Abstract
Lactobacillus equi, Lactobacillus hayakitensis, Lactobacillus johnsonii, and Weissella confusa/cibaria were the dominant species in 12 South African horses. The Bifidobacterium-group was detected in the feces of only one of the 12 horses. Sequencing of the nested-PCR amplicon identified the Bifidobacterium-group as Parascardovia denticolens. Cell numbers of L. equi, L. hayakitensis, and W. confusa/cibaria were consistent in all samples. P. denticolens, Bifidodobacterium pseudolongum, and a phylogenetic relative of Alloscardovia omnicolens were rarely detected. L. equigenerosi, a dominant species in Japanese horses, was detected in the fecal samples of only one horse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the first health benefits encountered by individuals that consumed certain strains of lactic acid bacteria in fermented milk [14], many lactic acid bacteria with probiotic properties have been described [17]. The probiotic application for humans varies from the prevention of infectious diseases [19], curing of irritable bowel syndrome, alleviation of allergies, digestion of lactose, and lowering of serum cholesterol levels [2], to the prevention of cancer [9]. In general, probiotic lactic acid bacteria do not cause immunological side effects [20]. However, Lactobacillus casei has been associated with symptoms of fever, arthritis, and hepatobiliary lesions in humans [22]. Symptoms such as these may be caused by cell wall components such as peptidoglycans that elicit cytokines [15]. Immunological side effects are often caused by cells that invade epithelial cells, migrate through mucus [24], and degrade mucus [20].
The human and animal gastrointestinal tracts are host to hundreds of bacterial species. Most research on intestinal microbiota has been done on humans [20] and ruminants [23, 28], but little is known about the microbiota in the equine gut, despite their important role in digestion. The bacteria, fungi, protozoa, and archaea in the equine hindgut are mostly anaerobic with strong cellulolytic activity. Celluloses are fermented to soluble sugars, which in turn are fermented to short chain fatty acids (SCFA) such as acetate, propionate, and butyrate. These SCFA are absorbed across the large intestinal epithelium and provide 60–70% of the horse’s energy [5].
Although Lactobacillus and Bifidobacterium spp. are regarded as beneficial to the general health of the host [17], not all lactic acid bacteria fall in the same category. Lactobacillus delbrueckii, L. fermentum, L. mucosae, L. reuteri, and L. salivarius are known to decarboxylate amino acids and have been implicated in equine laminitis [3, 16]. Lactobacillus pentosus WE7, isolated from the intestinal tract of horses, failed to prevent neonatal diarrhea in foals [27]. Instead, the strain stimulated the development of diarrhea and additional clinical abnormalities [27].
Bacteria–host interactions are specific. Lactobacillus rhamnosus strain GG (LGG), isolated from human feces and a well known probiotic, does not colonize in the intestinal tract of horses [26]. Lactobacillus salivarius, isolated from horse stomach, adhered to horse stomach tissue but not rat stomach [29]. Yuyama et al. [30] have shown that treatment of horses with L. crispatus, L. equi, L. johnsonii, and L. reuteri, which originated from horses, prevented diarrhea and enhanced growth. The 16S rRNA sequences recorded for 89% of the bacteria isolated from horses did not correspond to any known sequence, suggesting that the equine intestine may contain a number of yet-to-be cultured bacteria [5].
Culture-independent techniques based on PCR and PCR-Denaturing Gradient Gel Electrophoresis (PCR-DGGE) yield much more information on the diversity of microbiota. Furthermore, by performing real-time PCR (RT–PCR), the numbers of cells present for each genus or species can be determined. The aim of this study was to determine the Lactobacillus and Bifidobacterium population in horse feces by using PCR-DGGE and RT-PCR.
Materials and Methods
Reference Strains and Culture Conditions
The following strains were used as reference strains: Lactobacillus equi U6, Lactobacillus equigenerosi NRIC 0697T, Lactobacillus hayakitensis U46, Lactobacillus johnsonii NCFB 2241T, and Weissella confusa SU2. All these strains, except L. johnsonii NCFB 2241T, were isolated from horse feces [8] and were grown in MRS broth (Biolab, Biolab Diagnostics, Midrand, South Africa) at 37°C.
Fecal Samples
Fecal samples were collected from 12 healthy horses (5 stallions and 7 mares, ranging from 4 to 15 years old), stabled at Welgevallen experimental farm, Stellenbosch, South Africa. All horses were free from intestinal infections and did not receive antibiotics, probiotics, or prebiotics. The horses were fed barley hay, supplemented with commercial feed containing bran, maize, oat, and minerals. All animals were fed twice a day (9 h apart). Six horses were selected from which fecal samples were collected over a period of three months. Feces were collected immediately after defecation and were stored at −20°C until used.
DNA Extraction
DNA extraction from fecal samples and reference strains was performed by the method described previously [7], modified by using FastPrep FP120 (Savant Instruments, Farmingdale, NY, USA) for cell disruption, according to instructions of the supplier. DNA extracted from fecal samples was diluted ten times with TE buffer before subjected to PCR amplification.
PCR Amplification
All PCR primers used in this study are listed in Table 1. The Lactobacillus group community was analyzed with Lac1 and Lac2GC primers described by Walter et al. [25]. The reaction mixture was prepared as described by Endo and Okada [6]. DNA amplification was done according to the method of Walter et al. [25]. The community of the Bifidobacterium-group was analyzed with single-PCR and nested-PCR, as described previously [7]. For single-PCR, primers bif164-f and bif662-GC-r were used. For nested-PCR, primers Im26-f and Im3-r were used for the first-round PCR, and primers bif164-f and bif662-GC-r for the second-round PCR. After the first-round PCR, amplicons were purified by using the QIA quick PCR purification kit (QIAGEN Inc., Valencia, USA). Purified products were diluted ten times with TE buffer and the purified DNA was used as a template for the second-round PCR. The reaction mixture and the amplification program used for PCR of the Bifidobacterium group were as described by Satokari et al. [21].
DGGE Analysis and Excision of DNA Fragments
DGGE analysis of each PCR product was conducted with a DCode System (Bio-Rad Laboratories, Hercules, CA, USA) as described previously [7]. The denaturant gel gradient ranged from 35 to 50% for the Lactobacillus-group and from 45 to 60% for the Bifidobacterium-group. Electrophoresis was performed in Tris–acetate–EDTA buffer for 14 h at a constant voltage at 60 V. Gels were stained for 30 min with SYBR green I nucleic acid gel stain (BioWhittaker Molecular Applications, Rockland, ME, USA). Excision of bands from the gel was performed using sterilized toothpicks as described previously [6]. For the Lactobacillus-group, PCR amplicons of L. equi, L. equigenerosi, L. hayakitensis, L. johnsonii, and W. confusa with Lac1 and Lac2GC primers were used as markers of migration distances. Bands produced at the same migration distances to the reference strains were identified as the species. DNA bands that did not match the migration pattern were re-amplified by using the same primer set as used for generating the DGGE samples.
Sequence Analysis
The PCR products of the re-amplification products were purified by using a QIA quick PCR purification kit (QIAGEN Inc., Valencia, USA). Sequencing was according to the method described previously [6]. Blast analysis was used to determine similarities between sequences of the isolated DNA and those deposited at GenBank [1]. Accession numbers for the sequences were AB491611 to AB491613.
Calculation of the Number of Cells in the Lactobacillus-Group
The number of cells in the Lactobacillus-group present in each sample was determined by using RT-PCR with SYBR Green JumpStartTM Taq ReadyMixTM (Sigma, Missouri, USA) in a LightCylcler (Roche Diagonostics, Mannheim, Germany). The primer set Lac1 and S-G-Lab-0677-a-A-17 described by Rinttilä et al. [18] was used for the Lactobacillus group. The reaction mixture was prepared according to instructions of the supplier. Amplification was as follows: initial denaturation at 95°C for 30 s and 40 cycles of 94°C for 0 s, 58°C for 10 s, and 72°C for 15 s. DNA extracted from known amounts of cells of L. johnsonii NCFB 2241T was used as reference in preparing a standard curve. Cell counts of L. johnsonii NCFB 2241T was determined by serial dilution in saline and plating on MRS agar. The plates were incubated at 37°C for 3 days under anaerobic conditions using a gas generating kit (Anaerobic system BR0038B, Oxoid Ltd., Basingstoke, Hants, UK).
Results
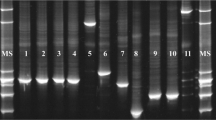
Separation of PCR amplicons by DGGE revealed several DNA bands for each of the 12 fecal samples (Fig. 1). Five of the DNA bands corresponded with the migration profile of the standard DNA (lane M), indicating that L. johnsonii, L. equi, and W. confusa/cibaria are the predominant species present in the fecal samples analyzed. L. hayakitensis was detected in seven out of 12 samples, and L. equigenerosi in only one sample (sample no. 9). Similar fecal microbiota was recorded among individual horses.
DGGE profiles of the Lactobacillus group in horse feces (numbered 1–12). M a combination of DNA from L. equi, L. equigenerosi, L. hayakitensis, L. johnsonii, and W.cibaria. Labeled bands with letters “a” to “e” were allotted to the following species: a: L. johnsonii, b: L. equi, c: W. cibaria, d: L. hayakitensis, e: L. equigenerosi. The gel was stained with SYBR Green I nucleic acid gel stain (BioWhittaker Molecular Applications)
No PCR amplicons were generated for bifidobacteria by single-PCR. However, nested-PCR produced an amplicon from sample no. 6 and DGGE profile generated a single band from the sample. The DNA band in the DGGE gel was excised with sterile toothpicks and re-amplified by PCR using the bif164-f and bif662-GC-r primer set. Sequence analysis of PCR amplified DNA revealed the amplicon originated from Parascardovia denticolens (Accession no. AB491611).
Concluded from the DGGE profiles, the Lactobacillus flora in the six horses remained stable during the 3-month test period. L. equi, L. hayakitensis, and W. confusa/cibaria were detected in more than 80% of samples analyzed. L. johnsonii and L. equigenerosi were detected in 40 and 20% of samples analyzed, respectively. Other Lactobacillus spp. have not been detected.
P. denticolens was detected in one or two time-course samples from horses numbered 2, 3, 6, 9, and 11 as bifidobacteria by DGGE analysis. Bifidobacterium pseudolongum was detected in a time-course sample of horse no. 7 (Accession no. 491613). A phylogenetic relative of Alloscardovia omnicolens (98% sequence similarity) was detected in a time-course sample of horse no. 11 (Accession no. 491612), which had a 100% similarity to the DNA sequence detected from the feces of a Japanese horse (Accession no. AB250251) [7].
A standard curve for real-time PCR, obtained with reference strain L. johnsonii NCFB 2241T is shown in Fig. 2. The number of cells (log10) recorded for Lactobacillus present in samples numbered 1–12 were 8.25, 7.34, 7.13, 6.95, 7.78, 8.12, 6.83, 7.85, 8.11, 7.12, 7.07, and 7.35 cells g−1 of feces, respectively. The mean ± standard deviation (SD) was 7.49 ± 0.50. The numbers of cells (log10) detected in time-course samples were stable and ranged from 6.85 to 7.30 for horse no. 2, from 6.64 to 7.28 for no. 3, from 7.47 to 8.17 for no. 6, from 6.57 to 7.15 for no. 7, from 7.43 to 7.77 for no. 9, and from 6.86 to 7.33 for no. 11.
Discussion
The diversity of Lactobacillus and Bifidobacterium spp. in horse feces is described using group-specific primers. PCR-DGGE with each primer set revealed target bacterial diversity. The Lactobacillus population in the feces of each horse was almost identical. L. johnsonii, L. equi, L. hayakitensis, and W. confusa/cibaria were predominant. These species have also been reported as dominant in feces of Japanese racehorses [7]. L. equigenerosi, a dominant species in Japanese horses [8], is a minority species in South African horses and was detected in the feces of only one of the 12 horses by DGGE. Cell numbers (log10) recorded for the Lactobacillus group ranged from 6.83 to 8.25 (mean SD = 7.49 ± 0.50). This was slightly lower than that the numbers recorded for Japanese horses (8.47 ± 0.62) [7]. This may be due to differences in forages and the environment. The Japanese horses were racehorses kept in stables and had several hard trainings [7].
No species allocated to the Bifidobacterium group were found by single-PCR, but a few species were detected by nested-PCR, suggesting that cell number of bifidobacteria was <103 g−1 of feces or 103 < cells < 105 g−1 of feces. This conclusion is based on limitations in the detection of single-PCR (103 cells g−1 of feces) and nested-PCR (101 cells g−1 of feces) as described previously [7]. P. denticolens was detected as a Bifidobacterium-group species in fecal samples from horse no. 6. P. denticolens was also detected in the feces of time-course samples collected from horse nos. 2, 3, and 6. This species has been formerly classified as Bifidobacterium denticolens [4], and it was later reclassified as P. denticolens [11]. Strains of the species have been detected in two out of six fecal samples collected from Japanese horses [7], suggesting that P. denticolens is a normal inhabitant of the horse GIT. This is an interesting characteristic, since P. denticolens had previously only been detected in human oral cavities. In time-course samples taken from horse no. 11, a phylogenetic relative of A. omnicolens was detected. The sequence shared 100% similarity to the sequence detected in feces of Japanese horse (Accession no. AB250251) [7]. This suggests that the species is a normal inhabitant of horses. B. pseudolongum was found in horse feces for the first time.
L. delbrueckii, L. fermentum, L. mucosae, L. reuteri, and L. salivarius, associated with equine laminitis [3], were not detected in the present study. These species are recorded in horses that have been on a high carbohydrate diet [3]. An excess of forage may thus lead to the disruption of complex and well-balanced microbiota, resulting in laminitis.
Lactobacillus spp. detected in the feces of South African horses have also been described in the feces of Japanese horses [7], suggesting that certain species are predominantly present, irrespective of the breed, forage, or environmental conditions. Further research is needed to develop strains of L. johnsonii, L. equi, L. hayakitensis, and W. confusa/cibaria into probiotics. B. pseudolongum, P. denticolens, and the phylogenetic relative of A. omnicolens are in the minority and may not play any role as probiotics.
References
Altschul SF, Gish W, Miller E, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andersson H, Asp N-G, Bruce A, Roos A, Wadström T, Wold AE (2001) Health effects of probiotics and prebiotics. A literature review on human studies. Food Nutr Res 45:58–75
Bailey SR, Baillon ML, Rycroft AN, Harris PA, Elliott J (2003) Identification of equine cecal bacteria producing amines in an in vitro model of carbohydrate overload. Appl Environ Microbiol 69:2087–2093
Crociani F, Biavati B, Alessandrini A, Chiarini C, Scardovi V (1996) Bifidobacterium inopinatum sp. nov. and Bifidobacterium denticolens sp. nov., two new species isolated from human dental caries. Int J Syst Bacteriol 46:564–571
Daly K, Stewart CS, Flint H, Shirazi-Beechey SP (2001) Bacterial diversity within the equine large intestine as revealed by molecular analysis of cloned 16S rRNA genes. FEMS Microbiol Ecol 38:141–151
Endo A, Okada S (2005) Monitoring the lactic acid bacterial diversity during shochu fermentation by PCR-denaturing gradient gel electrophoresis. J Biosci Bioeng 99:216–221
Endo A, Okada S, Morita H (2007) Molecular profiling of Lactobacillus, Streptococcus, and Bifidobacterium species in feces of active racehorses. J Gen Appl Microbiol 53:191–200
Endo A, Roos S, Satoh E, Morita H, Okada S (2008) Lactobacillus equigenerosi sp. nov., a coccoid species isolated from faeces of thoroughbred racehorses. Int J Syst Evol Microbiol 58:914–918
Gibson GR, Macfarlane GT (1994) Intestinal bacteria and disease. In: Gibson SAW (ed) Human health—the contribution of microorganisms. Springer-Verlag, London, pp 53–62
Heilig HG, Zoetendal EG, Vaughan EE, Marteau P, Akkermans AD, de Vos WM (2002) Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl Environ Microbiol 68:114–123
Jian W, Dong X (2002) Transfer of Bifidobacterium inopinatum and Bifidobacterium denticolens to Scardovi inopinata gen. nov., comb. nov., and Parascardovia denticolens gen. nov., comb. nov., respectively. Int J Syst Evol Microbiol 52:809–812
Kaufmann P, Pfefferkorn A, Teuber M, Meile L (1997) Identification and quantification of Bifidobacterium species isolated from food with genus-specific 16S rRNA-targeted probes by colony hybridization and PCR. Appl Environ Microbiol 63:1268–1273
Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis GR, Wilkinson MH, Welling GW (1995) Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol 61:3069–3075
Metchnikoff E (1907) The prolongation of life: optimistic studies. William Heinemann, London
Miettinen M, Vuopio-Varkila J, Varkila K (1996) Production of human necrosis factor a, interleukin 6, and interleukin 10 is induced by lactic acid bacteria. Infect Immun 64:5403–5405
Mungall BA, Kyaw-Tanner M, Pollitt CC (2001) In vitro evidence for a bacterial pathogenesis of equine laminitis. Vet Microbiol 79:209–223
Ouwehand AC, Salminen S, Isolauri E (2002) Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82:279–289
Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A (2004) Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 97:1166–1177
Rolfe RD (2000) The role of probiotic cultures in the control of gastro-intestinal health. J Nutrit 130(2S):396S–402S
Salminen S, Von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, Fondén R, Saxelin M, Collins K, Mogensen G, Birkeland SE, Matilla-Sandholm T (1998) Demonstration of safety of probiotics—a review. Int J Food Microbiol 44:93–106
Satokari RM, Vaughan EE, Akkermans AD, Saarela M, de Vos WM (2001) Bifidobacterial diversity in human feces detected by genus-specific PCR and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67:504–513
Schwab JH (1993) Phylogistic properties of peptide-glycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonise humans. Infect Immun 61:4535–4539
Tajima K, Aminov RI, Nagamine T, Ogata K, Nakamura M, Matsui H, Benno Y (1999) Rumen bacterial diversity as determined by sequence analysis of 16S rDNA libraries. FEMS Microbiol Ecol 29:159–169
Tang P, Foubister V, Pucciarelli MG, Finlay BB (1993) Methods to study bacterial invasion. J Microbiol Meth 23:119–125
Walter J, Hertel C, Tannock GW, Lis CM, Munro K, Hammes WP (2001) Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl Environ Microbiol 67:2578–2585
Weese JS, Anderson MEC, Lowe A, Monteith GJ (2003) Preliminary investigation of the probiotic potential of Lactobacillus rhamnosus strain GG in horses: fecal recovery following oral administration and safety. Can Vet J 44:299–302
Weese JS, Rousseau J (2005) Evaluation of Lactobacillus pentosus WE7 for prevention of diarrhea in neonatal foals. J Am Vet Med Assoc 226:2031–2034
Whitford MF, Forster RJ, Beard CE, Gong J, Teather RM (1998) Phylogenetic analysis of rumen bacteria by comparative sequence analysis of cloned 16S rRNA genes. Anaerobe 4:153–163
Yuki N, Shimazaki T, Kushiro A, Watanabe K, Uchida K, Yuyama T, Morotomi M (2000) Colonization of the stratified squamous epithelium of the nonsecreting area of horse stomach by lactobacilli. Appl Environ Microbiol 66:5030–5034
Yuyama T, Takai S, Tsubaki S, Kado Y, Morotomi M (2004) Evaluation of a host-specific Lactobacillus probiotics in training horses and neonatal foals. J Intest Microbiol 18:101–106
Acknowledgments
The authors thank Prof. S. Okada (NODAI Culture Collection Center, Tokyo University of Agriculture) for providing the NRIC strains. Dr. A. Endo received a postdoctoral grant from Claude Leon Foundation, Cape Town, South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Endo, A., Futagawa-Endo, Y. & Dicks, L.M.T. Lactobacillus and Bifidobacterium Diversity in Horse Feces, Revealed by PCR-DGGE. Curr Microbiol 59, 651–655 (2009). https://doi.org/10.1007/s00284-009-9498-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9498-4