Abstract
A study for screening and selection of mutants of Pseudomonas corrugata (NRRL B-30409) based on their phosphate solubilization ability, production of organic acids, and subsequent effect on plant growth at lower temperatures under in vitro and in situ conditions was conducted. Of a total 115 mutants tested, two (PCM-56 and PCM-82) were selected based on their greater phosphate solubilization ability at 21°C in Pikovskaya’s broth. The two mutants were found more efficient than wild-type strain for phosphate solubilization activity across a range of temperature from psychotropic (4°C) to mesophilic (28°C) in aerated GPS medium containing insoluble rock phosphate. High-performance liquid chromatography analysis showed that phosphate solubilization potential of wild-type and mutant strains were mediated by production of organic acids in the culture medium. The two efficient mutants and the wild strain oxidized glucose to gluconic acid and sequentially to 2-ketogluconic acid. Under in vitro conditions at 10°C, the mutants exhibited increased plant growth as compared to wild type, indicating their functionality at lower temperatures. In greenhouse trials using sterilized soil amended with either soluble or rock phosphate, inoculation with mutants showed greater positive effect on all of the growth parameters and soil enzymatic activities. To the best of our knowledge, this is the first report on the development of phosphate solubilizing mutants of psychotropic wild strain of P. corrugata, native to the Indian Himalayan region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting bacteria are soil and rhizosphere bacteria that can benefit plant growth by different mechanisms [5], and P-solubilization ability of the microorganisms is considered to be one of the most important traits associated with plant P nutrition [19]. These phosphate-solubilizing microorganisms (PSM) render insoluble phosphate into available forms by the process of acidification, chelation, and exchange reaction [6]. This process not only compensates for the higher cost of manufacturing fertilizers in industry but also mobilizes the fertilizers added to soil. Although PSM have been isolated from various environmental niches, including highly stressed conditions and extreme environmental factors [4, 8, 26] there are only a few reports on characterization of PSM for their activity at lower temperatures [2, 14, 15, 24].
There are several studies that indicate that seed or soil inoculation with PSM improves solubilization of fixed soil phosphorus and applied phosphates, resulting in higher crop yields [18]. The establishment and performance of these rhizosphere microbes is severely affected under abiotic stresses such as temperature, pH, and salinity [10]. High arctic and low temperature habitats such as mountain regions of the Indian Himalayan Region (IHR) require strains with greater adaptability to the particular environmental conditions. Das et al. [2] proposed exploitation of the mutant gene pool for higher crop productivity for development of efficient strains for different climatic conditions.
Previous studies have shown that Pseudomonas corrugata (NRRL B-30409) originally isolated from the rhizosphere of maize grown at a field in Sikkim Himalaya [13] improves the growth of plants by various mechanisms [13, 14, 25]. The aim of this study was to develop cold-tolerant mutants of P. corrugata for effective solubilization of phosphate at lower temperatures. The effect of temperature on phosphate solubilization, production of organic acids (2-ketogluconic acid, 2-KGA), and decrease in pH of the selected mutants were compared with the wild strain. The efficiency of the wild and selected mutants to promote plant growth was determined by paper towel (10°C) and pot assay in a growth chamber (10–15°C) using wheat (Triticum aestivum) as test crop.
Materials and Methods
Bacterial Strain
P. corrugata (NRRL B-30409) was obtained from the departmental cultural collection. The strain was maintained on Tryptone Yeast Extract (TY) Agar (HiMedia, Mumbai) slants at 4°C in a refrigerator and as glycerol stocks at −20°C in deep freezers.
Development of Mutants
Overnight-grown culture of P. corrugata was inoculated in PVK medium (HiMedia, Mumbai) and was subjected to N′-methyl-N′-nitro-N-nitrosoguanidine (NTG) treatment for mutagenesis [11]. After NTG treatment, the cell suspension (0.1 ml) was spread onto solid PVK medium (pH 6.0) and incubated at 21°C along with untreated control for 24 h. Afterwards, the colonies were observed on the media and were collected for further analysis. All of the possible mutants were screened for phosphate solubilization (compared to parent strains) after 24 h growth in PVK broth (pH 6.0) at 21°C. Mutants showing maximum phosphate solubilization were selected for further studies.
Determination of pH and P2O5 Concentration
Quantitative estimation of the phosphate solubilization of wild type and selected mutants was carried out in 250.0 ml Erlenmeyer flasks containing 100.0 ml of GRC broth (yeast extract 0.2 g, yeast autolysate 0.2 g, tryptone peptone 0.2 g/l, glucose 0.8 m, rock phosphate 5%, and CaCO3 1%). The broth was autoclaved after adjusting the pH to 6.0. The flasks were inoculated with 1.0 ml of bacterial suspension (106 cfu ml−1) growing in TY broth for 24 h at 21°C. Cultures were grown at 2000 rpm at various temperatures (4–28°C) for 30 days with an air supply. For air supply, the culture broth was aerated by filtered air through a 0.45-μm Whatman membrane at a flow rate of 2 vvm. The growth medium was withdrawn aseptically every 3 days for 30 days from each flask incubated at different temperatures and centrifuged at 10,000 g for 10 min. The supernatant was collected and was assayed for P2O5 content by chlorostannus reduced molybdo-phosphoric acid blue method [1]. The pH of the supernatant was recorded with a pH meter. The experiments were done in triplicate and were repeated three times.
Measurement of Organic Acids
The culture fluid of wild and selected mutants of P. corrugata was filtered through a 0.2-μm Whatman membrane filter. The organic acids in the filtrate were determined by high-performance liquid chromatography (HPLC) [7].
In Vitro Plant Growth Promotion
To test the efficacy of the mutants to promote plant growth at low temperatures, a paper towel assay was set up. P. corrugata wild-type strain and its respective mutants, grown to log phase at 21°C in TY broth (approximately 108 cfu per seed), were used for seed bacterization of wheat and incubated at 10°C as followed by Mishra and Goel [11]. Nonbacterized seeds served as control.
Greenhouse Experiments
Wheat plants were raised from surface-sterilized seeds (1 % NaOCl for 3 min) after subsequent washing with sterilized distilled water, in surface-sterilized plastic pots (500 ml) filled with steam-sterilized local soil (C = 0.19%; N = 0.042%; P = 0.0908%; pH = 7.2). The soil was sterilized by autoclaving at 121°C for 2 h on 3 consecutive days. For inoculation, 1 ml of the bacterial suspension (TY broth containing approximately 108 cells ml−1) was added to each seed at the time of sowing. After emergence of seedlings, they were thinned to three plants per pot. The experiment was carried out in 7 different treatments (see footnotes of Table 1). The pots were placed in a plant growth chamber with a constant temperature varying from 10 to 15°C (night–day). At harvest (30 days), the length and dry weight of the shoot and root were determined. P content of plants was determined [1]. Assay of acid and alkaline phosphatase activity in soil was done by methods as described in Tabatabai and Bremner [22].
For plant growth experiments, a randomized block design was used with 5 replicates (25 plants per replicate) per treatment. Subsamples of 10 plants from each replications were sacrificed and plant growth measurements were taken.
Statistical Analysis
The plant growth data were subjected to analysis of variance using SAS-9.1 software (SAS Institute, Cary, NC) [20]. Mean values among treatments were compared by Duncan’s Multiple Range (DMR) test at P ≤ 0.05 or 0.01 significance level. For broth studies, correlation values among different parameters were determined by a simple correlation coefficient regression equation. The result means were depicted diagrammatically by the computer program MSTACT Microsoft Excel version 5.0.
Results and Discussion
After subjecting P. corrugata (NRRL B-30409) to mutagenesis with NTG, several mutants were (115) were isolated. The mutants were then evaluated for their phosphate-solubilizing activity at 21°C using PVK broth. Of 115 strains, 90 were found to solubilize phosphate in broth-based assays. After 24-h incubation, phosphate solubilization ranged from 22 to 82 μg ml−1. Two mutants (PCM-56 amd PCM-82) showing maximum solubilization (≥80 μg ml−1) were selected for further studies. Katiyar and Goel [9] have reported development of cold-tolerant mutants of P. fluorescens (strains GRS1, PRS9, and ATCC 13525) for their ability to solubilize phosphate at lower temperatures. To the best of our knowledge, this is the first report on the development of phosphate-solubilizing mutants of the psychotropic wild strain of P. corrugata, native to the Indian Himalayan region.
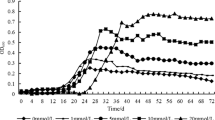
Quantitative estimation of solubilized phosphate, carried out from the 3rd to the 30th day of incubation (at 3-day intervals) in aerated GPS broth, indicated maximal activity at 21°C for wild-type as well as mutant strains. P2O5 release in broth was also found to take place at other temperatures (4, 9, and 28°C) (Fig. 1). The phosphate-solubilization activity of both of the mutants was significantly greater compared to wild type at all the incubation temperatures. The phosphate solubilization activity of both of the mutants at lower temperatures was greater than some wild [9, 14, 15] and mutant strains [2] of other bacterial species. The decrease in phosphate-solubilizing ability after a certain period of incubation (12–18 days) may be due to depletion of nutrients, production of certain toxic metabolites in the growth, or to autolysis of cells [4].
The pH of the broth was found to decline in each case due to the phosphate-solubilizing activity of the bacterial strains (Fig. 1). This lowering in pH indicated the production of organic acids during the metabolism of glucose, which is considered a mechanism responsible for the dissolution of inorganic insoluble phosphate [7]. Organic acids produced by the wild and the mutant strains were investigated in broth cultures. HPLC analysis of the culture filtrate revealed two major peaks. These two peaks were identified as gluconic acid and 2-KGA by comparing the retention time with those of authentic standards. During the initial period of growth, the concentration of 2-KGA was lower compared to that of gluconic acid, but it continuously increased during the growth period. Similar results have been described during the course of phosphate solubilization by other bacterial species [7, 12, 21].
Lowering of the pH and increase in the amount of 2-KGA coincided with an increase in the efficiency of phosphate solubilization (Fig. 1). Correlation coefficient values between phosphate solubilization and change in pH or amount of 2-KGA produced were significant in wild and both of the mutant strains at all the growth temperatures. Maximum acid production was observed at 21°C followed by 28, 14, and 4°C, respectively. The decrease in pH and the amount of 2-KGA produced by both of the mutants was significantly greater as compared to wild type at all the incubation temperatures. The phosphate solubilization ability of wild and mutant strains of P. fluorescens had been reported to be accompanied by decrease in pH of the medium [9]. Increase in the amount of organic acids by two UV-induced mutants of Penicillium rugulosum during the course of solubilization of phosphate rocks and minerals has been reported [16]. Rise in pH and decrease in the amount of 2-KGA was observed during the later incubation periods in all of the three bacterial strains, which may be due to the use of organic acids during prolonged growth in the culture medium [3].
In order to establish the relationship between mutants, phosphate solubilization, and plant growth at lower temperatures, a paper towel assay was set up. It was found that both of the mutants were effective in enhancing the growth of wheat plants significantly as compared to control (p ≤ 0.01) and wild type (p ≤ 0.05) (Fig. 2). These results are in good accordance with Katiyar and Goel [9], where they found stimulation in growth of wheat and mung bean by mutant strains of P. fluorescens at 10°C. In greenhouse assay, bacterial inoculation had a positive effect on all the growth parameters and soil enzymatic activities (Table 1). The addition of rock phosphate alone has no effect on the increase in plant growth. Inoculation of bacteria to the soil amended with rock phosphate had a significant effect on plant growth in all of the cases. This shows the functionality of the wild and mutants strains to solubilize insoluble forms of phosphate in actual soil conditions. The ability of the mutants to augment overall plant growth was greater compared to the wild strain. Genetically modified strains of P. rugulosum were able to stimulate growth of maize plants by means of increased P uptake [17].
The ability of any organism to produce novel compounds may be increased or decreased by unknown effects on genotype and also by disruption of normal physiological processes [23]. This study indicates that it is possible to select physiologically efficient strains of P. corrugata through mutagenesis and that resultant mutants strains may improve the use of soil phosphate by plants at lower temperatures. The mutants may provide a model for undertaking studies related to gene regulation at low temperature and for understanding the molecular basis of cold adaptation as well as to serve as a source of novel enzymes and cellular metabolites.
References
Allen SE (1974) Chemical analysis of ecological materials. 2nd edn. Oxford: Blackwell Scientific Publications
Das K, Katiyar V, Goel R (2003) ‘P’ solubilization potential of plant growth promoting Pseudomonas mutants at low temperature. Microbiol Res 158:359–362
Dave A, Patel HH (1999) Inorganic phosphate solubilizing soil Pseudomonads. Ind J Microbiol 39:161–164
Gaind S, Gaur AC (1991) Thermotolerant phosphate solubilizing microorganisms and their interaction with mung-bean. Plant Soil 133:141–149
Glick BR, Patten CL, Holguin G, Penrose DM (1999) Biochemical and genetic mechanisms used by plant growth promoting bacteria. 1st edn. London: Imperial College Press
Goldstein AH (1986) Bacterial solubilization of mineral phosphates: historical perspective and future prospects. Am J Alter Agri 1:51–57
Hwangbo H, Park RD, Kim YW, Rim YS, Park KH, Kim TH, Suh JS, Kim KY (2003) 2-Ketogluconic acid production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol 47:87–92
Johri JK, Surange S, Nautiyal CS (1999) Occurrence of salt, pH, and temperature tolerant, phosphatic solubilizing bacteria in alkaline soils. Curr Microbiol 39:89–93
Katiyar V, Goel R (2003) Solubilization of inorganic phosphate and plant growth promotion by cold tolerant mutants of Pseudomonas fluorescens. Microbiol Res 158:163–168
Kucey RMN, Janzen HH, Laggett ME (1989) Microbially mediated increases in plant available phosphorus. Adv Agron 42:199–228
Mishra M, Goel R (1999) Development of cold resistant mutant of plant growth promoting Ps. fluorescens and its functional characterization J Biotechnol 75:71–75
Neijssel OM, Tempest DW, Postma PW, Duine JA, Frank JJ (1983) Glucose metabolism by K+-limited Klebsiella aerogenes: evidence for the involvement of a quinoprotein glucose dehydrogenase. FEMS Microbiol Lett 20:35–39
Pandey A, Palni LMS (1998) Isolation of Pseudomonas corrugata from Sikkim Himalaya. World J Microbiol Biotech 14:411–413
Pandey A, Palni LMS, Mulkalwar P, Nadeem M (2002). Effect of temperature on solubilization of tricalcium phosphate by Pseudomonas corrugata. J Sci Ind Res (India) 61:457–460
Pandey A, Trivedi P, Kumar B, Palni LMS (2006) Characteristics of a phosphate solubilizing and antagonistic strain of Pseudomonas putida (BO) isolated from a sub-alpine location in the Indian central Himalaya. Curr Microbiol 53:102–107
Reyes I, Baziramakenga R, Bernier L, Autoun H (2001) Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biol Biochem 33:1741–1747
Reyes I, Bernier L, Autoun H (2002) Rock phosphate solubilization and colonization of maize rhizosphere by wild and genetically modified strains of Penicillium rugulosum. Microb Ecol 44:39–48
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Rodrìguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21
SAS Institute (2003) SAS/STAT guide for personal computers. Cary, NC: SAS Institute
Svitel JJ, Sturdik E (1995) 2-ketogluconic acid production by Acetobacter pasteurianus. Appl Biochem Biotechnol 53:53–63
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenol phosphate for assay of phosphatase activity. Soil Biol Biochem 1:301–307
Tiedje JM, Colwell RK, Grossman YL, Hodson RE, Lenski RE (1989) The planned introduction of genetically engineered organisms: ecological considerations and recommendations. Ecol 70:298–315
Trivedi P, Kumar B, Pandey A, Palni LMS (2007) Growth promotion of rice by phosphate solubilizing bioinoculants in a Himalayan location. In: Velázquez E, Rodríquez-Barrueco C (eds) First international meeting on microbial phosphate solubilization, vol 1. Dordrecht, Springer, pp 291–299
Trivedi P, Pandey A, Palni LMS (2007) In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol Res (in press)
Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semi arid coastal lagoon. Biol Fertil Soils 30:460–468
Acknowledgments
The Senior author (P.T.) gratefully acknowledges financial support from the Department of Science and Technology (DST), Government of India, in the form of a Young Scientist award under the SERC FAST TRACK Scheme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trivedi, P., Sa, T. Pseudomonas corrugata (NRRL B-30409) Mutants Increased Phosphate Solubilization, Organic Acid Production, and Plant Growth at Lower Temperatures. Curr Microbiol 56, 140–144 (2008). https://doi.org/10.1007/s00284-007-9058-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-9058-8