Abstract
Background
Prostate cancer is a prevalent cancer in men worldwide, and castration-resistant prostate cancer (CRPC) is characterized by disease progression despite androgen deprivation therapy. While clinical and prognostic biomarkers have been identified in CRPC, the significance of serum inflammatory markers remains unclear.
Materials and methods
This retrospective study included 79 CRPC patients treated with abiraterone or enzalutamide. Inflammatory markers, including the modified Glasgow prognostic score (mGPS), systemic immune-inflammation index (SII), and neutrophil-to-lymphocyte ratio (NLR), were assessed as predictive tools for treatment response. Patient data were obtained from medical charts, and statistical analyses were performed.
Results
The median age of the patients was 67 years, with most having a Gleason score of 8–10. The median values for NLR, PLR, and SII were 2.9, 168.5, and 713.5, respectively. The objective response rate (ORR) to abiraterone or enzalutamide therapy was 55.1%. mGPS showed a significant association with ORR, with the mGPS 0 group having the highest response rate (59.5%). Median progression-free survival (PFS) was 12.8 months, and median overall survival (OS) was 35.4 months. Palliative radiotherapy during therapy and PSA doubling time were independent prognostic factors for PFS.
Conclusions
mGPS and PSA doubling time significantly impacted survival, and mGPS significantly predicted the treatment response in mCRPC, which may lead to further prospective studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second commonly diagnosed cancer in men worldwide. In an advanced setting, androgen deprivation therapy can be used with or without treatments targeting androgen pathways or docetaxel. Many patients progress while receiving these treatments. Castration-resistant prostate cancer (CRPC) is defined as having evidence of disease progression (an increase in serum prostate-specific antigen (PSA), new metastases, or progression of existing metastases), and who have to castrate levels of serum testosterone (< 50 ng/dl) [1].
There are well-defined clinical and prognostic biomarkers in CRPC. The most extensive data come from circulating tumor cell analyses (CTCs) and gene expression profiles [2, 3]. Clinical parameters are prostate-specific antigen (PSA)-only recurrence (M0 disease with a rising PSA and no demonstrable metastases), and at the other end are males with extensive, high-volume, symptomatic, metastatic disease in visceral sites (especially the liver), and bone metabolism biomarkers [4,5,6].
On the other hand, the prognostic importance of serum inflammatory markers in CRPC remains unclear. Many studies have evaluated the modified Glasgow prognostic score (mGPS), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic inflammatory index (SII), and C-reactive protein (CRP) in CRPC. They showed that higher values of inflammatory markers were associated with worse survival [7,8,9]. Also, the pan-immune-inflammation marker was studied in CRPC patients treated with abiraterone or enzalutamide and was significantly associated with survival [10].
We aimed to assess the inflammatory markers as modified Glasgow prognostic score (mGPS), systemic immune-inflammation index (SII), and neutrophile-to-lymphocyte ratio (NLR) as practical tools for predicting response to abiraterone or enzalutamide treatments in metastatic CRPC patients.
Materials and methods
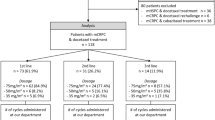
A total of 79 men diagnosed with metastatic castration-resistant (serum testosterone < 50 ng/dl) prostate cancer (CRPC) between 2015 and 2021, treated with abiraterone or enzalutamide, were included in this study. Patients’ data were retrospectively obtained from patients’ charts. The laboratory findings have been noted before abiraterone/enzalutamide treatment. Patients with Eastern Cooperative Oncology Group Performance Status (ECOG PS) 3 and 4, who could not continue to active follow-up, were excluded from data analysis.
The mGPS was defined as follows: an average Alb level ≧ of 3.5 g/dl and a CRP ≦ of 10 mg/dl was scored as 0, a low Alb and CRP ≥ 10 mg/dl was scored as 2, and only a low Alb or CRP ≥ 10 mg/dl was scored as 1. NLR is calculated as absolute neutrophil count (neutrophil count/ml)/absolute lymphocyte count (lymphocyte count/ml); PLR is calculated as absolute platelet count (platelet count/ml)/absolute lymphocyte count. The SII was defined as follows: SII = Platelets × neutrophil/lymphocytes.
The PSA progression was defined by a rise of over 25% in PSA measurements compared to the initial value. PSA doubling time was defined as the number of months for PSA to increase two-fold. Treatment response, encompassing partial response (PR), complete response (CR), stable disease (SD), and progressive disease (PD), along with objective response rates (PR and CR), were assessed following RECIST 1.1 guidelines. In this framework, disease progression is determined by a minimum 20% increase in the longest diameter of target lesions, referencing the smallest longest diameter noted from treatment commencement or the emergence of new lesions [11, 12].
Written informed consent was obtained from patients, and the Local Ethics Committee of Istanbul Medipol University approved the study.
Statistical analysis
SPSS 24.0 (SPSS Inc., Chicago, IL, USA) software was used for all statistical analyses. Parameters were described with their median values, and due to non-normal distribution, nonparametric tests were used. PFS was defined as the allocation date of enzalutamide or abiraterone to the radiological progression date. OS was defined as the time from CRPC diagnosis to the death or last seen date or loss to follow-up. Survival analysis and curves were performed using the Kaplan–Meier method and compared with the log-rank test. The multivariate COX regression analysis was performed to evaluate independent prognostic factors. Afterwards, binary logistic regression analysis was performed to assess all the significant characteristics for predicting treatment response. The 95% confidence interval (CI) was used to quantify the relationship between survival time and each independent factor. All p values were two-sided in tests, and p values less than or equal to 0.05 were considered statistically significant.
Results
A total of 79 CRPC patients with a median age of 67 (range, 39–85 years) were included in this study. All of the patients were treated with abiraterone or enzalutamide. Most patients’ Gleason scores were 8–10 (n = 59; 74.7%). The number of patients treated with docetaxel in a castration-sensitive setting was 47 (59.5%). Before abiraterone or enzalutamide therapy, 39 (49.4%) and 34 (43%) patients’ ECOG PS remained 0 and 1, respectively. The number of patients with visceral metastasis was ten (12.7%). Thirty patients (38.0%) had only bone metastatic disease. The baseline median PSA value before abiraterone or enzalutamide therapy was 10.7 (range, 0.4–902.0 ng/ml), and the median value of PSA response to abiraterone or enzalutamide therapy was 1.5 (range, 0.01–1536.0 ng/ml). The median PSA doubling time before the allocation of abiraterone or enzalutamide therapy was 8.8 months. Fifty-seven (73.1%) patients received palliative radiotherapy during abiraterone or enzalutamide therapy (Table 1). Fifty-seven (72.2%) patients progressed under treatment, and cancer-related death was observed in 43 patients (54.4%).
Median NLR, PLR, and SII values were determined as 2.9, 168.5, and 713.5, respectively. Of the 79 patients, seven (9.0%) had CR, 36 (46.2%) had PR, and 21 (26.9%) had SD. The ORR regarding RECIST 1.1 was 55.1%. When the patients were categorized according to achieving ORR to abiraterone or enzalutamide therapy, no significant difference was determined between NLR ≤ 2.9 vs. > 2.9; PLR ≤ 168.5 vs. > 168.5, and SII ≤ 713.5 vs. > 713.5 (Table 2). The ORR in mGPS 0, 1, and 2 groups were significantly different and were 59.5, 40.5, and 0%, respectively (p < 0.001) (Fig. 1).
At a median follow-up of 23.3 months, the median PFS was 12.8 months, and the median OS was 35.4 months. The univariate analysis for PFS showed no significant correlation between PFS and PLR, NLR, SII, pretreatment with docetaxel, visceral metastasis, and bone-only metastatic disease. PSA doubling time before abiraterone or enzalutamide therapy and palliative radiotherapy during therapy were significant prognostic factors for PFS (p < 0.001 and p < 0.001). The median PFS was 21.6, 11.8, and 5.3 months with respect to the mGPS 0, 1, and 2, respectively (p < 0.001) (Fig. 2). Multivariate analysis for PFS revealed that significant independent prognostic factors were palliative radiotherapy during abiraterone or enzalutamide therapy (p = 0.01, HR: 12.2 95%, CI 1.63–92.4) and PSA doubling time before treatment (p = 0.02, HR: 0.31, 95% CI 0.11–0.85).
The median OS was 43.5, 31.2, and 19.2 months in patients with mGPS 0, 1, and 2, respectively (p = 0.008) (Fig. 3). The absence of visceral metastasis (median OS 37.4 vs. 20.5 months, p = 0.006) and median PSA doubling time > 8.8 months before abiraterone or enzalutamide therapy (median OS 43.5 vs. 25.8 months, p = 0.003, respectively) were significantly related to prolonged survival. The univariate analysis for OS demonstrated no significant correlation between OS and NLR, PLR, SII, Gleason grade group, pre-docetaxel treatment, palliative radiotherapy, and the presence of bone-only metastasis. The independent prognostic factors for OS were the presence of visceral metastasis (p = 0.03, HR: 4.9, 95% CI 1.12–21.87) and median PSA doubling time before abiraterone or enzalutamide therapy (p = 0.01, HR: 0.22, 95% CI 0.67–0.74) in the multivariate analysis. Table 3 summarizes the univariate and multivariate analysis for both PFS and OS.
Logistic regression analysis was done to identify independent predictive factors for response to abiraterone or enzalutamide treatment. PSA doubling time before the commencement of abiraterone or enzalutamide treatment, along with SII, NLR, and PLR, did not significantly predict the response. The analysis revealed that only mGPS was an independent significant predictive factor for the abiraterone or enzalutamide therapy response (p = 0.001, OR: 0.75, CI 95% 0.19–0.30). Thus, the patients with mGPS = 0 were significantly good responders to the abiraterone or enzalutamide therapy in CRPC.
Discussion
High inflammation parameters significantly impact prognosis in many cancer types [13, 14]. In addition to inflammatory markers, malnutrition causes deaths in cancer patients, which is well described. mGPS is a marker derived from albumin, the major protein in the blood and the objective indicator for nutritional status, and CRP, the most common acute-phase serum protein and an indicator of the systemic inflammatory response [15]. Systemic inflammatory and nutritional biomarkers have also been studied in various cancers such as pancreatic, lung, and prostate [16,17,18]. In contrast, none have examined the prediction of abiraterone or enzalutamide therapy response yet for patients with metastatic CRPC.
Neuberger et al. studied the impact of NLR, SII, and mGPS on survival, biochemical, and radiological response in metastatic CRPC patients treated with docetaxel. They showed that no inflammatory marker was predictive of the radiological response. However, mGPS and PLR were significant predictive factors for the biochemical response. mGPS and NLR were significant independent factors for OS [9]. Yazgan et al. demonstrated that pan-immune-inflammation (PIV) value and PIV-LDH combined score were significant independent prognostic factors for OS in CRPC patients treated with abiraterone or enzalutamide [10].
Many studies have reported the importance of mGPS in metastatic castration-sensitive prostate cancer (CSPC), which has shown poor survival outcomes [7, 18, 19]. Also, mGPS was superior to NLR [19]. Additionally, mGPS was an independent predictive marker of OS and combined radiological response in metastatic CSPC treated with chemotherapy [8]. In the CRPC setting, Linton et al. reported that the mGPS 2 group had a significantly shorter OS. Still, the difference in mGPS 0 and mGPS 1 was insignificant in patients treated with docetaxel-based chemotherapy [18]. In another study, mGPS was a predictive factor for progression but statistically not significant for OS [21].
Our study assessed NLR, PLR, SII, and potentially other prognostic factors in metastatic CRPC. Our findings revealed that the median progression-free survival (PFS) was 21.6, 11.8, and 5.3 months, respectively. Additionally, the median overall survival (OS) was observed to be 43.5, 31.2, and 19.2 months in patients with mGPS scores of 0, 1, and 2 (p < 0.001 and p = 0.008, respectively). Moreover, the independent prognostic factors for PFS were the presence of palliative radiotherapy during abiraterone or enzalutamide therapy (p = 0.01, HR: 12.2 95% CI 1.63–92.4) and shorter PSA doubling time (< 8.8 months) before abiraterone or enzalutamide therapy (p = 0.02 HR: 0.31 95% CI 0.11–0.85). No inflammatory parameters had an impact on OS. The presence of visceral metastasis (p = 0.03, HR: 4.9 95% CI 1.12–21.87) and median PSA doubling time before abiraterone or enzalutamide therapy (p = 0.01, HR: 0.22 95% CI 0.67–0.74) were found to be independent prognostic factors for OS. ORR in mGPS 0, 1, and 2 groups were 59.5, 40.5, and 0%, respectively (p < 0.001). Additionally, we showed that mGPS was a significant predictor for ORR in logistic regression analysis (p = 0.001, OR: 0.75, 95% CI 0.19–1.30). Our findings were thus different from the previous studies [9, 19,20,21].
The Gleason score is a well-known risk factor [22, 23]. Additionally, in the CRPC setting, the Gleason score significantly impacted OS in several studies [24, 25]. We did not demonstrate a significant correlation between the Gleason score, OS, and PFS. This could be explained by the 7.8% missing data, and 74.7% of patients were in Gleason 8–10 group.
Howard et al. showed that PSA doubling time was associated with metastases, all-cause mortality, and prostate cancer-specific mortality (all p < 0.001). Specifically, doubling time < 3 months in men with non-metastatic CRPC was associated with an approximately ninefold increased risk of metastases (HR 8.63, 95% CI 5.07–14.7) and cancer-specific mortality (HR 9.29, 95% CI 5.38–16.0) [26]. A phase 3 trial demonstrated that in metastatic CSPC patients treated with abiraterone, PSA doubling time was significantly correlated with radiologic PFS and OS [27]. Additional studies showed a significant correlation between developing metastasis and PSA doubling time in CSPC [28, 29]. We evaluated the PSA doubling time before allocating abiraterone or enzalutamide therapy, and the median value was 8.8 months. The median PFS and OS time were 7.0 vs. 23.6 months (p < 0.001), 25.8 vs. 43.5 months (p = 0.003), according to the median PSA doubling time (≤ 8.8 vs. > 8.8 months), respectively.
Visceral metastasis was another prognostic factor for survival in metastatic CRPC [30, 31]. Similar to the literature, visceral metastasis was significantly associated with poor OS in our study. The prior chemotherapy before abiraterone in 1088 metastatic CRPC patients was not a significant factor for OS but reduced its effect on radiographic-PFS (p = 0.04) [32]. The activity of enzalutamide was blunted after abiraterone and docetaxel and still more after both in another study [33]. In our research, pre-docetaxel treatment did not significantly affect OS and PFS. The possible reason for this could be the small sample size and the high rate of patients (59.7%) treated with docetaxel before abiraterone or enzalutamide therapy, which may affect the analysis.
The several limitations of our study were the retrospective design and small sample size. In addition, we analyzed patients treated with enzalutamide or abiraterone in both the first-line and second-line treatments in metastatic castration-resistant settings, which might influence our findings. Although the impact of palliative therapy on patient outcomes is noteworthy, we acknowledge that its potential influence was not directly assessed in our study due to the limited availability of ECOG PS data. On the other hand, our study contributes to the literature by demonstrating the impact of both mGPS and PSA doubling time for survival in patients with metastatic CRPC receiving abiraterone or enzalutamide.
Conclusions
In conclusion, this study highlights the potential of mGPS as a practical tool for predicting response to abiraterone or enzalutamide treatment in metastatic CRPC patients. Monitoring inflammatory markers, particularly mGPS, may help clinicians assess treatment outcomes and personalize therapeutic strategies. Further research is warranted to validate these findings and explore the underlying mechanisms linking inflammation and treatment response in mCRPC.
Data availability
The data supporting this study’s findings are not openly available. Further inquiries can be directed to the corresponding author.
References
Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M et al (2021) EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 79(2):243–262
Heller G, McCormack R, Kheoh T, Molina A, Smith MR, Dreicer R et al (2018) Circulating tumor cell number as a response measure of prolonged survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. J Clin Oncol 36(6):572–580
Armstrong AJ, Lin P, Higano CS, Sternberg CN, Sonpavde G, Tombal B et al (2018) Development and validation of a prognostic model for overall survival in chemotherapy-naïve men with metastatic castration-resistant prostate cancer. Ann Oncol 29(11):2200–2207
Lara PN Jr, Ely B, Quinn DI, Mack PC, Tangen C, Gertz E et al (2014) Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst 106(4):dju013
Halabi S, Kelly WK, Ma H, Zhou H, Solomon NC, Fizazi K et al (2016) Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J Clin Oncol 34(14):1652–1659
Moreira DM, Howard LE, Sourbeer KN, Amarasekara HS, Chow LC, Cockrell DC et al (2016) Predictors of time to metastasis in castration-resistant prostate cancer. Urology 96:171–176
Ando K, Sakamoto S, Saito S, Maimaiti M, Imamura Y, Sazuka T et al (2021) Prognostic value of high-sensitivity modified Glasgow prognostic score in castration-resistant prostate cancer patients who received docetaxel. Cancers (Basel) 13(4):773
Neuberger M, Goly N, Skladny J, Milczynski V, Weiß C, Wessels F et al (2022) Systemic inflammatory biomarkers as predictive and prognostic factors in men with metastatic castration-refractory prostate cancer treated with docetaxel therapy: a comprehensive analysis in a German real-world cohort. J Cancer Res Clin Oncol 149(7):3371–3381
Neuberger M, Skladny J, Goly N, Wessels F, Weiß C, Egen L et al (2022) Baseline modified Glasgow prognostic score [mGPS] predicts radiologic response and overall survival in metastatic hormone-sensitive prostate cancer treated with docetaxel chemotherapy. Anticancer Res 42(4):1911–1918
Yazgan SC, Yekedüz E, Utkan G, Ürün Y (2022) Prognostic role of pan-immune-inflammation value in patients with metastatic castration-resistant prostate cancer treated with androgen receptor-signaling inhibitors. Prostate 82(15):1456–1461
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Arlen PM, Bianco F, Dahut WL, D’Amico A, Figg WD, Freedland SJ et al (2008) Prostate specific antigen working group guidelines on prostate-specific antigen doubling time. J Urol 179:2181–2185
Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ (2012) A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107(4):695–699
Kawahara T, Kato M, Tabata K, Kojima I, Yamada H, Kamihira O et al (2020) A high neutrophil-to-lymphocyte ratio is a poor prognostic factor for castration-resistant prostate cancer patients who undergo abiraterone acetate or enzalutamide treatment. BMC Cancer 20(1):919
McMillan DC (2009) Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 12(3):223–226
Gil M, Gomes A, Baptista M, Vale Martins R, Nunes V (2022) Inflammatory and nutritional biomarkers as predictors of non-resectability and early recurrence in pancreatic and periampullary cancer. Minerva Surg 77(2):130–138
Freitas C, Jacob M, Tavares N, Cruz-Martins N, Souto-Moura C, Araújo D et al (2021) Modified Glasgow prognostic score predicts survival among advanced non-small cell lung carcinoma patients treated with anti-PD1 agents. Anticancer Drugs 32(5):567–574
Shafique K, Proctor MJ, McMillan DC, Qureshi K, Leung H, Morrison DS (2012) Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow inflammation outcome study. Prostate Cancer Prostatic Dis 15:195–201
Shafique K, Proctor MJ, McMillan DC, Leung H, Smith K, Sloan B, Morrison DS (2013) The modified Glasgow prognostic score in prostate cancer: results from a retrospective clinical series of 744 patients. BMC Cancer 13:292
Linton A, Pond G, Clarke S, Vardy J, Galsky M, Sonpavde G (2013) Glasgow prognostic score as a prognostic factor in metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Clin Genitourin Cancer 11:423–430
Stangl-Kremser J, Mari A, Suarez-Ibarrola R, D’Andrea D, Korn SM, Pones M et al (2020) Development of a prognostic model for survival time prediction in castration-resistant prostate cancer patients. Urol Oncol 38:600.e9-600.e15
Zhou Y, Lin C, Hu Z, Yang C, Zhang R, Ding Y et al (2021) Differences in survival of prostate cancer Gleason 8–10 disease and the establishment of a new Gleason survival grading system. Cancer Med 10(1):87–97
Gordetsky J, Epstein J (2016) Grading of prostatic adenocarcinoma: current state and prognostic implications. Diagn Pathol 11:25
Chen WJ, Kong DM, Li L (2021) Prognostic value of ECOG performance status and Gleason score in the survival of castration-resistant prostate cancer: a systematic review. Asian J Androl 23(2):163–169
Nishimoto M, Fujita K, Yamamoto Y, Hashimoto M, Adomi S, Banno E et al (2022) Prognostic factors in Japanese men with high-Gleason metastatic castration-resistant prostate cancer. Transl Cancer Res 11(8):2681–2687
Howard LE, Moreira DM, De Hoedt A, Aronson WJ, Kane CJ, Amling CL et al (2017) Thresholds for PSA doubling time in men with non-metastatic castration-resistant prostate cancer. BJU Int 120(5B):E80–E86
Matsubara N, Chi KN, Özgüroğlu M, Rodriguez-Antolin A, Feyerabend S, Fein L, Alekseev BY et al (2020) Correlation of prostate-specific antigen kinetics with overall survival and radiological progression-free survival in metastatic castration-sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: post hoc analysis of phase 3 LATITUDE study. Eur Urol 77(4):494–500
Markowski MC, Chen Y, Feng Z, Cullen J, Trock BJ, Suzman D et al (2019) PSA doubling time and absolute PSA predict metastasis-free survival in men with biochemically recurrent prostate cancer after radical prostatectomy. Clin Genitourin Cancer 17(6):470-475.e1
Klaassen Z, Howard L, Wallis CJD, Janes JL, De Hoedt A, Aronson WJ et al (2022) Is time to castration resistant prostate cancer a potential intermediate end-point for time to metastasis among men initiating androgen deprivation therapy for non-metastatic prostate cancer with rapid PSA doubling time[< 9 months]? Prostate Cancer Prostatic Dis 26(1):151–155
Cui PF, Cong XF, Gao F, Yin JX, Niu ZR, Zhao SC, Liu ZL (2020) Prognostic factors for overall survival in prostate cancer patients with different site-specific visceral metastases: a study of 1358 patients. World J Clin Cases 8(1):54–67
Satapathy S, Mittal BR, Sood A (2020) Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate cancer treated with 177Lu-labeled prostate-specific membrane antigen radioligand therapy: a systematic review and meta-analysis. Clin Nucl Med 45(12):935–942
Shameem R, Hamid MS, Xu KY, Wu S (2015) Comparative analysis of the effectiveness of abiraterone before and after docetaxel in patients with metastatic castration-resistant prostate cancer. World J Clin Oncol 6(4):64–72
Cheng HH, Gulati R, Azad A, Nadal R, Twardowski P, Vaishampayan UN et al (2015) Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis 18(2):122–127
Funding
There have been no financial or other relationships. There was no funding.
Author information
Authors and Affiliations
Contributions
All authors read and gave their stamp of approval for the submission of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
The Local Ethics Committee of Istanbul Medipol University approved the study in October of 2022 (E-10840098-772.02-6509). Further inquiries can be directed to the corresponding author.
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goktas Aydin, S., Kutlu, Y., Muglu, H. et al. Predictive significance of inflammatory markers and mGPS in metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide. Cancer Chemother Pharmacol 93, 71–78 (2024). https://doi.org/10.1007/s00280-023-04592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04592-x