Abstract
Plasma cells are terminally differentiated B cells that secrete antibodies, important for immune protection, but also contribute to any allergic and autoimmune disease. There is increasing evidence that plasma cell populations exhibit a considerable degree of heterogeneity with respect to their immunophenotype, migration behavior, lifetime, and susceptibility to immunosuppressive drugs. Pathogenic long-lived plasma cells are refractory to existing therapies. In contrast, short-lived plasma cells can be depleted by steroids and cytostatic drugs. Therefore, long-lived plasma cells are responsible for therapy-resistant autoantibodies and resemble a challenge for the therapy of antibody-mediated autoimmune diseases. Both lifetime and therapy resistance of plasma cells are supported by factors produced within their microenviromental niches. Current results suggest that plasma cell differentiation and survival factors such as IL-6 also signal via mammalian miRNAs within the plasma cell to modulate downstream transcription factors. Recent evidence also suggests that plasma cells and/or their immediate precursors (plasmablasts) can produce important cytokines and act as antigen-presenting cells, exhibiting so far underestimated roles in immune regulation and bone homeostasis. Here, we provide an overview on plasma cell biology and discuss exciting, experimental, and potential therapeutic approaches to eliminate pathogenic plasma cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: plasma cells in immunopathology
For nearly a century, plasma cells had been associated with the pathogenesis of many different diseases [1]. At that time, it was known that plasma cells are present in tissues of healthy individuals but typically increase in numbers around various pathological lesions. Only much later it was discovered that plasma cells represent the source for antibodies present in our body fluids [2]. Although the secretion of protective and pathogenic antibodies resemble major effector mechanisms for immune protection and the pathogenesis of autoimmune and allergic diseases, non-neoplastic plasma cells were not in the focus of immunology and research between the mid 1960s and the end of 1990s. During this period, textbooks said that antibody responses are solely controlled at the level of B-cell activation while plasma cells were merely believed to be terminally differentiated short-lived effector B cells that undergo apoptosis within a few days [3]. Only later it was discovered that plasma cells come in at least two flavors—short- and long-lived—and that long-lived plasma cells can provide memory antibodies for years, in the absence of ongoing immune stimulation [4, 5]. Most importantly, long-lived plasma cells are refractory to immunosuppressive drugs such as cyclophosphamide or dexamethasone [6, 7]. The production of autoantibodies by long-lived plasma cells provides a rational explanation for the observation that conventional therapy efficiently suppresses autoantibodies in some patients, but not in others, or suppresses autoantibodies in one patient only incompletely [8]. In addition to their pathogenic role in autoimmunity, long-lived plasma cells have been shown to maintain allergy-specific IgE in a mouse model for allergic asthma [9]. Hence, suggesting that the depletion of pathogenic long-lived plasma cells is a pre-requisite for a successful cure of this and possible other allergic diseases.
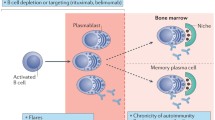
Despite their resistance to conventional immunosuppressive drugs, long-term survival is not an intrinsic feature of plasma cells but depends on factors provided by their microenvironmental niches [10–12]. As discussed below, novel experimental strategies, allowing for the depletion of pathogenic long-lived plasma cells, that are able to eliminate pathogenic antibody memory include proteasome inhibitors and drugs that target the plasma cell niche.
There is accumulating evidence that plasma cells and/or their immediate precursors do not only secrete antibodies but also cytokines with important functions for the regulation of immunity and bone homeostasis [13–17]. Moreover, plasma cells may resemble potent antigen-presenting cells with the capacity to alter T-cell responses [18]. Possible consequences of antigen presentation and cytokine production by plasma cells might be underestimated and have a greater impact on immunopathology than expected so far.
Plasma cell migration and homing
Specific antibodies and plasma cell populations rapidly decline after the peak immune reaction induced by immunization/vaccination, but an estimated 10 % of plasma cells become long-lived and can maintain specific memory antibodies in serum [4, 5]. B-cell activation and early plasma cell differentiation mainly takes place within activated lymph nodes, Peyers patches, and spleen, depending on the localization and nature of the antigenic stimulation. Newly generated plasma cells can leave the secondary lymphoid tissues, transiently circulate through blood and eventually migrate to and accumulate in deposit tissues [19, 20]. Both the egress from the secondary tissues and immigration into tissues are highly regulated by adhesion molecules and chemokines/chemokine receptors [21]. Emigration of early plasma cells from peripheral lymph nodes depends on the expression of beta2 integrin. The chemokines CXCL9, CXL10, CXCL11, and CXCL12 (also known as SDF-1) are major attractants for early plasma cells from lymph nodes and the spleen. CXCR4, the receptor for CXCL12, is expressed on the precursors of long-lived plasma cells accumulating in the murine bone marrow and also on early tetanus-specific plasma cells transiently migrating through the peripheral blood a few days after vaccination with the respective antigen [22]. Likewise, B lineage-specific genetic deletion of the CXCL12 receptor CXCR4 in mice results in a drastic delay in the accumulation of immunization-induced plasma cells in the bone marrow, as well as to an altered plasma cell localization within the splenic red pulp [3]. Plasma cell homing to the bone marrow also involves the adhesion molecules LFA-1 and VLA-4 and sphingosine-1-phosphate (S1P) receptor-1 [21, 23]. Combined blockade of LFA-1 and VLA-4, but not of one of these molecules, depletes plasma cells from the bone marrow and leads to a partial loss of specific antibodies [23], indicating that adhesion molecules play important but redundant functions for the retention of plasma cells within this tissue.
CXCL9, CXL10, and CXCL11 resemble inflammatory cytokines that signal via their common receptor CXCR3. This receptor is expressed on subpopulations of plasma cells from normal donors and autoimmune patients as well as on murine plasma cells in various models [24–26]. Genetic deletion of CXCR3 considerably reduced lymphocyte infiltrations, nephritis, and tissue damage in the MRL/lpr mice, a model for lupus nephritis [27], suggesting that CXCR3 and its ligands resemble promising therapeutic targets for the treatment of this disease. However, the beneficial effect of CXCR3 ablation in MRL/lpr mice was mediated through the suppression of the accumulation of inflammatory IFN-gamma and IL-17-producing T cells within the kidneys. A possible direct effect on the migration and homing of pathogenic plasma cells was not tested. In contrast to the observations in MRL/lpr mice, CXCR3 ablation had no therapeutic effect in NZB/W mice, another model for lupus nephritis [25]. The inflamed kidneys and the spleen resemble a major site of accumulation and persistence of plasma cells in this model [28]. Despite the fact that approximately 40 % of plasma cells and effector T cells, present within the inflamed kidneys of NZB/W mice, express CXCR3 [26], CXCR3 deficiency did neither result in a reduced plasma cell nor T-cell infiltrates. Older CXCR3-deficient NZB/NZW mice also exhibited a massive kidney pathology similar to that seen in CXCR3-competent NZB/NZW mice. Interestingly, during renal disease onset, the total and autoreactive IgG1 serum antibodies were transiently reduced in CXCR3-deficient NZB/W mice to approximately 50 % of that in CXCR3-competent NZB/W mice. In contrast, antibodies of other subclasses were not affected [25]. Hence, suggesting that CXCR3 may also permit another so far unknown function during establishing an antibody response.
Of note, these findings do not exclude a role for CXCR3 and its ligand for the attraction of migratory plasma cells (and T cells) to the inflamed kidneys. Attraction by ligands for other chemokine receptors may substitute for the loss of CXCR3 signaling. Candidates are CCR2 and CXCR4. Plasma cells can express the chemokine receptor CCR2 that mediates migration towards the inflammatory chemokine “monocyte chemoattractant protein1” (CCL2). This chemokine is upregulated in serum and urine of lupus patients [29, 30]. However, in our studies, we could not demonstrate plasma cell migration towards CCL2 [3], and another study suggested that CCR2-mediated signals suppress immunoglobulin production rather than mediating plasma cell migration [31]. Thus, despite plasma cells expressing CCR2, a role for CCR2 in mediating plasma cell migration remains to be established. Since apart from CXCR3, CXCR4 is the only chemokine receptor that mediates the chemoattraction of plasma cells newly generated in systemic immune responses, and because the CXCR4 ligand CXCL12 is expressed in the glomeruli of NZB/W mice (Manz unpublished results), we speculate that CXCR4 permits the accumulation of CXCR3-deficient plasma cells in this mouse model. The roles of these chemokines and their cognate receptors in the inflammatory lesions of human diseases still need to be elucidated.
Both immunization/vaccination via the parenteral and mucosal routes induce migratory plasma cells transiently found in the peripheral blood. The relative contribution of systemic- and mucosal-derived migratory plasma cells depends on the immunization and health status. Under physiological conditions, the great majority of plasma cells found in the peripheral blood of healthy donors are derived from mucosal immune responses. However, approximately 1 week after parenteral tetanus/diphtheria vaccination, vaccine-specific plasma cells that migrate to the bone marrow and possibly also to inflamed tissues transiently dominate the pool of peripheral blood plasma cells [32]. Plasma cells induced by B-cell activation within mucosa exhibit distinct adhesion molecule and chemokine receptor expression profiles, mediating their preferential re-location to mucosa [33].
Tissue specific heterogeneity of plasma cell properties and phenotypes
Efficient rituximab-mediated B-cell depletion results in the eradication of some, but not all, autoantibodies [8]. Moreover, antibodies to vaccine antigens are completely stable for at least 1 year after initial treatment, even in the complete absence of B cells in the peripheral blood. These results indicate that long-lived plasma cells that can provide persistent antibody production in the absence of B cells and continuous plasma cell formation also exist in humans, as it had been demonstrated before in mice. The presence of functionally distinct plasma cell subtypes such as short- or long-lived cells is reflected by a high degree of heterogeneity of antibody-secreting plasma cells present in different tissues and also among the plasma cell population within the same tissue [34, 35]. Tonsilar plasma cells exhibit the typical phenotype of an early plasma cell stage which still expresses high levels of the B-cell markers CD19 and CD20 and CD22 [34]. Antibody-secreting cells in this tissue also exhibit heterogeneity in the expression of CD38, a marker often used to define human plasma cells [35]. Tonsilar plasma cells express very low levels of Bcl-2, suggesting that these cells are prone to apoptosis and may mainly represent short-lived plasma cells. In contrast, the bone marrow plasma cells uniformly exhibit Bcl-2 expression at high levels [34]. Hence, they are likely to represent long-lived cells, as it had been shown for the murine bone marrow plasma cells [4, 5]. Nevertheless, subpopulations exist even within the bone marrow plasma cell compartment, as evidenced by the existence of populations showing distinct expression levels of CD19, heterogeneic expression of CD9, and cell size [35].
The nature of splenic plasma cells is even less clear. Based on some morphologic, molecular, and functional characteristics, they share with the bone marrow plasma cells; it had been postulated that human splenic plasma cells also contain long-lived cells [36]. Indeed, murine spleens contain considerable proportions of long-lived plasma cells (Manz et al, unpublished observation). Precursors of long-lived plasma cells induced after immunization in adult mice mostly leave the spleen to find a survival niche in the bone marrow [3]. It is possible that long-lived plasma cells in the spleen are formed early in life but not later. However, this idea needs to be further elucidated. Also, little is known about the phenotype of plasma cells in human gut-associated tissues. A recent study showed that intestinal IgA-producing plasma cells in mice contain at least two functionally distinct subsets of CD11b-positive and CD11b-negative cells. The former are found in Peyer’s patches, but not in the lamina propria mediating the early phase of the intestinal IgA responses induced by oral immunization with protein antigen. CD11b-positive IgA-secreting plasma cells are also found in the systemic compartments of the mouse, e.g., in the spleen, but these cells seem to exhibit other immunological characteristics than those of the CD11b-positive plasma cells in the intestine [37]. In conclusion, plasma cells exhibit a considerable degree of heterogeneity. The relevance and function of the distinct plasma cell subtypes are not completely understood. In particular, their roles in immune protection and/or the production of pathogenic antibodies, their susceptibility to immunosuppressive therapy, and the role their niches within their various deposit tissues play for the development of these plasma cell subpopulations need to be further elucidated.
Survival niches
Survival of pathogenic plasma cells even under therapy is so far an unsolved problem [38]. The anti-apoptotic factors supporting the persistence of long-lived plasma cells within their niches are likely to protect them also during therapy [20]. Studies in mice indicate that the great majority of long-lived plasma cells induced after vaccination/immunization reside in the bone marrow [3–5]. There is some evidence that long-lived plasma cells are also present in human spleens from healthy donors [36]. In mice, immunization induces very few long-lived plasma cells that are found in spleen months after immunization [4]. In contrast, spleens from the F1 generation of crosses between New Zealand Black and New Zealand White (NZB/W) mice which spontaneously develop hypergammaglobulinemia and a lupus-like disease contain 1–2 % of plasma cells, with a large proportion resembling long-lived cells [6], some of them producing autoantibodies to dsDNA. In patients suffering from SLE and mouse models of this disease, plasma cells can be also detected within the inflamed kidneys [28]. Bromodeoxyuridine (BrdU) incorporation experiments in mice indicate that many of them resemble long-lived cells [39]. Aside from the spleen and bone marrow, plasma cells can also reside in other chronically inflamed tissues. In the CNS in induced multiple sclerosis [40], in the lung during chronic airway inflammation induced by allergy [9], in the salivary glands of patients with Sjögrens syndrome [41], as well as in the synovium in rheumatoid arthritis [42].
The needed factors for plasma cells to survive in these tissues are provided by their microenvironmental niches within the various deposit organs. Distinct cellular and molecular compositions of plasma cell survival niches and differential availability of survival factors provide a likely explanation for the tissue-specific variations of plasma cell half-lives in splenic red pulp, bone marrow, and lamina propria of the intestine as determined by BrdU incorporation experiments [43].
The so far best characterized niche supporting plasma cell growth and survival lies within the bone marrow. This niche seem to provide an optimal combination of survival factors relevant for plasma cells and may be crucial for maintaining protein-specific antibodies induced after immunization/vaccination and infection and also for the production of therapy-resistant autoantibodies [7]. Fibroblast-like stromal cells expressing high levels of the chemokine CXCL12 were the first component of plasma cell niches to be identified. These cells differ from other stromal cells by a distinct cytokine expression profile [20]. More recent results show that in addition to stromal cells, hematopoietic lineage cells such as macrophages, dendritic cells, megakaryocytes, and eosinophils also contribute to the function of these microcompartments [11, 12, 44, 45]. These hematopoietic cells represent a second component of plasma cell niches, essential for their function [46]. Whether these distinct hematopoietic cells provide specific functions within plasma cell niches or can substitute for each other remains unclear. Some experiments indicate that their roles may change during ontogeny and differ between tissues [11, 44, 45]. In inflamed tissues, the survival factors are considered to be produced either by immune cells attracted to the inflammation or by epithelial cells activated by the inflammation. For instance, dendritic cells, monocytes/macrophages, and neutrophils are capable of IL6 and/or proliferation-inducing ligand (APRIL) production. Inflamed lymph nodes of immunized mice show high levels of APRIL, TACI, BCMA, and CXCL12 mRNA in the medullary cords, where plasma cells reside during antibody production. These factors are considered to be produced by Gr1 + CD11b + F4/80 + monocyte/macrophages [45], creating the microenvironment needed for prolonged plasma cell survival. If mouse lymph nodes are chronically inflamed, autoreactive plasma cells can even survive for several weeks to months as we have recently shown in an autoimmune model for epidermolysis bullosa acquisita (Manz and Tiburzy, unpublished results).
Plasma cells can also be generated and show prolonged survival in inflamed non-lymphatic tissues, like the synovial tissues of rheumatoid arthritis patients and the salivary glands in Sjögren’s syndrome [47]. In Sjögrens syndrome, the ductal epithelium and acini of the salivary glands can express high levels of CXCL12, while IL-6 is provided by interstitial cells, creating a survival niche for non-proliferating plasma cells [41]. In rheumatoid arthritis, plasma cells reside in niches provided by the inflamed synovial tissue [42], providing an environment rich in APRIL produced by infiltrating neutrophils and macrophages [48].
There are distinct types of plasma cell niches that provide the discussed factors necessary for plasma cell survival, even within a given tissue, as indicated by our recent finding that plasma cells that produce antibodies of the IgA subclass are found in other niches in the bone marrow than plasma cells that produce IgM antibodies [11]. Distinct cellular and molecular compositions of plasma cell survival niches and differential availability of survival factors provide a likely explanation for the tissue-specific variations of plasma cell half-lives in the splenic red pulp, bone marrow, and lamina propria of the intestine as determined by BrdU incorporation experiments [43].
Plasma cells in mucosal tissues
Although IgG represents the main isotype in the serum, antibodies of IgA isotype are produced in higher quantities [3]. In a steady state, the main fraction of antibody-secreting cells circulating in the peripheral blood is derived from mucosal immune responses [32]. IgA present in the mucus layer serves as a first line of immune defense against pathogen invasion through mucosal surfaces: it neutralizes pathogenic microbes and their products, e.g., toxins, acts as a barrier for commensal bacteria [49], and seem to be involved in the induction of tolerogenic dendritic cells [50]. However, mucosal plasma cells can also produce IgA autoantibodies that contribute to immune disorders such as celiac disease [51] and autoimmune blistering skin diseases [52]. Class switch of B cells to IgA is induced in isolated lymphoid follicles, Peyer’s patches, and mesenteric lymph nodes. Factors like TGF-ß and APRIL are abundant in these tissues and promote class switching of plasma cell precursors to IgA [53]. Mucosal IgA + plasmablasts originating from Peyer’s patches migrate to the mesenteric lymph nodes via lymph vessels and then enter the blood circulation through the thoracic duct to the home to the diffuse lamina propria. The chemokine receptors CCR9 and CCR10 on plasmablasts are responsible for homing to the small and large intestine, respectively, and mucosal plasmablasts express the mucosa-specific adhesion molecule α4β7 [19]. Another fraction of IgA + plasma cells is not induced in Peyer’s patches but generated locally within the lamina propria, without the need for re-circulation [54]. A recent study has described that some lamina propria plasma cells are able to produce tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (INOS), revealing an unexpected heterogeneity in the antibody-secreting cell compartment in the gut [55].
IgA production by mucosal plasma blasts critically depends on the presence of gut microbiota in the gastrointestinal tract [49]. Moreover, the gut flora has been shown to influence the IgA repertoire, leading to diverse, unique repertoires with a low degree of overlap between individuals at least in the murine system [56]. However, little is known about the reactivity of antibodies generated in mucosal immune responses. Cloning and in vitro expression of antibodies from ileal plasma blasts of healthy donors revealed that the majority of IgA- and also IgG-secreting plasma blasts were generated in response to intestinal microbes. The majority of mucosal antibodies were somatically mutated, and their sequences showed evidence of antigenic selection, indicative of their generation in mucosal immune responses. Interestingly, a fraction of these plasma blasts also secreted self-reactive antibodies [57], some of them were directed against tissue structures in the intestine. In a recent study, the antibody repertoire from the intestinal plasma blasts of patients with celiac disease was analyzed. This chronic inflammatory disease is characterized by the presence of antibodies against transglutaminase 2 (TG2) in the serum and intestine, which are used as a diagnostic marker. In line with this, the frequency of TG2-specific antibody-secreting cells was significantly expanded in the small intestine lamina propria of patients with active disease. Although these self-reactive antibodies bound TG2 with a high affinity, they showed a rather low degree of somatic hypermutation, indicating that the germline repertoire in these individuals is decisive in the generation of autoreactive plasma cells in these patients [51]. The authors suggested the TG2-specific antibody-secreting cells in the lamina propria to be rather long-lived. This conclusion is based on the observations that these cells lack the proliferation marker Ki-67and that TG2-specific plasma blasts could not be detected in the peripheral blood of those patients. Although the exact lifetime of plasma cells generated in mucosal immune responses has not been determined yet, there is evidence from the murine system that long-lived plasma cells may be present in the intestine [58, 59]. However, after intestinal plasma cells have been depleted in mice using bortezomib, the intestine was re-colonized with plasma cell clones that were identical to the previously depleted ones. This could be explained by the rapid differentiation of intestinal memory B cells into antibody-secreting cells that replenish plasma cells present in the lamina propria [56]. In line with this, a population of α4β7 + memory B cells has been shown to be present for at least 9 months in Peyer’s patches of the small intestine after rotavirus infection in mice [60]. The presence of memory B cells in mucosa associated lymphoid tissues and their prominent role in preserving an individual’s antibody repertoire may have implications for the design of therapeutic strategies against autoreactive plasma cells in these tissues.
Plasma cell susceptibility to existing and experimental therapies
Drug-based standard therapies often fail to completely eliminate pathogenic autoantibodies [7, 38, 61, 62]. A promising novel strategy that eliminates mature plasma cells is small molecule-mediated proteasome inhibition. Treatment with the proteasome inhibitor Velcade/bortezomib showed very promising results in mouse models for systemic autoimmune lupus, ANCA-induced murine glomerulonephritis, and experimental autoimmune myasthenia gravis in rats [63–65]. Preliminary results testing this strategy in patients with refractory autoimmune disease are also promising, indicating that plasma cell depletion provides a reasonable approach to treat antibody-mediated autoimmune diseases [38].
However, its relative severe side effects prevent Velcade/bortezomib from application in most patients that do not suffer from an acute, life-threatening disease. Development of novel proteasome inhibitors which promise less site effects are on their way, but not all of them seem to have the same profound capacity to deplete long-lived plasma cells than was initially reported for Velcade/bortezomib [66].
In order to serve the needs for more specific therapies that deplete pathogenic antibodies, the humanized monoclonal antibody rituximab that targets the B-cell specific molecule CD20 has been approved for the treatment of various autoimmune diseases such as rheumatoid arthritis or anti-neutrophil cytoplasmic antibody-associated vasculitides. An alternative to this approach is belimumab, a drug that blocks BAFF. This reagent is licensed for the treatment of active, seropositive SLE. Both rituximab and belimumab efficiently eliminate activated B cells, early plasma cell stages, and prevent plasma cell formation. However, as described above, CD20 is completely downregulated during plasma cell differentiation. Accordingly, like current drug-based standard therapies, rituximab and belimumab fail to eliminate long-lived plasma cells. Based on the observation that even mature plasma cells retain various high or low levels of CD19 expression, therapeutic anti-CD19 antibodies are promising candidates to directly target pathogenic plasma cells [67]. As discussed below, the therapeutic manipulation of plasma cell niches may offer alternative and much milder approaches for the treatment of antibody-mediated autoimmune diseases and may possibly also allow the elimination of allergen-specific IgE.
Plasma cell niches as potential therapeutic targets
The supportive environment of their niche—i.e., the local production of anti-apoptotic survival factors—is likely to protect mature plasma cells from conventional therapy [20, 38]. Potent anti-apoptotic factors for human and murine plasma cells are IL-6 and CXCL12, signaling via CD44 and the “B-cell maturation antigen” (BCMA), a receptor for the cytokines’ APRIL and “B-cell activating factor” (BAFF) [10, 68]. While B cells express the receptors for IL-6, CXCL12, and CD44, BCMA is absent on B cells, but upregulated during their terminal differentiation into plasma cells. BCMA-deficient mice show a normal formation and expansion of antigen-induced plasma cell populations in secondary lymphoid tissues, but exhibit a 70 to 80 % reduction of immunization-specific plasma cells accumulating in the bone marrow [68]. Studies in APRIL- and BAFF-deficient mice showed that APRIL, but not BAFF, is required for generating a normal bone marrow plasma cell compartment [44]. Together, these studies indicate an essential role for APRIL-mediated BCMA signaling for the formation and/or survival of bone marrow plasma cells. Mice deficient for IL-6 exhibit a considerable delay in the formation of the immunization-induced population of bone marrow plasma cells, while their capacity to sustain plasma cell survival is not much impaired [10]. Hence, IL-6 is not required for long-term plasma cell survival in vivo, but its profound capacity to suppress plasma cell apoptosis might be crucial for the survival and expansion of early plasma cells and for the abnormal expansion of plasma cell populations, as observed under some pathogenic conditions, such as autoimmune lupus.
Importantly, as independently shown by us and others, the composition of distinct plasma cell niches offers novel options to manipulate plasma cell populations and antibody responses: (I) Very recently, the group of Claudia Berek showed that eosinophils produce large quantities of the major plasma cell survival factor APRIL and contribute to the formation of plasma cell niches. Accordingly, a single injection of an antibody that specifically depletes eosinophils led to a 50 % drop in plasma cell numbers in the bone marrow, the major site for the production of memory antibodies [12]. (II) Additionally, our group demonstrated that megakaryocytes also contribute to at least 30 % of plasma cell niches in this organ and that injection of thrombopoietin—a megakaryocyte-specific growth factor—boosts antibody responses because it allows newly formed plasma cells to home to the bone marrow and persist there in increased numbers [11]. Together, these results provide a first line of evidence and the proof-of-principle that plasma cell niches represent a promising target to suppress pathogenic antibodies that are not sufficiently suppressed by current therapies. Importantly, eosinophil and megakaryocyte deficiencies lead to a specific reduction of plasma cells in the bone marrow, but not in other tissues. Hence, the therapeutic targeting of their niches may offer the possibility to deplete plasma cells in a tissue-specific manner.
MicroRNA regulation of plasma cells
As discussed below, plasma cell survival factors such as IL-6 and CXCL12 provide anti-apoptotic signals via mammalian microRNAs (miRNAs), indicating that these pathways represent potential therapeutic targets for the suppression of pathogenic antibodies. Since their discovery, miRNAs have emerged as critical regulators of many cellular processes including the development, differentiation, and survival of immune cells by post-transcriptional gene regulation [69]. miRNAs are endogenously encoded 19–23 nucleotides long, in many cases evolutionary conserved, single-stranded RNAs, and several of them exhibit highly specific, regulated expression patterns. They bind partially complementary to their target sequences and thereby causing the downregulation of the target by either translational repression or degradation. Deletions or dysregulated expression of individual miRNA species are associated with altered cellular functions and consequently with immune defects and malignancies. In the last years, a fraction of miRNAs was identified to be important in many aspects of plasma cell biology. The highly conserved miRNA-155, which is encoded within the B-cell integration cluster (bic) and expressed at high levels in germinal center (GC), was described to be elementary in B-cell immunity [70]. Mice harboring a germline deletion of miR-155 show a defective extracellular response for switched isotypes to both thymus-independent (TI) and thymus-dependent (TD) antigens including decreased numbers of GC B cells, decreased memory B cells, reduced IgG1 production, and decreased IgG1 + plasma cells in the bone marrow [71, 72]. Molecular mechanisms responsible probably include the target proteins AID, SHIP1, HGAL, and PU.1 [72–75]. Along with miR-155, the members of the miRNA cluster miR-125b and miR-125a are preferentially expressed by actively proliferating centroblasts within GC, and particular miR-125b affects plasma cell differentiation and survival by targeting the transcription factors BLIMP-1 and IRF4 [76]. Another plasma cell-relevant miRNA, miR-24-3p, is highly conserved and strongly expressed in human B cells, plasma blasts (Pb), and plasma cells as well as in myeloma cells, a neoplasm of mature plasma cells specifically residing within the bone marrow. Functional studies revealed an anti-apoptotic function of mature miR-24-3p in plasma cells. Similarly, miR-24-3p expression becomes upregulated by IL-6 and CXCL12, both part of the bone marrow survival niche, and is associated with IL-6-mediated survival under endoplasmic reticulum (ER) stress conditions. One possible mechanism on how miRNA-24-3p contributes to plasma cell survival is suggested in directly targeting the mitochondrial and ER stress-induced protein Bim [77].
In the last years, it has become clear that the dysregulation of miRNA expression and function, caused by mutations, epigenetic inactivation, and gene amplification, is associated with plasma cell malignancies and autoimmunity [78]. In current genome-wide expression studies, a potential association between genetic abnormalities and miRNA expression in multiple myeloma was described. Multiple myeloma, characterized by the accumulation of clonal malignant plasma cells in the bone marrow, abnormal Ig production, and severe bone lesions. Serial studies identified, e.g., the miR-17 ∼ 92 cluster, miR-19a, 19b, miR-21, and miR-181a/b as important regulators of myeloma transformation, proliferation, and survival by targeting SOCS-1, Bim, E2F1, and other genes critical in pathogenesis [79–81]. Mice engineered for the high expression of the miR-17 ∼ 92 cluster in lymphocytes developed lymphoproliferative disease and autoimmunity. Strong repression of the tumor-suppressor PTEN and anti-apoptotic Bim through miR-17 ∼ 92-mediated silencing led to increased proliferation and less apoptosis parental for the development of both lymphoproliferative disease and autoimmunity [82]. Another prominent miRNA mechanism in myeloma biology represents the p53-mediated miRNA regulation. The p53-induced miRNAs 194, 192, and 215 have been reported to be downregulated in the subsets of newly diagnosed multiple myeloma (MM) and to modulate the expression of MDM2, a negative regulator of p53. Enhanced expression of these miRNAs reinforced p53 activity and was associated with impaired myeloma cell growth. Additional to their role in the p53-mediated regulation of myeloma development, miR-215 and miR-192 prevent the increased migration of plasma cells to the bone marrow by targeting the members of the IGF pathway [83]. Although in the last years, many links between miRNAs and malignancy emerged, important questions remain open to clearly define the mechanisms of miRNA-induced silencing in MM.
In contrast to their role in plasma cell neaoplasm, the role of miRNAs in plasma cell-mediated chronic inflammatory disease and autoimmunity is almost unknown. Several miRNAs were identified as markers in a variety of autoimmune diseases, but many of them address T-cell function. To date, only a few are known to be associated with plasma cell-mediated autoimmunity. Conditional deletion of the endoribonuclease dicer in murine B cells causes an overrepresentation of transitional and marginal zone (MZ) B-cell compartments with the simultaneous reduction of follicular B cells. Impaired generation of follicular B cells is supposed to be a result of reduced BCR signaling as a consequence of Btk downregulation through miR-185. Furthermore, dicer deletion in mature B cells led to a skewed Ig repertoire, and female mice developed an autoimmune disease with high titers of autoantibodies in serum and severe progression [84]. Recently, a miR-146a null mouse has been described to develop a severe autoimmune disorder with enlarged spleen and lymph nodes, high autoantibody titers against double-stranded DNA together with increased B- and T-cell activation [85]. MiR-146a is suggested to be involved in the pathogenesis of other autoimmune diseases like rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) Patients with RA have high amounts of miR-146a in their synovial tissue compared to patients with osteoarthritis and normal tissue [86, 87]. Furthermore, Tang et al. found out that miR-146a expression in blood leukocytes of patients suffering from SLE leads to an abnormal activation of the type I IFN pathway. In the same study, miR-146a was identified to be a regulator of multiple IFN signaling proteins, including TLR-7/-9, STAT1, and TRAF6/IRAK-1 and as a potential biomarker for disease activity [88]. MiR-146a is one of the best characterized miRNAs, but a defined role in plasma cell-mediated autoimmunity is not clearly determined. Future studies will certainly reveal additional links between the mechanisms of miR-146a-mediated gene silencing and other still unknown miRNAs and development/progression of PC-mediated autoimmune disease.
The role of miRNAs in plasma cell- mediated autoimmunity, chronic inflammatory disease, and malignancy is an area of intensive research with high potential for the development of new therapeutic strategies. But many questions, addressing the understanding of miRNA biology in plasma cells, remain open. First, which single miRNAs or miRNA clusters are involved in fundamental cellular processes, whose dysregulation leads to pathogenesis? A second question is how miRNA expression and turnover are regulated in general. Insights into these processes will help us to understand how miRNA-induced silencing by a given miRNA is able to regulate specific expression programs without affecting another. For example, future investigations in miRNA-mediated regulation of specific survival pathways in autoreactive or malignant plasma cells could provide new ways for drug targeting, e.g., by the identification of novel key factors associated with pathogenicity. To date, there are promising approaches for the use of miRNAs as therapeutic agents. The use of chemically engineered oligonucleotides to inhibit the function of dysregulated miRNA species is one possible approach for miRNA-based therapy [89]. A major limitation in the use of the so-called antagomirs is the transfer into target cells. To date, viral vector-based delivery as well as the use of chemically modified RNA molecules are under intensive research.
Another role of miRNAs is their use as biomarkers for disease characterization, stage, and prognosis. Thus, recent comparative examinations of miRNA expression patterns in multiple myeloma revealed that the high expression of miR-15a, miR-16-1, and miR-17 ∼ 92 cluster is linked to a shorter progression-free survival and general prognosis [90].
Plasma cells as cellular modulators of immunity and bone homeostasis
In recent years, B lineage-derived cytokine production has gained much attention, but which B-cell subpopulations actually produce these cytokines often remained obscure. Current evidence suggests that at least in some cases, the phenotype of the cytokine producing B cells resembles that of plasma cells/plasmablasts [14–17]. Many different studies have suggested that B cells regulate adaptive immunity by the provision of cytokines [91]. While B cells have been shown to produce different cytokines like IL-6 [92], IL-17 [17], IL-12 [93], OPG [14], and TGF-beta [13], the most extensively studied B cell-derived cytokine in mouse models as well as in humans is IL-10. The IL-10-expressing B cells have been termed “regulatory B cells” since they can negatively regulate immune responses [94] mainly by the provision of IL-10, a known immune regulatory cytokine [95, 96]. Regulatory B cells have been shown to attenuate many inflammatory and autoimmune conditions in different mouse models and in humans [95, 97]. The regulatory role played by B cells can also explain the exacerbated disease observed after rituximab treatment in humans in the case of ulcerative colitis and psoriasis and also in a mouse model for multiple sclerosis. Different phenotypes have been associated with the immunoregulatory functions of B cells [98]. However, whether they resemble distinct immunoregulatory B cell subtypes or rather certain developmental stages remains to be elucidated. Interestingly, one prominent mouse regulatory B-cell type characterized by an CD1dhiCD5 + CD19hi phenotype was shown to resemble plasma cell precursors [16].
Other studies provide evidence that IL-10 expression is not only detectable in plasma cell precursors but is also maintained at least to an early plasma cell stage [13, 15]. In an attempt to convert naïve spleen B cells from mice into regulatory B cells using a fusokine of GM-CSF and IL-15 called GIFT-15, it was shown that the developed regulatory B cells upregulated the plasma cell marker CD138 and downregulated the transcription factor PAX5 which is required for the development and maintenance of a B-cell phenotype but lost in plasma cells [99]. In another study, by using the IL-10 transcriptional eGFP-reporter VertX mice, it was shown that B cells with plasmablasts/plasma cell phenotype predominantly express IL-10 in peripheral lymphoid tissues [15]. IL-10 expressing plasmablasts/plasma cells were found already in naïve mice but in much higher quantities after challenges with LPS, CpG, goat anti-mouse IgD, or during mouse cytomegalovirus (MCMV) infection. Among B-cell subpopulations, the fraction of cells expressing eGFP was highest in CD138 + plasma cells/plasmablasts. In the same study, the lack of B cell-derived IL-10 increased virus specific CD8+ T cells and plasma cell numbers during MCMV infection. Hence, suggesting that the IL-10 from plasma cells has an impact on the cytotoxic T cell response. Whether IL-10 from plasmablasts/plasma cells acts directly on T cells or provides an immune regulatory activity at the level of other antigen-presenting cells is not yet known. IL-10 + CD138hi plasma cells also developed in mice 1 day post-Salmonella infection. Mice lacking IL-10 specifically in B cells displayed an improved survival after infection with this pathogen. Together, these data suggest that IL-10 from plasma cells may suppress immunity against the infection [100]. Apart from infection models, the IL-10 + plasma cells were observed in Lyn kinase-deficient mice that develop an autoimmune disease similar to SLE [101]. In IL-10 reporter lyn knockout mice, CD138hi plasma cells again were the major producers of IL-10. Adoptive transfer of CD19 + B cells from WT but not from IL-10 knockout mice reduces the lupus-like disease in lyn knockout mice suggesting that the protective effect from B cells is IL-10-dependent. The observation that among B lineage cells, plasma cells are the dominant source of IL-10 suggests that plasma cell-derived IL-10 mediates a so far unrecognized immunomodulatory function. Consistent with the finding that normal plasma cells produce IL-10, elevated IL-10 levels were observed in multiple myeloma patients, and this cytokine was found to be expressed by various myeloma cell lines [102]. Whether the myeloma-derived IL-10 contributes to the increased susceptibility of myeloma patients to bacterial infections needs to be further elucidated. Apart from IL-10, plasma cells have been shown to secrete TGF-beta1 [13], another immunoregulatory cytokine known to suppress the activity of various lymphoid and hematopoietic cells [103].
B lineage cells also contribute to bone homeostasis by the provision of osteoprotegerin (OPG), also called osteoclastogenesis-inhibitory factor. It was shown that 64 % of bone marrow OPG in mice is expressed by B cells. Accordingly, B cell-deficient mice are osteopenic and display decreased bone marrow OPG concentrations [14]. While all B lineage cells produce OPG, plasma cells produce the highest concentrations of this cytokine and approximately 5-fold higher quantities of OPG per cell compared to naïve B cells.
Interestingly, plasma cells are capable of producing OPG that is known to have a prominent role in bone protection, but they can also secrete IL-17 that is associated with pathogenic bone destruction, e.g., in rheumatoid arthritis. Until now, IL-17 was considered mainly a T-cell cytokine, but recently it was found that B lineage cells resembling a CD138 + plasmablast/plasma cell phenotype can also express IL-17 in response to Trypanosoma cruzi infection [17]. Importantly, in the course of this infection, the IL-17 + plasmablasts are higher in numbers than IL-17-producing T cells and are required for an immune response towards the infection in vivo. The exposure to parasite-derived trans-sialidase in vitro was sufficient to trigger IL-17A and IL-17F expression in mouse and human B cells/plasma cells. This data suggests that the IL-17 from plasma cells or plasmablasts should be further explored in the context of immunity against pathogens and also in IL-17-mediated autoimmune diseases like psoriasis, rheumatoid arthritis, and multiple sclerosis. Since rituximab treatment has been successfully used as therapy in these diseases, one might speculate that the therapeutic effect of rituximab may also be partly due to the deletion of IL-17-producing plasmablasts.
Apart from the regulation of immunity by the expression of cytokines, plasma cells were shown to retain the ability to present antigen by their continued expression of MHCII and to express the co-stimulatory molecules CD80 and CD86. Through these functions, plasma cells inhibited IL-21 production and decreased Bcl-6 expression in follicular helper T cells (Tfh) in an antigen-specific manner [18]. Tfh are characterized by CXCR5 and PD-1 expression and resemble a subset of CD4 + T cells specialized to migrate to the B-cell follicles and provide T-cell help to activated B cells, hence determining the quantity and quality of the plasma cell response [104]. In the absence of plasma cells in B cell-specific Blimp-1-deficient mice, Tfh cells accumulate in significantly greater numbers in draining lymph nodes and spleen. These data provide evidence for a negative feedback loop mediated by the cognate interaction of plasma cells/plasmablasts with T cells, which eventually controls the ongoing B cell/plasma cell response and may also constrain antibody-mediated autoimmunity.
So far, plasma cells were primarily considered as producers of antibodies. However, as discussed above, plasma cells/plasmablasts also play important roles as drivers and regulators of immunity via the secretion of cytokines and as antigen-presenting cells important in infectious and autoimmune diseases.
Conclusions
Despite their important role in many autoimmune and allergic diseases, therapies directly targeting pathogenic plasma cells are not available. Current therapies mostly suppress B-cell activation and plasma cell formation resulting in the extinction of short-lived plasma cell populations but do not deplete long-lived plasma cells. Moreover, their effects on the distinct plasma cell populations which are localized in lymphoid, mucosal, and inflamed tissues need to be further elucidated. Experimental data from mouse models suggest that some existing and experimental therapies preferentially deplete plasma cells from one tissue but spare those in others. Optimal therapeutics would either selectively destroy only the pathogenic plasma cells or eliminate merely plasma cells in those tissues where pathogenic antibodies are produced, hence sparing protective antibodies and other effects plasma cells contribute to protective immunity and bone homeostasis. Multiple cell types of mesenchymal and hematopoietic origin seem to contribute to the function of plasma cell niches in a tissue-specific manner, thus offering novel approaches for the tissue-specific targeting of plasma cell niches and the selective elimination of pathogenic plasma cells.
References
Miller FR (1931) The induced development and histogenesis of plasma cells. J Exp Med 54:333–347
Ehrich WE, Drabkin DL, Forman C (1949) Nucleic acids and the production of antibody by plasma cells. J Exp Med 90:157–168
Manz RA, Hauser AE, Hiepe F, Radbruch A (2005) Maintenance of serum antibody levels. Annu Rev Immunol 23:367–386. doi:10.1146/annurev.immunol.23.021704.115723
Manz RA, Thiel A, Radbruch A (1997) Lifetime of plasma cells in the bone marrow. Nature 388:133–134. doi:10.1038/40540
Slifka MK, Antia R, Whitmire JK, Ahmed R (1998) Humoral immunity due to long-lived plasma cells. Immunity 8:363–372
Hoyer BF (2004) Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med 199:1577–1584. doi:10.1084/jem.20040168
Mumtaz IM, Hoyer BF, Panne D et al (2012) Bone marrow of NZB/W mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: implications for the treatment of autoimmunity. J Autoimmun 39:180–188. doi:10.1016/j.jaut.2012.05.010
Ferraro AJ, Drayson MT, Savage COS, MacLennan ICM (2008) Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with rituximab. Eur J Immunol 38:292–298. doi:10.1002/eji.200737557
Luger EO, Fokuhl V, Wegmann M et al (2009) Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol 124:819–826.e4. doi:10.1016/j.jaci.2009.06.047
Cassese G, Arce S, Hauser AE et al (2003) Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol 171:1684–1690
Winter O, Moser K, Mohr E et al (2010) Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood 116:1867–1875. doi:10.1182/blood-2009-12-259457
Chu VT, Fröhlich A, Steinhauser G et al (2011) Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol 12:151–159. doi:10.1038/ni.1981
Matthes T, Werner-Favre C, Zubler RH (1995) Cytokine expression and regulation of human plasma cells: disappearance of interleukin-10 and persistence of transforming growth factor-beta 1. Eur J Immunol 25:508–512. doi:10.1002/eji.1830250230
Li Y, Toraldo G, Li A et al (2007) B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109:3839–3848. doi:10.1182/blood-2006-07-037994
Madan R, Demircik F, Surianarayanan S et al (2009) Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol 183:2312–2320. doi:10.4049/jimmunol.0900185
Maseda D, Smith SH, DiLillo DJ et al (2012) Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo. J Immunol 188:1036–1048. doi:10.4049/jimmunol.1102500
Bermejo DA, Jackson SW, Gorosito-Serran M et al (2013) Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells. Nat Immunol 14:514–522. doi:10.1038/ni.2569
Pelletier N, McHeyzer-Williams LJ, Wong KA et al (2010) Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol 11:1110–1118. doi:10.1038/ni.1954
Kunkel EJ, Campbell DJ, Butcher EC (2003) Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation 10:313–323. doi:10.1038/sj.mn.7800196
Moser K, Tokoyoda K, Radbruch A et al (2006) Stromal niches, plasma cell differentiation and survival. Curr Opin Immunol 18:265–270. doi:10.1016/j.coi.2006.03.004
Kabashima K, Haynes NM, Xu Y et al (2006) Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med 203:2683–2690. doi:10.1084/jem.20061289
Odendahl M, Mei H, Hoyer BF et al (2005) Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105:1614–1621. doi:10.1182/blood-2004-07-2507
DiLillo DJ, Hamaguchi Y, Ueda Y et al (2008) Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 180:361–371
Muehlinghaus G, Cigliano L, Huehn S et al (2005) Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105:3965–3971. doi:10.1182/blood-2004-08-2992
Moser K, Kalies K, Szyska M et al (2012) CXCR3 promotes the production of IgG1 autoantibodies but is not essential for the development of lupus nephritis in NZB/NZW mice. Arthritis Rheum 64:1237–1246. doi:10.1002/art.33424
Lacotte S, Decossas M, Le Coz C et al (2013) Early differentiated CD138(high) MHCII + IgG + plasma cells express CXCR3 and localize into inflamed kidneys of lupus mice. PLoS ONE 8:e58140. doi:10.1371/journal.pone.0058140
Panzer U, Steinmetz OM, Paust H-J et al (2007) Chemokine receptor CXCR3 mediates T cell recruitment and tissue injury in nephrotoxic nephritis in mice. J Am Soc Nephrol 18:2071–2084. doi:10.1681/ASN.2006111237
Espeli M, Bökers S, Giannico G et al (2011) Local renal autoantibody production in lupus nephritis. J Am Soc Nephrol 22:296–305. doi:10.1681/ASN.2010050515
Noris M, Bernasconi S, Casiraghi F et al (1995) Monocyte chemoattractant protein-1 is excreted in excessive amounts in the urine of patients with lupus nephritis. Lab Investig 73:804–809
Amoura Z, Combadiere C, Faure S et al (2003) Roles of CCR2 and CXCR3 in the T cell-mediated response occurring during lupus flares. Arthritis Rheum 48:3487–3496. doi:10.1002/art.11350
Rafei M, Hsieh J, Fortier S et al (2008) Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 112:4991–4998. doi:10.1182/blood-2008-07-166892
Mei HE, Yoshida T, Sime W et al (2009) Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 113:2461–2469. doi:10.1182/blood-2008-04-153544
Kantele A, Kantele JM, Savilahti E et al (1997) Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol 158:574–579
Medina F, Segundo C, Campos-Caro A et al (2002) The heterogeneity shown by human plasma cells from tonsil, blood, and bone marrow reveals graded stages of increasing maturity, but local profiles of adhesion molecule expression. Blood 99:2154–2161
Arce S, Luger E, Muehlinghaus G et al (2004) CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J Leukoc Biol 75:1022–1028. doi:10.1189/jlb.0603279
Ellyard JI, Avery DT, Phan TG et al (2004) Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood 103:3805–3812. doi:10.1182/blood-2003-09-3109
Kunisawa J, Gohda M, Hashimoto E et al (2013) Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun 4:1772. doi:10.1038/ncomms2718
Hiepe F, Dörner T, Hauser AE et al (2011) Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol 7:170–178. doi:10.1038/nrrheum.2011.1
Starke C, Frey S, Wellmann U et al (2011) High frequency of autoantibody-secreting cells and long-lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice. Eur J Immunol 41:2107–2112. doi:10.1002/eji.201041315
Pachner AR, Li L, Lagunoff D (2011) Plasma cells in the central nervous system in the Theiler’s virus model of multiple sclerosis. J Neuroimmunol 232:35–40. doi:10.1016/j.jneuroim.2010.09.026
Szyszko EA, Brokstad KA, Oijordsbakken G et al (2011) Salivary glands of primary Sjögren’s syndrome patients express factors vital for plasma cell survival. Arthritis Res Ther 13:R2. doi:10.1186/ar3220
Doorenspleet ME, Klarenbeek PL, de Hair MJH et al (2013) Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity. Ann Rheum Dis. doi:10.1136/annrheumdis-2012-202861
Ho F, Lortan JE, MacLennan IC, Khan M (1986) Distinct short-lived and long-lived antibody-producing cell populations. Eur J Immunol 16:1297–1301. doi:10.1002/eji.1830161018
Belnoue E, Pihlgren M, McGaha TL et al (2008) APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111:2755–2764. doi:10.1182/blood-2007-09-110858
Mohr E, Serre K, Manz RA et al (2009) Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol 182:2113
Winter O, Mohr E, Manz RA (2011) Alternative cell types form a multi-component-plasma-cell-niche. Immunol Lett 141:145–146. doi:10.1016/j.imlet.2011.07.006
Corsiero E, Bombardieri M, Manzo A et al (2012) Role of lymphoid chemokines in the development of functional ectopic lymphoid structures in rheumatic autoimmune diseases. Immunol Lett 145:62–67. doi:10.1016/j.imlet.2012.04.013
Gabay C, Krenn V, Bosshard C et al (2009) Synovial tissues concentrate secreted APRIL. Arthritis Res Ther 11:R144. doi:10.1186/ar2817
Macpherson AJ, Gatto D, Sainsbury E et al (2000) A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222–2226
Diana J, Moura IC, Vaugier C et al (2013) Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J Immunol 191:2335–2343. doi:10.4049/jimmunol.1300864
Di Niro R, Mesin L, Zheng N-Y et al (2012) High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med 18:441–445. doi:10.1038/nm.2656
Ludwig RJ, Recke A, Bieber K et al (2011) Generation of antibodies of distinct subclasses and specificity is linked to H2s in an active mouse model of epidermolysis bullosa acquisita. J Invest Dermatol 131:167–176. doi:10.1038/jid.2010.248
Cerutti A, Rescigno M (2008) The biology of intestinal immunoglobulin A responses. Immunity 28:740–750. doi:10.1016/j.immuni.2008.05.001
Fagarasan S, Kinoshita K, Muramatsu M et al (2001) In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature 413:639–643. doi:10.1038/35098100
Fritz JH, Rojas OL, Simard N et al (2012) Acquisition of a multifunctional IgA + plasma cell phenotype in the gut. Nature 481:199–203. doi:10.1038/nature10698
Lindner C, Wahl B, Föhse L et al (2012) Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. J Exp Med 209:365–377. doi:10.1084/jem.20111980
Benckert J, Schmolka N, Kreschel C et al (2011) The majority of intestinal IgA + and IgG + plasmablasts in the human gut are antigen-specific. J Clin Invest 121:1946–1955. doi:10.1172/JCI44447
Hapfelmeier S, Lawson MAE, Slack E et al (2010) Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328:1705–1709. doi:10.1126/science.1188454
Mesin L, Di Niro R, Thompson KM et al (2011) Long-lived plasma cells from human small intestine biopsies secrete immunoglobulins for many weeks in vitro. J Immunol 187:2867–2874. doi:10.4049/jimmunol.1003181
Youngman KR, Franco MA, Kuklin NA et al (2002) Correlation of tissue distribution, developmental phenotype, and intestinal homing receptor expression of antigen-specific B cells during the murine anti-rotavirus immune response. J Immunol 168:2173–2181
Manz RA, Arce S, Cassese G et al (2002) Humoral immunity and long-lived plasma cells. Curr Opin Immunol 14:517–521
Yoshida T, Mei H, Dörner T et al (2010) Memory B and memory plasma cells. Immunol Rev 237:117–139. doi:10.1111/j.1600-065X.2010.00938.x
Neubert K, Meister S, Moser K et al (2008) The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 14:748–755. doi:10.1038/nm1763
Bontscho J, Schreiber A, Manz RA et al (2011) Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol 22:336–348. doi:10.1681/ASN.2010010034
Gomez AM, Vrolix K, Martínez-Martínez P et al (2011) Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol 186:2503–2513. doi:10.4049/jimmunol.1002539
Kasperkiewicz M, Müller R, Manz R et al (2011) Heat-shock protein 90 inhibition in autoimmunity to type VII collagen: evidence that nonmalignant plasma cells are not therapeutic targets. Blood 117:6135
Mei HE, Schmidt S, Dörner T (2012) Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther 14(Suppl 5):S1. doi:10.1186/ar3909
O’Connor BP, Raman VS, Erickson LD et al (2004) BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 199:91–98. doi:10.1084/jem.20031330
Baltimore D, Boldin MP, O’Connell RM et al (2008) MicroRNAs: new regulators of immune cell development and function. Nat Immunol 9:839–845. doi:10.1038/ni.f.209
Georgantas RW 3rd, Hildreth R, Morisot S et al (2007) CD34+ hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A 104:2750–2755. doi:10.1073/pnas.0610983104
Rodriguez A, Vigorito E, Clare S et al (2007) Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611. doi:10.1126/science.1139253
Vigorito E, Perks KL, Abreu-Goodger C et al (2007) microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity 27:847–859. doi:10.1016/j.immuni.2007.10.009
Teng G, Hakimpour P, Landgraf P et al (2008) MicroRNA-155 is a negative regulator of activation-induced cytidine deaminase. Immunity 28:621–629. doi:10.1016/j.immuni.2008.03.015
Dorsett Y, McBride KM, Jankovic M et al (2008) MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity 28:630–638. doi:10.1016/j.immuni.2008.04.002
Dagan LN, Jiang X, Bhatt S et al (2012) miR-155 regulates HGAL expression and increases lymphoma cell motility. Blood 119:513–520. doi:10.1182/blood-2011-08-370536
Gururajan M, Haga CL, Das S et al (2010) MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int Immunol 22:583–592. doi:10.1093/intimm/dxq042
Gabler J, Wittmann J, Porstner M et al (2013) Contribution of microRNA 24-3p and Erk1/2 to interleukin-6-mediated plasma cell survival. Eur J Immunol. doi:10.1002/eji.201243271
Iborra M, Bernuzzi F, Invernizzi P, Danese S (2012) MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmun Rev 11:305–314. doi:10.1016/j.autrev.2010.07.002
Pichiorri F, Suh S-S, Ladetto M et al (2008) MicroRNAs regulate critical genes associated with multiple myeloma pathogenesis. Proc Natl Acad Sci U S A 105:12885–12890. doi:10.1073/pnas.0806202105
Löffler D, Brocke-Heidrich K, Pfeifer G et al (2007) Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood 110:1330–1333. doi:10.1182/blood-2007-03-081133
O’Donnell KA, Wentzel EA, Zeller KI et al (2005) c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435:839–843. doi:10.1038/nature03677
Xiao C, Srinivasan L, Calado DP et al (2008) Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol 9:405–414. doi:10.1038/ni1575
Pichiorri F, Suh S-S, Rocci A et al (2010) Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 18:367–381. doi:10.1016/j.ccr.2010.09.005
Belver L, de Yébenes VG, Ramiro AR (2010) MicroRNAs prevent the generation of autoreactive antibodies. Immunity 33:713–722. doi:10.1016/j.immuni.2010.11.010
Boldin MP, Taganov KD, Rao DS et al (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 208:1189–1201. doi:10.1084/jem.20101823
Stanczyk J, Pedrioli DML, Brentano F et al (2008) Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum 58:1001–1009. doi:10.1002/art.23386
Nakasa T, Miyaki S, Okubo A et al (2008) Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 58:1284–1292. doi:10.1002/art.23429
Tang Y, Luo X, Cui H et al (2009) MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum 60:1065–1075. doi:10.1002/art.24436
Krützfeldt J, Rajewsky N, Braich R et al (2005) Silencing of microRNAs in vivo with “antagomirs”. Nature 438:685–689
Gao X, Zhang R, Qu X et al (2012) MiR-15a, miR-16-1 and miR-17-92 cluster expression are linked to poor prognosis in multiple myeloma. Leuk Res 36:1505–1509. doi:10.1016/j.leukres.2012.08.021
Fillatreau S (2013) Cytokine-producing B cells as regulators of pathogenic and protective immune responses. Ann Rheum Dis 72(Suppl 2):ii80–ii84. doi:10.1136/annrheumdis-2012-202253
Barr TA, Shen P, Brown S et al (2012) B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 209:1001–1010. doi:10.1084/jem.20111675
Sugimoto K, Ogawa A, Shimomura Y et al (2007) Inducible IL-12-producing B cells regulate Th2-mediated intestinal inflammation. Gastroenterology 133:124–136. doi:10.1053/j.gastro.2007.03.112
Mizoguchi A, Bhan AK (2006) A case for regulatory B cells. J Immunol 176:705–710
Moore KW, de Waal MR, Coffman RL, O’Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765. doi:10.1146/annurev.immunol.19.1.683
Fillatreau S, Sweenie CH, McGeachy MJ et al (2002) B cells regulate autoimmunity by provision of IL-10. Nat Immunol 3:944–950. doi:10.1038/ni833
Iwata Y, Matsushita T, Horikawa M et al (2011) Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117:530–541. doi:10.1182/blood-2010-07-294249
Vitale G, Mion F, Pucillo C (2010) Regulatory B cells: evidence, developmental origin and population diversity. Mol Immunol 48:1–8. doi:10.1016/j.molimm.2010.09.010
Rafei M, Hsieh J, Zehntner S et al (2009) A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med 15:1038–1045. doi:10.1038/nm.2003
Neves P, Lampropoulou V, Calderon-Gomez E et al (2010) Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 33:777–790. doi:10.1016/j.immuni.2010.10.016
Scapini P, Lamagna C, Hu Y et al (2011) B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc Natl Acad Sci U S A 108:E823–E832. doi:10.1073/pnas.1107913108
Otsuki T, Yata K, Sakaguchi H et al (2002) IL-10 in myeloma cells. Leuk Lymphoma 43:969–974
Yoshimura A, Muto G (2011) TGF-β function in immune suppression. Curr Top Microbiol Immunol 350:127–147. doi:10.1007/82_2010_87
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG (2009) Follicular helper T cells: lineage and location. Immunity 30:324–335. doi:10.1016/j.immuni.2009.03.003
Acknowledgments
The authors’ laboratory is supported by the SPP 1468 Osteoimmunology—IMMUNOBONE (DFG grant MA 2273/8-1); the GRK1727/1, the “Deutsche Krebshilfe project 108658”; the “Werner und Klara Kreitz-Stiftung”; and the Excellence Cluster “Inflammation at Interfaces”. Anja E. Hauser is supported by DFG HA5354/4-1 and DFG TRR130/P17.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a contribution to the special issue on B cell-mediated autoimmune diseases - Guest Editors: Thomas Winkler and Reinhard Voll
Rights and permissions
About this article
Cite this article
Tiburzy, B., Kulkarni, U., Hauser, A.E. et al. Plasma cells in immunopathology: concepts and therapeutic strategies. Semin Immunopathol 36, 277–288 (2014). https://doi.org/10.1007/s00281-014-0426-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-014-0426-8