and elastase enzymes play as pathogenic factors of P. aeruginosa to the host [1, 3, 14, 21]. As shown in Fig. 1, the test compound PAA showed a reduction in the above said virulence factors production, without any impact on PAO1 growth. In the earlier studies, a reduction in the production of protease and elastase enzymes was observed when treated with the known anti-QS compounds such as salicylic acid, nifuroxazide and chlorzoxazone [31], with the extract of marine bacteria [18] and edible fruits [17]. Similarly, reduction in the pyocyanin production was observed when PAO1 was treated with the extract of edible fruits [17] and marine bacteria [18].
Pseudomonas aeruginosa requires yet other important factors such as flagellar motility and production of EPS for its biofilm mode of growth [15, 20, 28]. The development of biofilm is highly essential for the survival of P. aeruginosa inside the host system. The flagella-driven motility helps the P. aeruginosa cells to adhere to the host tissue. The adhered cells then secrete the EPS to its surroundings which forms the protective barrier around the P. aeruginosa and thereby prevents the action of host immune system and antibiotics [6, 20, 28, 30]. Therefore, the interruption in the motility and EPS production could possibly prevent the survival of P. aeruginosa cells within the host. Thus, in continuation of the assessment of anti-QS activity of PAA on virulence factors production, the compound was further tested for its ability to inhibit EPS production and swimming motility of PAO1. The attained results demonstrated a reduction in both of these AHL-regulated phenomena when treated with PAA (Figs. 1, 3). The findings in the present study go well with the earlier reports wherein, anti-QS compound azithromycin inhibited the swimming motility [20], and the extract of Cuminum cyminum reduced the EPS production in PAO1 [22].
In in vivo analysis, C. elegans has been successfully employed as an alternative host to investigate the virulence of a variety of bacterial pathogens [16]. It has also been well established that the virulence factors of P. aeruginosa responsible for killing C. elegans are also relevant to mammalian systems [24, 27]. The strain PAO1 causes nematode death through cyanide poisoning and neuromuscular paralysis [12]. The AHL-dependent hcn operon produces cyanide in PAO1, which leads to paralysis and death of C. elegans [12]. Hence, in the present investigation, an attempt was made to study the potential of test compound in reducing the mortality of PAO1-preinfected C. elegans. As shown in Fig. 4, an enhanced survival of preinfected C. elegans was observed against PAO1 infection. Thus, from the attained result, it is envisaged that the enhanced survival of C. elegans is probably due to the interference in the AHL system of PAO1 by PAA which leads to the reduced death of C. elegans caused by cyanide poisoning.
In summary, though PAA has been known to have various bioactive potential, to the best of authors’ knowledge, so far no reports are available on the potential of this compound in reducing the QS-dependent factors production in bacterial pathogens. This is the first report demonstrating the anti-QS property of PAA against PAO1. Pertaining to the structural activity relationship, the chemical compound PAA shows the structural similarity with the previously reported anti-QS compound salicylic acid. Thus, based on the attained result in this study and structural similarity of PAA, it is envisaged that the inhibitory activity of PAA might probably be due to the inhibition of AHL-regulated behaviors by binding competitively to the AHL receptor protein.
References
Adonizio A, Kong KF, Mathee K (2008) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob Agents Chemother 52:198–203
Atkinson DC, Leach EC (1976) Anti-inflammatory and related properties of 2-(2,4-dichlorophenoxy)phenylacetic acid (fenclofenac). Agents Actions 6:657–666
Bjarnsholt T, Givskov M (2007) Quorum-sensing blockade as a strategy for enhancing host defences against bacterial pathogens. Phil Trans R Soc B 362:1213–1222
Chandel AK, Rao LV, Narasu ML, Singh OV (2008) The realm of penicillin G acylase in β-lactam antibiotics. Enzyme Microb Tech 42:199–207
Clinical and Laboratory Standards Institute (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th edn. Clinical and Laboratory Standards Institute document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA
de Kievit TR (2009) Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol 11:279–288
Essar DW, Eberly L, Hadero A, Crawford IP (1990) Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol 172:884–900
Favre-Bonte S, Kohler T, Delden CV (2003) Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J Antimicrob Chemother 52:598–604
Fothergill JL, Panagea S, Hart CA, Walshaw MJ, Pitt TL, Winstanley C (2007) Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol 7:45
Fuqua WC, Winans SC, Greenberg EP (1994) Quorum sensing in bacteria: the LuxR-LuxI family of cell density responsive transcriptional regulators. J Bacteriol 176:269–275
Fuqua C, Parsek MR, Greenberg EP (2001) Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet 35:439–468
Gallagher LA, Manoil C (2001) Pseudomonas aeruginosa PAO1 kills Caenorhabditis elegans by cyanide poisoning. J Bacteriol 183:6207–6214
Hwang BK, Lim SW, Kim BS, Lee JY, Moon SS (2001) Isolation and in vivo and in vitro antifungal activity of phenylacetic acid and sodium phenylacetate from Streptomyces humidus. Appl Environ Microbiol 67:3739–3745
Kessler E, Safrin M, Olson JC, Ohman DE (1993) Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508
Kohler T, Curty LK, Barja F, Delden CV, Pechere JC (2000) Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signalling and requires flagella and pili. J Bacteriol 182:5990–5996
Moy T, Ball I, Anklesaria AR, Casadei Z, Lewis GK, Ausubel FM (2006) Identification of novel antimicrobials using a live-animal infection model. Proc Natl Acad Sci 103:10414–10419
Musthafa KS, Ravi AV, Annapoorani A, Packiavathy ISV, Pandian SK (2010) Evaluation of anti-quorum-sensing activity of edible plants and fruits through inhibition of the N-acyl homoserine lactone system in Chromobacterium violaceum and Pseudomonas aeruginosa. Chemotherapy 56:333–339
Musthafa KS, Saroja V, Pandian SK, Ravi AV (2011) Antipathogenic potential of marine Bacillus sp. SS4 on N-acyl homoserine lactone mediated virulence factors production in Pseudomonas aeruginosa (PAO1). J Biosci 36:55–67
Nahar L, Russell WR, Middleton M, Shoeb M, Sarker SD (2005) Antioxidant phenylacetic acid derivatives from the seeds of Ilex aquifolium. Acta Pharm 55:187–193
Nalca Y, Jansch L, Bredenbruch F, Robert GR, Buer J, Haussler S (2006) Quorum sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother 50:1680–1688
Ohman DE, Cryz SJ, Iglewski BH (1980) Isolation and characterization of a Pseudomonas aeruginosa PAO1 mutant that produces altered elastase. J Bacteriol 142:836–842
Packiavathy IASV, Agilandeswari P, Musthafa KS, Pandian SK, Ravi AV (2012) Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against gram negative bacterial pathogens. Food Res Int 45:85–92
Pesci EC, Pearson JP, Seed PC, Iglewski BH (1997) Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol 179:3127–3132
Rahme LG, Stevens EJ, Wolfort SF, Shao J, Tompkins RG, Ausubel FM (1995) Common virulence factors for bacterial pathogenicity in plants and animals. Science 268:1899–1902
Sajid I, Shaaban KA, Hasnain S (2011) Identification, isolation and optimization of antifungal metabolites from the Streptomyces malachitofuscus CTF9. Braz J Microbiol 42:592–604
Sivamaruthi BS, Ganguli A, Kumar M, Bhaviya S, Pandian SK, Balamurugan K (2011) Caenorhabditis elegans as a model for studying Cronobacter sakazakii ATCC BAA-894 pathogenesis. J Basic Microbiol 51:1–10
Tan MW, Miklos SM, Ausubel FM (1999) Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc Natl Acad Sci 96:715–720
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extra cellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554
Williams P, Winzer K, Chan WC, Camara M (2007) Look who’s talking: communication and quorum sensing in the bacterial world. Phil Trans R Soc B 362:1119–1134
Wu H, Lee B, Yang L, Wang H (2011) Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol Med Microbiol 62:49–56
Yang L, Rybtke MT, Jakobsen TH, Hentzer M, Bjarnsholt T, Givskov M, Nielsen TT (2009) Computer-aided identification of recognized drugs as Pseudomonas aeruginosa quorum-sensing inhibitors. Antimicrob Agents Chemother 53:2432–2443
Acknowledgments
The authors gratefully acknowledge the opportunity to use the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by the Department of Biotechnology, Government of India; Grant No. BT/BI/25/001/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musthafa, K.S., Sivamaruthi, B.S., Pandian, S.K. et al. Quorum Sensing Inhibition in Pseudomonas aeruginosa PAO1 by Antagonistic Compound Phenylacetic Acid. Curr Microbiol 65, 475–480 (2012). https://doi.org/10.1007/s00284-012-0181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0181-9