Abstract
Complement-mediated cell death is caused by C5b-9, the membrane attack complex (MAC) composed of the five complement proteins C5b, C6, C7, C8, and C9. Assembly of the C5b-9 complex initiates oligomerization of C9 and production of a transmembrane protein channel that inflicts damage to target cells. For protection, cells eliminate the MAC from their surface either by ectocytosis (direct emission of membrane vesicles) or by endocytosis (internalization). The process of ectosome release is rapid and involves cytosolic Ca2+ and activation of protein kinases, such as protein kinase C (PKC) and extracellular signal-regulated protein kinase (ERK). Recently, the involvement of mortalin (also known as GRP75 and mitochondrial hsp70) in MAC elimination has been suggested. Extracellular application of antibodies directed to mortalin increases cell sensitivity to MAC-mediated lysis. Release of membrane vesicles is ubiquitous and enhanced in apoptotic or tumor cells and upon cell activation. Composition of the ectosomes (also often referred to as microparticles) membrane proteins and lipids appears to be different from those of the original plasma membrane, indicating involvement of a selective sorting process during ectosome formation. Exosomes (unlike ectosomes) are membrane vesicles generated by endocytosis, endosome sorting into perinuclear multivesicular bodies (MVB) and exocytosis of MVBs. Exosomes appear to be different in size and composition from ectosomes. Exosome-associated MAC has also been described. Although research on ectosomes and exosomes is still limited, physiological roles in coagulation, vascular functions, angiogenesis, wound healing and development have been attributed to these shed membrane vesicles. On the other hand, there are indications that elevated levels of ectosomes and exosomes may predispose to morbidity. Membrane vesicles released by cells exposed to complement MAC may play roles in health and disease beyond protection from cell death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The complement system plays important roles in activation, regulation, and execution of innate and acquired immune responses. Initiation of the complement activation cascade may occur through the classical, alternative, or lectin pathway. These three initiation pathways end up splitting complement C5 into C5a and C5b. C5b triggers a spontaneous, sequential assembly of the terminal complement complexes C5b-6, C5b-7, C5b-8, and C5b-9 followed by their membrane adhesion and insertion (reviewed in [62]). The C5b-9 complex, also known as the membrane attack complex (MAC), is composed of a C5b-8 complex attached to a transmembrane C9 oligomer (poly-C9, n=1–18) [62, 94]. The MAC is a potent membrane-damaging agent and a pore-former. The lytic capacity of the MAC was studied in depth with erythrocytes. The conclusion of these early studies was that a single functional MAC is sufficient to cause death of an erythrocyte [54]. Later studies have suggested that nucleated cells are more resistant to MAC-mediated lysis compared with erythrocytes and their lysis requires multiple MACs [44, 57]. The mechanism of cell death induced by the MAC is still not fully clear. MAC insertion is known to enhance dramatically [Ca2+ i] level [61], and this may be responsible for the ensuing mitochondrial damage, depletion of ATP, and osmotic cell lysis [71]. More recently, the MAC has also been implicated in induction of caspase-dependent apoptotic cell death in certain target cells in vitro and in vivo [21, 63, 83].
Unlike high doses of MAC that are lytic or apoptotic to nucleated cells, low, sublytic doses of MAC induce a variety of biological responses (reviewed in [6]). Depending on the cell studied, sublytic MAC has been shown to induce enhanced expression of adhesion molecules [92], release of proinflammatory mediators [40], entry into cell cycle [79], production of active oxygen metabolites [57], or increased DNA synthesis and cell proliferation [33, 65]. Sublytic MAC induces these activities by triggering several signaling pathways including Ca+2 influx, activation of phospholipases, generation of diacylglycerol (DAG) and ceramide, and activation of protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) pathways [46, 58, 66, 84].
Nucleated cells employ several defensive molecular mechanisms against complement (reviewed in [38]). Resistance is conferred by constitutive expression of membrane-associated complement regulatory proteins, such as CD46, CD55, and CD59 [43, 55, 64]. These complement regulators restrict binding of complement components to the cell surface. Defense is also conferred by ecto-proteases [24, 37] and ecto-protein kinases [5, 69]. Basal resistance of cells to complement can be augmented by treatment with various agents. Thus, treatment with sublytic MAC doses leads to desensitization to lytic MAC doses [76]. This phenotypic conversion depends on Ca+2 influx, activation of PKC and ERK and protein synthesis [46, 76]. Both PKC and ERK [16, 23, 45, 46] as well as heat shock proteins [22, 88] are involved in cell protection from complement-mediated lysis. Sublytic MAC has also been shown to exert antiapoptotic effects [87].
Elimination of the MAC by membrane vesiculation
Cells also possess the ability to evade complement attack by elimination of the MAC. After a sublytic complement attack, cells emit the MAC rapidly and actively from their plasma membrane [12, 59, 74]. Bound MAC is also removed from the surface of erythrocytes, primarily by membrane vesiculation [36]. Following a complement attack, multiple membrane protrusions appear on the cell surface [68], and membrane vesicles are shed from the cells (Fig. 1). Membrane vesicles can be released from dying cells; however, release of the MAC-containing membrane vesicles appears to be clearly associated with cell recovery from complement attack. Such complement-induced membrane vesiculation was reported with neutrophils, oligodendrocytes, platelets, glomerular epithelial cells, and with the tumor cell lines Ehrlich, U937, and K562 [12, 57, 60, 82]. As described later in this review, many cells release plasma membrane vesicles either spontaneously or after a trigger unrelated to the insertion of the C5b-9 complex. Membrane vesiculation may occur by ectocytosis or exocytosis (Fig. 2). Ectocytosis leads to formation of ectosomes, often referred to as microparticles. These are vesicles generated upon budding out and pinching off from the cell surface. In contrast, exosomes are vesicles generated following an endocytic process. They are loaded within multivesicular bodies (MVB) and released by exocytosis. Ectosomes and exosomes differ in size and composition. It is conceivable that most cells produce both ectosomes and exosomes, yet erythrocytes can probably produce only ectosomes.
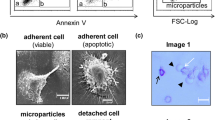
Examples of vesicles released from activated cells. a and b Membrane vesicles (see arrows) released from K562 cells treated with a sublytic dose of antibody and complement. Light microscopy, vesicles size ∼1 μm. c Ectosomes (see arrows) released from PMNs activated by fMLP. TEM, negative staining, vesicles size ∼50 nm
Schematic presentation of modes of emission of ectosomes vs. exosomes. Under sublytic conditions, the complement membrane attack complexes (MAC) may either be released by ectocytosis (a), a budding-off process, or exocytosis (b) following endocytosis and packaging in multivesicular bodies (MVB). Currently, data support MAC emission by ectocytosis
Rapid clearance of the C5b-9 complexes from the cells occurs under nonlytic conditions [10]. The membrane vesicles collected from complement-attacked neutrophils contain much of the cell-bound C9 but exclude normal cell surface constituents, suggesting selective disposal of the MAC [59]. High concentrations of the MAC cylinder-like structures, containing 12 C9 molecules per one C8 molecule, were visualized by electron microscopy on membrane vesicles shed from neutrophils following a sublytic complement attack [59]. Such ring lesions were also shown on vesicles recovered from the cerebrospinal fluid of patients with multiple sclerosis [82]. Analysis of the composition of the MAC-containing vesicles indicated a sorting process of membrane proteins and lipids in neutrophils attacked by sublytic complement [89]. The content of cholesterol and DAG was found higher in the shed vesicles. In contrast, the same quantity of sphingomyelin, phosphatidylcholine and phosphatidylinositol was found in shed vesicles and in the plasma membrane. Platelets attacked by sublytic MAC shed vesicles enriched in the plasma membrane glycoproteins GP Ib and GP IIb-IIIa, the membrane protein GMP-140, the receptor for coagulation factor Va, and the receptor for factor VIII [29, 85]. Membrane vesicles shed from erythrocytes after calcium ionophore treatment or complement attack are enriched in proteins anchored to the plasma membrane via glycosyl-phosphatidylinositol (GPI), such as acetylcholinesterase and decay-accelerating factor (DAF, CD55) [7, 100]. The presence of GPI-anchored proteins appears to be essential for ectocytosis from erythrocytes [100], but not from platelets [102].
Nucleated cells can also eliminate the terminal complement complexes from their surface by internalization or endocytosis. This was demonstrated with neutrophils, glomerular epithelial cells, and Ehrlich tumor cells [13, 39, 59]. The internalization may be followed by proteolytic degradation of the C5b-9 complex [61]. C5b-8 complexes deposited on the surface of Ehrlich cells were seen in endocytic vesicles and in MVB [13]. C5b-9 complexes deposited on glomerular epithelial cells in kidneys of rats with experimental membranous nephropathy were found in clathrin-coated pits and also in MVB [39]. The latter report suggested that C5b-9 complexes deposited in vivo on epithelial cells are endocytosed, packed into MVB, and then released by exocytosis into the urine in a degraded form, attached to membrane vesicles.
Insights into the mechanism of MAC-induced membrane vesiculation
The mechanism of the MAC-induced vesiculation process is largely unknown. The earliest signal reported in cells undergoing a MAC attack is an increase in intracellular Ca+2 levels [8, 9]. It was suggested that this rapid rise in intracellular Ca+2 could signal MAC removal from the plasma membrane [12]. Chelation of intracellular Ca+2 inhibited MAC removal and increased cell death. Elevation of intracellular calcium concentration is known to activate certain protein kinases that regulate activity of key proteins by phosphorylation. Involvement of protein kinases in MAC elimination has indeed been suggested [14, 101, 103]. Activation of protein kinases, such as PKC and extracellular signal-regulated protein kinase (ERK), by sublytic MAC was demonstrated in several cell types [16, 45, 46]. MAC removal was suggested to be dependent on PKC activation [14]. Cell resistance to complement involves PKC-dependent activation of ERK [46]. The emission of proteins from K562 cells following a trigger by a sublytic complement attack can be reduced by treatment with an ERK inhibitor (Pilzer and Fishelson, unpublished). This suggests the involvement of ERK in the MAC shedding process.
Which proteins act downstream of PKC and ERK to eliminate the MAC from the cell surface? Recent data have suggested that mortalin is involved in MAC vesiculation [73]. Mortalin (also known as GRP75, PBP74, or mitochondrial hsp70) is a member of the hsp70 family (reviewed in [99]). It is found mainly in mitochondria but to some extent also in the cytoplasm, endoplasmic reticulum, and cytoplasmic vesicles [75]. Mortalin is constitutively expressed in cells, participating in stress response [11] and mitochondrial import [98], and is frequently upregulated in tumors [18, 90]. We (Pilzer and Fishelson) found that mortalin is released from K562 cells within minutes after an attack by sublytic complement together with the MAC, probably on membrane vesicles. Mortalin release depends on C5b-8 and C5b-9 formation and on PKC and ERK activation. Interestingly, the presence of anti-mortalin antibodies outside the cells during complement attack reduces release of C9 and mortalin and augments cell death [73]. Therefore, it appears that mortalin is involved in the process of MAC elimination and in cell defense from complement. The mode of action of mortalin is still not known, yet its ability to bind C8 and C9 [73] may suggest a role for mortalin in MAC assembly prior to vesicle formation. The contribution of plasma membrane remodeling to MAC vesiculation still awaits further examination.
Complement-independent release of vesicles by normal and malignant cells
As described above, complement is a potent inducer of release of membrane vesicles. Ectocytosis and exocytosis are a general biological property of most eukaryotic cells. For instance, polymorphonuclear leukocytes (PMN) release ectosomes after stimulation with calcium ionophores, phorbol esters, and microbial peptides such as formyl-methionyl-leucyl-phenylalanine (fMLP) [26, 35] (Fig. 1c). Chondrocytes continuously release ectosomes loaded with annexin I, which induce calcium precipitation and bone formation [2]. The number of different cells which have been described to undergo ectocytosis in vitro as well as in vivo has been growing over recent years and now covers a large spectrum of cell types, in particular, many tumor cells and cell lines, endothelial cells, and almost all blood cell types. Ectocytosis has also been described in platelets, monocytes, and lymphocytes [52, 81, 85]. The physiological distinction between ectosomes and exosomes in platelets might be difficult since both are released concomitantly when platelets are activated [34]. Formation of exosomes and ectosomes can be well distinguished in developing erythrocytes. The loss of transferrin receptors occurs during erythrocyte maturation in part through the formation of exosomes, which are then continuously shed until complete loss of transferrin receptors [70]. In contrast, stressed erythrocytes shed ectosomes (e.g., upon ATP depletion activating in vitro aging or a complement attack) [72]. Whether or not ectosomes released in response to diverse stimuli have distinct structure and composition remains to be determined.
Recent studies, mainly of PMN and erythrocyte ectosomes, have characterized some of the biological properties of ectosomes. First, ectosomes express an array of proteins different from that present on the surface of the cell from which the ectosomes have been released. For example, ectosomes released by PMN express selectins, integrins, complement regulators, HLA-1, FcγRIII, and CD66b, but lack CD14 and FcγRII, which are found on the surface of resting PMNs [26]. Ectosomes released by a monocytic cell line THP-1 have been reported to be loaded with IL-1β [51]. Thus, the transfer of molecules into ectosomes is a selective process. Very little is known about the mechanism responsible for such a protein sorting. The selection of the proteins incorporated into ectosomes appears to be unrelated to the type of membrane anchor since both GPI-linked as well as transmembrane proteins have been found on the surface of ectosomes [26]. Both complement receptor type 1 (CD35, a transmembrane protein) and DAF (CD55, GPI-anchored) are similarly enriched in ectosomes released from erythrocytes [72]. However, the fact that major differences are observed in both lipid composition of ectosome vs. plasma membranes and the distribution of the lipids within the membranes suggests that certain membrane lipids play a major role in ectosome formation [49]. The process of ectosome formation, like apoptotic cell death, involves externalization of phosphatidylserine (PS). Interestingly, proteins that are degraded on activated PMNs, may remain intact on ectosomes. For example, L selectin is cleaved on activated PMNs to permit migration through the endothelial cell layer. In contrast, on PMN ectosomes, it remains intact, suggesting that the L selectin protease has been excluded from the ectosomes. Another surprising finding is that PMN ectosomes express on their surface enzymes, e.g., myeloperoxidase, which are normally stored in granules in resting PMNs. The expression of proteins in ectosomes may therefore depend on both packaging of proteins during ectosome formation and further on binding of soluble proteins after their release.
Ectosomes and exosomes express membrane complement regulatory proteins that have originated either from the cell surface or from intracellular stores, respectively. PMN-derived ectosomes express CR1 (CD35), MCP (CD46), DAF (CD55), and CD59. The expression of membrane regulators has been shown to confer protection from complement on the released membrane vesicles [15, 32]. Thus, membrane regulators might significantly modify the fate of microvesicles, allowing them to remain intact and functional. Synthetic liposomes containing PS activate complement and are consequently lysed. In contrast, PMN-derived ectosomes activate complement and fix C1q, C4, and C3 as well as a small amount of C5b-9, but are not lysed [28, 47]. Thus, ectosomes opsonised with C3 fragments may bind to cells expressing C3 receptors, in particular CR1. In blood, the bulk of CR1 is expressed by erythrocytes. Recent studies have indicated that PMN ectosomes attach rapidly to erythrocytes when formed in whole blood [28]. Thus, ectosomes, like immune complexes, are probably transported in the circulation and may reach the liver/spleen, where they are likely to be downloaded similarly to immune complexes. This observation may explain why only few ectosomes can be isolated from blood plasma, even in diseases such as sepsis, in which continuous and strong activation of PMN is a central feature.
Release of membrane microparticles, i.e., 0.05- to 1-μm membrane vesicles, from stressed and/or apoptotic cells has been shown to be accompanied by plasma membrane changes in the form of membrane blebbing and externalization of phosphatidylserine (PS) (reviewed in [25]). However, this is probably not a general phenomenon. Studies with endothelial cells suggest that a large fraction of microparticles released from activated and apoptotic cells do not bind measurable amounts of annexin V, implying that the expression of PS on them is absent or very low [1]. Release of microparticles from apoptotic neutrophils depends on different signals from those required for membrane blebbing and PS externalization and is therefore occurring independently of these two hallmarks of apoptosis [67]. It still remains to be determined whether apoptosis-derived microparticles are comparable to ectosomes [56] or exosomes recycling through MVB [67].
Biological consequences of vesicle shedding
Should the released membrane vesicles be regarded simply as waste products, which are disposed of by macrophages, or perhaps they play important roles in inflammation and other biological reactions? Exosomes are capable of eliciting specific immune responses [3, 86, 93]. Ectosomes of endothelial cells, monocytes, and platelets have been shown to induce and enhance thrombosis [80, 81], whereas ectosomes of chondrocytes support ossification [2]. Many other essential functions might be related to vesicles in general. Monocytes may release IL-1β by ectocytosis. Recently, the cell-to-cell transfer of chemokine receptor has been suggested to be due to ectosomes shuttling from one cell to another [50]. PMN ectosomes express enzymes capable of attacking microorganisms, and they may well be doing so at sites of acute infection [26]. On the other hand, the same ectosomes, when ingested by monocytes/macrophages, downregulate the inflammatory potential of these cells [27]. It is conceivable that this latter property will protect the organism from excessive inflammation at a site of infection.
Release of membrane vesicles is also a common feature of tumor cells [91]. It is conceivable that these cells release both exosomes and ectosomes. Prostasomes released by prostate carcinoma cells and normal epithelial cells are found in semen [78]. Like exosomes, they are enriched in raft molecules found intracellularly in MVB, and their release is sensitive to wortmannin. Hence, prostasomes could actually represent specific exosomes of the prostate tissue [48]. The high cholesterol and sphingomyelin content of the extracellular membrane vesicles [4, 89, 95, 104] indicates increased membrane rigidity. Membrane vesicles released from tumor cells have been suggested to facilitate tumor growth, tissue invasion, and immune evasion by bearing matrix-degrading proteases [17, 31], angiogenic activity [41], and Fas ligand [91]. These membrane vesicles also contain protein kinase CK2 that has been shown to phosphorylate complement C9 and protect from complement-mediated lysis [5]. Membrane vesicles collected from malignant tumor fluids correlate positively with tumor malignancy. Therefore, measurement of membrane vesicle content and associated metalloproteinase level may serve as markers for tumor aggressiveness and progression [30].
Conclusions
Despite the fact that MAC removal by vesiculation is a widely accepted resistance mechanism against complement, still very little is known about the process of activation and regulation. Perhaps through mortalin [73], a deeper insight into the machinery of MAC vesiculation will be achieved. Mortalin is an intracellular hsp70-related protein, and it will be intriguing to realize whether and how such a protein supports membrane vesiculation. Hsc70, another intracellular hsp70-related protein that is externalized upon treatment with sublytic C5b-9 [22], is involved in clathrin-dependent endocytosis [19]. Mortalin may be similarly involved in the inverse activity, ectocytosis. To answer that, it is important to determine first whether the MAC vesicles are released from the plasma membrane by direct ectocytosis or following endocytosis, formation of MVB, and exocytosis pending fusion of the MVB with the plasma membrane (Fig. 2). At present, concomitant MAC vesiculation by ectocytosis and exocytosis cannot be excluded.
Exosomes derived from dendritic or cancer cells are also being considered as immunomodulators for cancer immunotherapy [3, 20]. Such exosomes express MHC-peptide complexes that can be targeted to antigen-presenting cells, thus amplifying specific immune responses [96]. Given that mortalin is capable of presenting antigens to T cells [42, 97] and that membrane vesicles released after sublytic complement attack may contain mortalin-peptide/protein complexes, it is intriguing to propose that, like MHC-loaded exosomes, mortalin-loaded membrane vesicles may play a role in immune regulation in normal and pathological immune responses. It is also important to note that, under certain conditions, instead of augmenting immune responses, exosomes can suppress antitumor immune responses [77, 91] and ectosomes can have anti-inflammatory effects [27]. Therefore, MAC-induced vesicles may be added to the growing list of complement-derived immunomodulators produced at complement activation sites, and future studies will reveal their effects. Furthermore, MAC-induced vesicles are likely to have numerous effects beyond the immune system, as predicted from the reported effects of membrane microparticles (ectosomes) on coagulation, vascular functions, angiogenesis, wound healing, and development (reviewed in [53, 56]). Elevated levels of microparticles may be pathogenic [25] and have already been implicated in endothelial and vascular/heart dysfunction, inflammation, autoimmunity, and tumor escape.
Abbreviations
- ERK:
-
Extracellular signal-regulated protein kinase
- fMLP:
-
Formyl-methionyl-leucyl-phenylalanine
- GPI:
-
Glycosyl-phosphatidylinositol
- GRP75:
-
75-kDa glucose-regulated protein
- MAC:
-
Membrane attack complex of complement
- MVB:
-
Endocytic multivesicular bodies
- PKC:
-
Protein kinase C
References
Ahn YS, Jy W, Jimenez JJ et al (2004) More on: cellular microparticles: what are they bad or good for? J Thromb Haemost 2:1215
Anderson HC (2003) Matrix vesicles and calcification. Curr Rheumatol Rep 5:222
Andre F, Escudier B, Angevin E et al (2004) Exosomes for cancer immunotherapy. Ann Oncol 15(Suppl 4):iv141
Arvidson G, Ronquist G, Wikander G et al (1989) Human prostasome membranes exhibit very high cholesterol/phospholipid ratios yielding high molecular ordering. Biochim Biophys Acta 984:167
Bohana-Kashtan O, Pinna LA, Fishelson Z (2005) Extracellular phosphorylation of C9 by protein kinase CK2 regulates complement-mediated lysis. Eur J Immunol 35:1939
Bohana-Kashtan O, Ziporen L, Donin N et al (2004) Cell signals transduced by complement. Mol Immunol 41:583
Butikofer P, Kuypers FA, Xu CM et al (1989) Enrichment of two glycosyl-phosphatidylinositol-anchored proteins, acetylcholinesterase and decay accelerating factor, in vesicles released from human red blood cells. Blood 74:1481
Campbell AK, Daw RA, Hallett MB et al (1981) Direct measurement of the increase in intracellular free calcium ion concentration in response to the action of complement. Biochem J 194:551
Campbell AK, Daw RA, Luzio JP (1979) Rapid increase in intracellular free Ca2+ induced by antibody plus complement. FEBS Lett 107:55
Campbell AK, Morgan BP (1985) Monoclonal antibodies demonstrate protection of polymorphonuclear leukocytes against complement attack. Nature 317:164
Carette J, Lehnert S, Chow TY (2002) Implication of PBP74/mortalin/GRP75 in the radio-adaptive response. Int J Radiat Biol 78:183
Carney DF, Hammer CH, Shin ML (1986) Elimination of terminal complement complexes in the plasma membrane of nucleated cells: influence of extracellular Ca2+ and association with cellular Ca2+. J Immunol 137:263
Carney DF, Koski CL, Shin ML (1985) Elimination of terminal complement intermediates from the plasma membrane of nucleated cells: the rate of disappearance differs for cells carrying C5b-7 or C5b-8 or a mixture of C5b-8 with a limited number of C5b-9. J Immunol 134:1804
Carney DF, Lang TJ, Shin ML (1990) Multiple signal messengers generated by terminal complement complexes and their role in terminal complement complex elimination. J Immunol 145:623
Clayton A, Harris CL, Court J et al (2003) Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. Eur J Immunol 33:522
Cybulsky AV, Bonventre JV, Quigg RJ et al (1990) Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int 38:803
Dolo V, D'Ascenzo S, Violini S et al (1999) Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis 17:131
Dundas SR, Lawrie LC, Rooney PH et al (2005) Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol 205:74
Dworniczak B, Mirault ME (1987) Structure and expression of a human gene coding for a 71 kd heat shock ‘cognate’ protein. Nucleic Acids Res 15:5181
Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16:415
Fishelson Z, Attali G, Mevorach D (2001a) Complement and apoptosis. Mol Immunol 38:207
Fishelson Z, Hochman I, Greene LE et al (2001b) Contribution of heat shock proteins to cell protection from complement-mediated lysis. Int Immunol 13:983
Fishelson Z, Kopf E, Paas Y et al (1989) Protein phosphorylation as a mechanism of resistance against complement damage. Prog Immunol 7:205
Frade R (1999) Structure and functions of proteases which cleave human C3 and are expressed on normal or tumor human cells: some are involved in tumorigenic and metastatic properties of human melanoma cells. Immunopharmacology 42:39
Freyssinet JM (2003) Cellular microparticles: what are they bad or good for? J Thromb Haemost 1:1655
Gasser O, Hess C, Miot S et al (2003) Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res 285:243
Gasser O, Schifferli JA (2004) Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 104:2543
Gasser O, Schifferli JA (2005) Microparticles released by human neutrophils adhere to erythrocytes in the presence of complement. Exp Cell Res 307:381. DOI 10.116/j.yexcr.2005.03.011
Gilbert GE, Sims PJ, Wiedmer T et al (1991) Platelet-derived microparticles express high affinity receptors for factor VIII. J Biol Chem 266:17261
Ginestra A, Miceli D, Dolo V et al (1999) Membrane vesicles in ovarian cancer fluids: a new potential marker. Anticancer Res 19:3439
Graves LE, Ariztia EV, Navari JR et al (2004) Proinvasive properties of ovarian cancer ascites-derived membrane vesicles. Cancer Res 64:7045
Hakulinen J, Junnikkala S, Sorsa T et al (2004) Complement inhibitor membrane cofactor protein (MCP; CD46) is constitutively shed from cancer cell membranes in vesicles and converted by a metalloproteinase to a functionally active soluble form. Eur J Immunol 34:2620
Halperin JA, Taratuska A, Nicholson-Weller A (1993) Terminal complement complex C5b-9 stimulates mitogenesis in 3T3 cells. J Clin Invest 91:1974
Heijnen HF, Schiel AE, Fijnheer R et al (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94:3791
Hess C, Sadallah S, Hefti A et al (1999) Ectosomes released by human neutrophils are specialized functional units. J Immunol 163:4564
Iida K, Whitlow MB, Nussenzweig V (1991) Membrane vesiculation protects erythrocytes from destruction by complement. J Immunol 147:2638
Jurianz K, Ziegler S, Donin N et al (2001) K562 erythroleukemic cells are equipped with multiple mechanisms of resistance to lysis by complement. Int J Cancer 93:848
Jurianz K, Ziegler S, Garcia-Schuler H et al (1999) Complement resistance of tumor cells: basal and induced mechanisms. Mol Immunol 36:929
Kerjaschki D, Schulze M, Binder S et al (1989) Transcellular transport and membrane insertion of the C5b-9 membrane attack complex of complement by glomerular epithelial cells in experimental membranous nephropathy. J Immunol 143:546
Kilgore KS, Schmid E, Shanley TP et al (1997) Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol 150:2019
Kim CW, Lee HM, Lee TH et al (2002) Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res 62:6312
Kim HT, Nelson EL, Clayberger C et al (1995) Gamma delta T cell recognition of tumor Ig peptide. J Immunol 154:1614
Kojima A, Iwata K, Seya T et al (1993) Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol 151:1519
Koski CL, Ramm LE, Hammer CH et al (1983) Cytolysis of nucleated cells by complement: cell death displays multi-hit characteristics. Proc Natl Acad Sci U S A 80:3816
Kraus S, Fishelson Z (2000) Cell desensitization by sublytic C5b-9 complexes and calcium ionophores depends on activation of protein kinase C. Eur J Immunol 30:1272
Kraus S, Seger R, Fishelson Z (2001) Involvement of the ERK mitogen-activated protein kinase in cell resistance to complement-mediated lysis. Clin Exp Immunol 123:366
Liu D, Liu F, Song YK (1995) Recognition and clearance of liposomes containing phosphatidylserine are mediated by serum opsonin. Biochim Biophys Acta 1235:140
Llorente A, de Marco MC, Alonso MA (2004) Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J Cell Sci 117:5343
Lutz HU, Liu SC, Palek J (1977) Release of spectrin-free vesicles from human erythrocytes during ATP depletion. I. Characterization of spectrin-free vesicles. J Cell Biol 73:548
Mack M, Kleinschmidt A, Bruhl H et al (2000) Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 6:769
MacKenzie A, Wilson HL, Kiss-Toth E et al (2001) Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity 15:825
Martin S, Tesse A, Hugel B et al (2004) Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation 109:1653
Martinez MC, Tesse A, Zobairi F et al (2005) Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol 288:H1004
Mayer MM (1961) On the destruction of erythrocytes and other cells by antibody and complement. Cancer Res 21:1262
Meri S, Morgan BP, Davies A et al (1990) Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 71:1
Morel O, Toti F, Hugel B et al (2004) Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol 11:156
Morgan BP (1989) Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J 264:1
Morgan BP, Campbell AK (1985) The recovery of human polymorphonuclear leucocytes from sublytic complement attack is mediated by changes in intracellular free calcium. Biochem J 231:205
Morgan BP, Dankert JR, Esser AF (1987) Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol 138:246
Morgan BP, Imagawa DK, Dankert JR et al (1986) Complement lysis of U937, a nucleated mammalian cell line in the absence of C9: effect of C9 on C5b-8 mediated cell lysis. J Immunol 136:3402
Morgan BP, Luzio JP, Campbell AK (1986) Intracellular Ca2+ and cell injury: a paradoxical role of Ca2+ in complement membrane attack. Cell Calcium 7:399
Muller-Eberhard HJ (1986) The membrane attack complex of complement. Annu Rev Immunol 4:503
Nauta AJ, Daha MR, Tijsma O et al (2002) The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol 32:783
Nicholson-Weller A, Wang CE (1994) Structure and function of decay accelerating factor CD55. J Lab Clin Med 123:485
Niculescu F, Badea T, Rus H (1999) Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis 142:47
Niculescu F, Rus H, Shin S et al (1993) Generation of diacylglycerol and ceramide during homologous complement activation. J Immunol 150:214
Nusbaum P, Laine C, Bouaouina M et al (2005) Distinct signaling pathways are involved in leukosialin (CD43) down-regulation, membrane blebbing, and phospholipid scrambling during neutrophil apoptosis. J Biol Chem 280:5843
Ohanian SH, Schlager SI, Borsos T (1978) Molecular interactions of cells with antibody and complement: influence of metabolic and physical properties of the target on the outcome of humoral immune attack. Contemp Topics Mol Immunol 7:153
Paas Y, Bohana-Kashtan O, Fishelson Z (1999) Phosphorylation of the complement component, C9, by an ecto-protein kinase of human leukemic cells. Immunopharmacology 42:175
Pan BT, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967
Papadimitriou JC, Drachenberg CB, Shin ML et al (1994) Ultrastructural studies of complement mediated cell death: a biological reaction model to plasma membrane injury. Virchows Arch 424:677
Pascual M, Lutz HU, Steiger G et al (1993) Release of vesicles enriched in complement receptor 1 from human erythrocytes. J Immunol 151:397
Pilzer D, Fishelson Z (2005) Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int Immunol 17:1239
Ramm LE, Whitlow MB, Koski CL et al (1983) Elimination of complement channels from the plasma membranes of U937, a nucleated mammalian cell line: temperature dependence of the elimination rate. J Immunol 131:1411
Ran Q, Wadhwa R, Kawai R et al (2000) Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun 275:174
Reiter Y, Ciobotariu A, Fishelson Z (1992) Sublytic complement attack protects tumor cells from lytic doses of antibody and complement. Eur J Immunol 22:1207
Riteau B, Faure F, Menier C et al (2003) Exosomes bearing HLA-G are released by melanoma cells. Hum Immunol 64:1064
Ronquist G, Brody I (1985) The prostasome: its secretion and function in man. Biochim Biophys Acta 822:203
Rus HG, Niculescu F, Shin ML (1996) Sublytic complement attack induces cell cycle in oligodendrocytes. J Immunol 156:4892
Sabatier F, Roux V, Anfosso F et al (2002) Interaction of endothelial microparticles with monocytic cells in vitro induces tissue factor-dependent procoagulant activity. Blood 99:3962
Satta N, Toti F, Feugeas O et al (1994) Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol 153:3245
Scolding NJ, Morgan BP, Houston WA et al (1989) Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature 339:620
Shimizu A, Masuda Y, Kitamura H et al (2000) Complement-mediated killing of mesangial cells in experimental glomerulonephritis: cell death by a combination of apoptosis and necrosis. Nephron 86:152
Shirazi Y, McMorris FA, Shin ML (1989) Arachidonic acid mobilization and phosphoinositide turnover by the terminal complement complex, C5b-9, in rat oligodendrocyte x C6 glioma cell hybrids. J Immunol 142:4385
Sims PJ, Faioni EM, Wiedmer T et al (1988) Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem 263:18205
Skokos D, Botros HG, Demeure C et al (2003) Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 170:3037
Soane L, Cho HJ, Niculescu F et al (2001) C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J Immunol 167:2305
Sreedhar AS, Mihaly K, Pato B et al (2003) Hsp90 inhibition accelerates cell lysis. Anti-Hsp90 ribozyme reveals a complex mechanism of Hsp90 inhibitors involving both superoxide- and Hsp90-dependent events. J Biol Chem 278:35231
Stein JM, Luzio JP (1991) Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J 274(Pt 2):381
Takano S, Wadhwa R, Yoshii Y et al (1997) Elevated levels of mortalin expression in human brain tumors. Exp Cell Res 237:38
Taylor DD, Gercel-Taylor C (2005) Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 92:305
Tedesco F, Pausa M, Nardon E et al (1997) The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med 185:1619
Thery C, Duban L, Segura E et al (2002) Indirect activation of naive CD4+T cells by dendritic cell-derived exosomes. Nat Immunol 3:1156
Tschopp J, Podack ER, Muller-Eberhard HJ (1982) Ultrastructure of the membrane attack complex of complement: detection of the tetramolecular C9-polymerizing complex C5b-8. Proc Natl Acad Sci U S A 79:7474
van Blitterswijk WJ, Emmelot P, Hilkmann HA et al (1979) Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). Int J Cancer 23:62
Van Niel G, Mallegol J, Bevilacqua C et al (2003) Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut 52:1690
Vanbuskirk A, Crump BL, Margoliash E et al (1989) A peptide binding protein having a role in antigen presentation is a member of the HSP70 heat shock family. J Exp Med 170:1799
Voisine C, Craig EA, Zufall N et al (1999) The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell 97:565
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7:309
Whitlow M, Iida K, Marshall P et al (1993) Cells lacking glycan phosphatidylinositol-linked proteins have impaired ability to vesiculate. Blood 81:510
Wiedmer T, Ando B, Sims PJ (1987) Complement C5b-9-stimulated platelet secretion is associated with a Ca2+-initiated activation of cellular protein kinases. J Biol Chem 262:13674
Wiedmer T, Hall SE, Ortel TL et al (1993) Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood 82:1192
Wiedmer T, Sims PJ (1991) Participation of protein kinases in complement C5b-9-induced shedding of platelet plasma membrane vesicles. Blood 78:2880
Wubbolts R, Leckie RS, Veenhuizen PT et al (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 278:10963
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilzer, D., Gasser, O., Moskovich, O. et al. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immun 27, 375–387 (2005). https://doi.org/10.1007/s00281-005-0004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-005-0004-1