Abstract
Purpose
Head and neck squamous cell carcinoma (HNSCC) ranks as the sixth most prevalent cancer. In recent years, the modification of platinum(II) into platinum(IV) derivative compounds, by introducing biologically active molecules, has been extensively employed to develop novel platinum-based prodrugs. We investigated the anti-proliferative activity against HNSCC of a new veratric acid (COX-2 inhibitor)-platinum(IV) complex.
Methods
In this study, a new veratric acid (COX-2 inhibitor)–platinum(IV) complex, termed veratricplatin, was synthesized. We evaluated the anti-tumor effect of in vitro and in vivo by western blotting, flow cytometry and DNA damage analysis.
Results
Veratricplatin displayed remarkable anti-proliferative activity against various cancer cell lines, including A549, FaDu, HeLa, and MCF-7. Furthermore, veratricplatin demonstrated significantly stronger cytotoxicity than either platinum(II) or veratric acid monotherapy or their combination. Importantly, the synthesized prodrug exhibited less toxicity toward normal cells (MRC-5), while dramatically enhanced DNA damage in FaDu cells inducing apoptosis. Moreover, veratricplatin markedly reduced the migration ability of FaDu cells compared to the control or monotherapy. In vivo, veratricplatin displayed potent anti-tumor activity with no apparent toxicity in BALB/c nude mice bearing FaDu tumors. In addition, tissue immunofluorescence analysis revealed that veratricplatin could substantially inhibit the formation of tumor blood vessels.
Conclusion
Veratricplatin demonstrated remarkable drug efficacy, in terms of increased cytotoxicity in vitro and high efficiency with low toxicity in vivo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer (HNC) represents one of the ten frequently occurring cancers globally, with its incidence rate ranking as the sixth highest among all cancers, posing a serious threat to public health [6]. Head and neck squamous cell carcinoma (HNSCC) accounts for approximately 90% of reported HNC cases [21]. The oropharynx, larynx, and hypopharynx are the common sites for HNSCC progression [19]. Because of the lack of reliable diagnostic indicators, early detection of HNSCC is challenging and more than 50% of patients with HNSCC already reach an advanced stage during treatment initiation [5]. In addition, HNSCC is characterized by a high proliferation rate and inferior prognosis, making it an arduous task to treat this disease effectively [20].
Multidisciplinary treatment for HNSCC mainly involves three methods: surgery, anti-cancer drug therapy, and radiotherapy. Platinum-based chemotherapy is the standard treatment choice for HNSCC [28]. Cisplatin (CDDP; cis-diamminedichloroplatinum(II)) has been widely applied as a single chemotherapeutic agent or in combination with other antineoplastic agents for treating several solid tumors [14, 31]. Pt(II)-based regimens act by damaging genomic DNA (gDNA) or mitochondrial DNA (mtDNA), and trigger a variety of cellular processes (including transcription inhibition), ultimately causing cell apoptosis [30]. However, the cure rate for advanced HNSCC patients remains low [4]; the primary reason remains the development of resistance to conventional chemotherapy and radiotherapy by tumor cells, which severely limits the therapeutic application of Pt(II) compounds. Moreover, treatment with Pt(II) drugs can cause serious side effects impacting the kidneys, ears, liver, gastrointestinal tract, and peripheral nerves [8]. Studies have reported that less than 10% of Pt(II) compounds can only enter tumor cells and cross-link with DNA when administered alone, with the majority rest bonding to other biomolecules [11]. Therefore, designing and developing novel platinum-based drugs that are also effective in overcoming platinum (II) resistance and minimizing adverse side reactions is imperative and a key research focus.
According to previous literature, Pt(IV) complexes can function as prodrugs and are effective in overcoming CDDP-associated drawbacks [9]. Octahedral Pt(IV) complexes in their oxidized state exhibit increased inertness compared to Pt(II) complexes, thereby rendering greater stability when administered orally [15]. Moreover, the introduction of functional groups with multi-targeting properties to modify axial ligands can enhance pharmacological properties and reduce side effects and drug resistance [17, 34]. In this study, a Pt(IV) prodrug, combined with non-steroidal anti-inflammatory drugs (NSAIDs) as axial ligands, has been developed. This prodrug can efficiently enter tumor cells due to high lipophilicity and release the cytotoxic metabolite and NSAID intracellularly, thus, improving the therapeutic efficacy of platinum-based chemotherapy while reducing side effects. In recent years, numerous NSAID-Pt(IV) prodrugs have been designed and synthesized, exhibiting high therapeutic efficacy both in vitro and in vivo [27]. We previously synthesized tetravalent platinum nanoformulations (BSA@Ben-Pt(IV) NPs) which could synergistically enhance anti-tumor effects by activating anti-tumor immune responses in vivo and inhibiting tumor migration, invasion, and angiogenesis through DNA damage [32]. Multiple studies have shown that COX-2 inhibitors, such as celecoxib or nimesulide, can promote chemosensitivity in HNSCC; therefore, COX-2 may be a crucial target for HNSCC treatment [13, 24].
Veratric acid (3,4-dimethoxy benzoic acid), a hydrophobic benzoic acid derivative, is another COX-2 inhibitor with reported anti-inflammatory, anti-bacterial, anti-tumor, and anti-oxidant properties [26]. Accordingly, we aimed to design and synthesize a new veratric acid-derived NSAID-Pt(IV) prodrug as an axial ligand to investigate its potential in HNSCC therapy. To our knowledge, Pt(IV) complex has not been explored previously in HNSCC treatment. We conjugated veratric acid with CDDP to yield a novel Pt(IV) prodrug, termed veratricplatin, and evaluated its pharmacological effects on HNSCC both in vitro and in vivo. Our findings demonstrated that veratricplatin offers significant advantages related to anti-proliferation, pro-apoptosis, reduced invasion, and reduced tumor angiogenesis, which could provide valuable insights for further studies on HNSCC.
Materials and methods
Materials
CDDP was purchased from Shandong Boyuan Pharmaceutical Co., Ltd (China). Veratric acid and dimethyl sulfoxide (DMSO) were procured from J & amp; K Science Co., Ltd. Triethylamine (tea), H2O2 solution (30% water) and 2-(1h-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) were acquired from Shanghai Aladdin Biochemical Technology Co., Ltd. (China). Nuclease enzyme, propidium iodide (PI), 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetraammonium bromide (MTT), 4′,6-diamidino-2-phenylindole (DAPI), annexin V-FITC (fluorescein isothiocyanate)/PI apoptosis detection kit, and mouse VEGF ELISA kit were purchased from Beijing Solarbio Science & Technology Co., Ltd. (China). The γH2AX and alexafluor488 antibodies were purchased from Protein Technology Group, while antibodies for Western blotting (anti-Bcl-2 and anti-E-cad) were obtained from Cell Signaling Technology, Inc. (US). Additional antibodies, including APC anti-mouse CD3, PE anti-mouse CD8a, and FITC anti-mouse CD4, were bought from BioLegend (US). The 1H and 13C spectra were recorded on a Bruker Avance 400 spectrometer, and ESI-MS was performed on an AB Sciex API 4000 system. HPLC analysis was conducted using a Shimadzu LC-20A equipped with a Waters XBridge Shield RP18 column (150 × 4.6 mm, 3.5 µm). Infrared spectra (IR) were recorded on a Shimadzu IRAffinity-1.
Cell lines and animals
Human cell lines, including pharyngeal squamous cell carcinoma (FaDu), non-small cell lung cancer (A549), cervical cancer (HeLa), breast cancer (MCF-7), and embryonic lung fibroblasts (MRC-5) were obtained from the National Cell Line Resource in Chinese Academy of Sciences. A549 and HeLa were cultured using the final 1640 medium (containing 1% penicillin–streptomycin solution and 10% fetal bovine serum), while MCF-7 and FaDu cells were cultured using the final DMEM medium (with 1% penicillin–streptomycin solution and 10% fetal bovine serum) at 37 °C in a 5.0% CO2 incubator. Female Balb/C mice (aged 6–8 weeks) were acquired from Beijing Sibeifu Biotechnology Co., Ltd.
Synthesis of Pt(IV) complex c,c,t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ]
H2O2 (30 wt%, 2.0 mL) was added dropwise to a round bottom flask containing CDDP (200 mg, 0.67 mmol). The reaction mixture was heated to 75 °C for 5 h to obtain a bright yellow solution, which was then kept at 4 °C in the dark to yield yellow crystals. The crystals were recovered by filtration and washed with cold water, ethanol, and ether, followed by drying under vacuum, achieving a product yield of 84.7% (189 mg, 0.56 mmol). IR (KBr): 3461(s, OH stretch), 1074 (m, Pt–OH bend), 540 (m, Pt–N(O) stretch) (Fig. S6).
Synthesis of veratricplatin
Veratric acid (164 mg, 0.90 mmol), triethylamine (130 μL, 0.94 mmol), and TBTU (288 mg, 0.90 mmol) were added to a suspension of c,c,t-[Pt(NH3)2Cl2(OH)2] (100 mg, 0.30 mmol) in dry DMF (2 mL). The reaction mixture was stirred in the dark at room temperature for 96 h to obtain a clear yellow solution, which was then precipitated after the addition of 20 mL ice-cold water, and the precipitate was collected through filtration. Finally, the product was isolated by column chromatography using a mixture of 20/1 dichloromethane/methanol as eluent to afford a pale-yellow solid, with a yield of 40.3%. 1H NMR (400 MHz, DMSO-d6): δ 7.58–7.50 (m, 2H), 7.44 (s, 2H), 7.00 (d, J = 8.5 Hz, 2H), 6.76 (s, 6H), 3.81 (s, 6H), 3.78 (s, 6H).13C NMR (101 MHz, DMSO-d6): 173.74, 152.29, 148.19, 125.83, 123.81, 112.98, 110.99, 56.08, 56.01. MS (m/z): calcd for C18H24Cl2N2O8Pt (M-H)−, 661.38; found, 661.34.
In vitro cytotoxicity
A549, HeLa, MCF-7, FaDu, and MRC-5 cells were inoculated into 96-well plates (200 μL, 5 × 103 cells/well) overnight (37 °C, 5%CO2). Consecutively, the cells were treated with veratric acid, CDDP, veratric acid/CDDP combination, and veratricplatin at concentrations ranging from 0.16 to 20.0 μM. After 48 h, MTT (10 μL 50 mg/ml) was added to each well and further incubated for 4 h. The medium was then removed and the resulting formazan crystals were dissolved using DMSO (100 μL). The absorbance of each sample was measured at 570 nm using a microplate reader. The semi-inhibitory concentration value was determined using GraphPad Prism 7.0 software by measuring the cell inhibition rate as follows:
where ODcontrol, ODtreated and ODblank referred to the optical density of the control, blank and sample group, separately.
Cellular uptake and DNA platination
FaDu cells (2000 μL, 1 × 106 cells/well) were seeded in 6-well plates overnight (37 °C, 5%CO2). After incubation, cells were treated with veratric acid (14 μM), CDDP (7 μM), a combination of veratric acid/CDDP (14/7 μM), and veratricplatin (7 μM) for 3, 6, or 9 h. Subsequently, the cells were washed with PBS and harvested for quantification of platinum levels employing ICP-MS, following standardized pre-processing.
HPLC analysis
Solutions containing veratricplatin alone (1 mM) and veratricplatin (1 mM) + AsA or GSH (10 mM) were prepared in PBS-acetonitrile (9:1) mixture and incubated in the dark at 37 °C for 0, 12, 24, and 48 h, respectively. HPLC profiles were recorded using a UV detector at 260 nm and a mobile phase comprising acetonitrile (CH3CN) with 0.1% trifluoroacetic acid (TFA)/ water (H2O) with 0.1%TFA (v/v).
Cell apoptosis
FaDu cells were inoculated into 6-well plates (2000 μL, 1 × 106 cells/well) for overnight incubation (37 °C, 5%CO2). The cells were then treated with different drug compounds at a concentration of 2.0 μM for 48 h, followed by PBS washing and resuspension in binding buffer. Lastly, cells were stained with annexin V-FITC (5 μL) and PI (10 μL) for 15 min before detection using flow cytometry (BD FACSaria).
Scratch experiments
FaDu cells were cultivated into 12-well plates (1000 μL, 5 × 105 cells/well). Following overnight incubation, monolayer scratches were created and cleaned with PBS. Next, the cells were treated with different drug complexes (2.0 μM) for 24 h, washed with PBS, and observed under a microscope.
Western blotting
FaDu cells were seeded in a 6-well plate and subjected to drug exposure for 24 h. In the subsequent steps, cells were harvested, lysed, and buffered for 30 min before centrifugation at 12,000 rpm for 2 min. The protein concentration in the supernatant was determined using the BCA protein concentration detection kit (Solarbio). A total protein extract of 40 mg was separated running SDS-PAGE (10% separating gel and 5% concentrating gel). The gel was then transferred to a PVDF membrane (microporous membrane) and blocked with 5% (w/v) skim milk in TBST for 3 h. Next, the membrane was incubated with the primary antibody at a dilution of 1:3000 overnight and washed 4 times with TBST for 10 min each, followed by incubation with the secondary antibody conjugated to horseradish peroxidase (1:5000 diluted) for 1 h at room temperature and washing 4 times with TBST. The immune response bands were visualized using Pierce ECL Western blotting substrate (Thermo) and analyzed employing the Tanon Automatic Chemiluminescence Imaging System.
DNA damage analysis
FaDu cells were cultured overnight in 6-well plates (2000 μL, 5 × 105 cells/well) and were exposed to different drug complexes (2.0 μM) for 24 h. Consecutively, the cells were washed with PBS and fixed with 4% paraformaldehyde before incubating with primary antibodies (γH2AX) for 12 h, and then with Alexa 488 coupled secondary antibody (Alexa Fluor 488) for an additional 2 h. Finally, cells were stained using DAPI for 15 min and observed under a confocal microscope.
In vivo anti-tumor activity
Animal experiments were approved by Medical Ethics Committee of Shandong Second Provincial General Hospital and carried out according to the Health Guide for the Care and Use of Laboratory the Animals of National Institutes. The female Balb/C mice (aged 6–8 weeks) were injected subcutaneously with 1 × 106 FaDu cells into the right buttock. Once the tumor volume increased to 50–100 m3, the mice were randomly divided into 5 groups (n = 11) and intravenously administered with 1) NS (control), 2) veratric acid, 3) CDDP, 4) veratric acid/CDDP combination, and 5)Veratricplatin with dose at 2.5 mg CDDP/kg, respectively. The body weight and tumor volume of the mice were measured every 2 days for a total of 15 days. The mice were killed after 15 days, following the excision of their tumor and organs, which were assessed using H&E staining and ICP-MS.
In vivo anti-tumor mechanism analysis
The tumors, collected from killed mice, were used to analyze the immune response by immunofluorescence after stained with different antibodies.
Statistical analysis
GraphPad Prism (7.0) software was employed to evaluate statistical significance with *P < 0.05, **P < 0.01, and ***P < 0.001 as criteria.
Results
Synthesis and characterization of veratricplatin
Veratricplatin prodrug was synthesized following a previously reported method [14]. CDDP was oxidized with hydrogen peroxide (H2O2) to obtain Pt(IV) intermediate oxoplatin, which consecutively reacted with veratric acid in the presence of 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU), triethylamine, and dimethyl formamide (DMF) at 25 °C to yield veratricplatin. The product was characterized by 1H/13C nuclear magnetic resonance (NMR), and electrospray ionization mass spectrometry (ESI-MS), and its purity of > 95% was confirmed using high-performance liquid chromatography (HPLC) (Fig. S1–S4).
Cellular uptake of platinum drugs estimated by ICP-MS
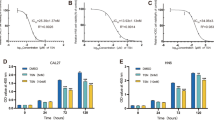
Adequate cellular uptake is a crucial factor that affects the therapeutic efficacy of drugs. The platinum content in FaDu cells was determined using inductively coupled plasma mass spectrometry (ICP-MS) [16], revealing that all samples accumulated in the cells in a time-dependent manner (Fig. 1). In addition, cells treated with veratricplatin exhibited higher platinum accumulation than those treated with free CDDP. Thus, the newly synthesized compound veratricplatin could enhance the platinum content in cells. Furthermore, the platinum concentration detected in DNA was consistent with the cell experiment results. These findings suggest that structural modification of drug can alter lipid solubility which may promote the cellular uptake of drugs.
Platinum concentration in DNA and FaDu was determined using ICP-MS after control, CDDP, and veratricplatin treatment for 3 h. Platinum content in A whole cells and B DNA. The values represent mean ± SD from at least three independent experiments performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001
In vitro cytotoxicity
The cytotoxicity of veratricplatin was analyzed on various tumor cells, such as A549, MCF-7, HeLa, and normal lung fibroblast cells (MRC-5), using the 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetraammonium bromide (MTT) assay. We observed a stronger cytotoxic effect of veratricplatin, a synthetic compound comprising veratric acid and CDDP, on tumor cells compared to the combined application of veratric acid and CDDP; however, lower cytotoxicity on normal cells was noticed (Table 1). Veratric acid alone did not exhibit any apparent cytotoxicity, while in combination with CDDP, a significantly enhanced inhibitory effect on cells was visible, with higher cytotoxicity than CDDP alone. This indicates that veratric acid can enhance the sensitivity of tumor cells to CDDP, thus, enhancing its efficacy.
Effect of veratricplatin on DNA damage and apoptosis
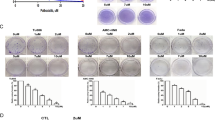
Platinum drugs are known to exert toxicity by inducing DNA damage, causing blockage of replication and transcription, as well as inhibiting tumor growth, proliferation, and metastasis [12]. γH2AX has been established to be a reliable DNA damage marker, mediating cell cycle arrest and participating in DNA damage repair [23]. Therefore, the concentration of γH2AX in cells treated with different drugs was analyzed using immunofluorescence (Fig. 2). Treatment with cisplatin remarkably enhanced the fluorescence of γH2AX. Likewise, veratricplatin treatment led to increased fluorescence intensity of γH2AX, with its up-regulated expression compared to cisplatin (Fig. 2A). This indicates that the newly synthesized prodrug veratricplatin can induce DNA damage and affect the proliferation and repair of tumor cells. Besides, we speculated that veratricplatin could also affect the expression of the anti-apoptotic protein Bcl-2; thus, Bcl-2 expression levels were assessed by Western blot analysis. Significant reduction in active Bcl-2 protein levels was observed in the presence of veratricplatin compared to CDDP, veratric acid, and their combination (Fig. 2B, C).
A Immunofluorescence staining images of FaDu cells. The scale bars represent 10 μm. B Protein expression and C quantification of Bcl-2 in FaDu cells after drug administration. D The statistical analysis of E flow cytometry analysis of apoptosis in FaDu cells induced with veratric acid, CDDP, veratric acid/CDDP (2:1) and Veratricplatin for 24 h, using Annexin V-FITC/PI staining. F Cell cycle analysis of FaDu cells induced with induced with veratric acid, CDDP, veratric acid in combination with CDDP (2:1) and veratricplatin for 24 h using flow cytometry. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group
Next, we detected apoptosis using flow cytometry, and the results indicated no apparent pro-apoptotic effect of veratric acid. Conversely, veratric acid in combination with CDDP could promote late cell apoptosis (Fig. 2D, E). Veratric acid has been reported to reverse cell damage caused by external factors [26]. Here, we suspected that the combined application of CDDP and veratric acid alleviated the cellular toxicity of CDDP, also consistent with immunofluorescence results. Hence, the addition of veratric acid weakens DNA damage induced by CDDP. Furthermore, the overall apoptosis rate increased after treatment with the new drug veratricplatin, with a marked enhancement in the proportion of early apoptosis. These findings indicate that veratricplatin could induce early cell apoptosis and inhibit the growth of tumor cells. Concomitantly, the effect of veratricplatin on the cell cycle was examined, revealing that veratricplatin treatment significantly blocked cells in the S phase, while other drugs showed no noticeable effect (Fig. 2E). This suggests that veratricplatin may inhibit the growth of FaDu cells by blocking the cell cycle.
Veratricplatin treatment effectively inhibited tumor cell migration and invasion
The poor prognosis of human pharyngeal squamous cell carcinoma is often attributed to metastasis [7]. Therefore, we have conducted scratch experiments to monitor whether veratricplatin could inhibit tumor cell metastasis in the presence of COX-2 inhibition, which is closely related to the epithelial–mesenchymal transition (EMT) mechanism [18]. Notably, veratricplatin significantly inhibited the migration of FaDu cells, determined based on the distance traveled by the damaged cell monolayer photographed at 0 and 24 h (Fig. 3A). The migration rate (0.67%) was considerably lower than that of the control group (34.03%), veratric acid (33.77%) and CDDP (13.10%), and their combination (12.27%) (Fig. 3). The results demonstrated that veratricplatin could be an effective inhibitor of FaDu cell metastasis.
A Scratch experiments showing migration inhibition of FaDu cells induced by PBS (control), veratric acid (14.0 μM), CDDP (7.0 μM), veratric acid/CDDP (14.0 μM/7.0 μM), and veratricplatin (7.0 μM) treatment for 24 h. The scale bars represent 200 μm. B Statistical analysis of migration rate in FaDu cells. C Protein expression and D quantification of E-cad in FaDu cells after drug administration. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control group
Veratricplatin treatment remarkably enhanced E-cadherin expression in FaDu cells
CDDP has been reported to affect the progression of EMT transformation in tumors [1]; accordingly, we determined the expression levels of the EMT marker E-cadherin (E-cad), a calcium-dependent transmembrane protein mainly involved in cell–cell adhesion, in response to treatment with different drug compounds. Western blot analysis illustrated that veratric acid treatment did not produce any noticeable alterations in FaDu cells (Fig. 3C, D); however, its combination with CDDP up-regulated the expression of E-cad compared to CDDP alone. Furthermore, even higher E-cad expression levels were observed in veratricplatin-treated cells than those subjected to combined treatment. Although other proteins involved in EMT were not examined, the results satisfactorily indicated that veratricplatin could inhibit EMT process. We speculate that resveratrol does not participate in the EMT conversion process, which explains the lack of significant difference between the combination of veratric acid with CDDP and CDDP alone. Inversely, veratricplatin may block the TGF-β and PI3K signaling pathways to inhibit EMT transformation and down-regulate the expression of Bcl-2 and up-regulate the expression of E-cad [29].
In vivo anti-tumor activity
To evaluate the anti-tumor activity of veratricplatin, a mouse xenograft tumor model was established. Vetroplatin showed significantly higher Pt accumulation in tumors compared with CDDP, but there was no significant difference in other (Fig. 4A). This demonstrates that veratricplatin exhibits a better targeting effect in tumors. The main organs of the mouse model were stained with hematoxylin and eosin (H&E), followed by pathological examination, showing that there was no significant difference in tissue and organ damage between the veratricplatin-treated group and other groups (Fig. 4B). Moreover, veratricplatin administration inhibited tumor growth, as evidenced by a reduction in the tumor weight of mice in the veratricplatin group (42%) compared to the control group (76%) after 15 days of intravenous injection (Fig. 4C–E); however, the overall body weight of mice showed no significant change (Fig. 4F). These findings indicate the best inhibitory effect on tumors and the minimal effect of veratricplatin administration on normal tissues and other organs compared to CDDP alone and its combination with veratric acid. Thus, veratricplatin can alleviate toxic side effects caused by CDDP.
A Bio-distribution of platinum in various mice tissue. B H&E staining images of tumor paraffin sections from the heart, liver, spleen, lung, and kidney of mice in various treatment groups. C Tumor weight of mice recorded after 15 days of intravenous injection of PBS (control), veratric acid, CDDP, veratric acid/CCDP, and veratricplatin. D Tumor volume of mice in different treatment groups. E Modifications in tumor volume during administration. F Changes in body weight during treatment. G Images of excised tumors treated with different drugs. Statistical significance: *P < 0.05, **P < 0.01
Treatment with veratricplatin inhibited angiogenesis
Tumor angiogenesis is a critical marker of solid tumor growth, invasion, and metastasis [29]. In this study, tumor vessels were labeled with CD34, and the fluorescence intensity of veratricplatin-treated mice was dramatically reduced compared to the control and CDDP-treated groups (Fig. 5). In addition, the expression of CD34 was down-regulated, indicating that veratricplatin can potentially inhibit tumor angiogenesis.
Stability and intracellular release of veratricplatin
The stability and intracellular release of veratricplatin were detected via HPLC [9], obtaining retention times of 10.2 and 8.4 min for standard veratricplatin and veratric acid (Figs. S4, S5), respectively. As depicted in Fig. 6, veratricplatin gradually produced veratric acid upon reduction over time, suggesting that both ascorbic acid (AsA) and glutathione (GSH) could reduce veratricplatin to yield the bioactive molecules veratric acid and CDDP. However, the reduction was evident in the presence of AsA than GSH.
Discussion
Currently, despite the diverse array of drug combination regimens available, the clinical chemotherapy approach for HNSCC continues to revolve around Pt(II) compounds. CDDP, carboplatin, and oxaliplatin are representative platinum drugs widely employed in the treatment of clinical tumors and established as crucial chemotherapeutic agents for clinical cancer therapy [33]. However, Pt(II) compounds exhibit characteristic drawbacks, such as poor stability, low bioavailability, significant toxic side effects, and severe cross-resistance, which limit their clinical efficacy and application. In addition, CDDP resistance poses a significant hurdle in the management of advanced HNSCC. Therefore, developing novel platinum-based drugs to overcome the inherent defects and resistance associated with original drugs and enhance their therapeutic efficacy is a major challenge for drug development and chemists.
Over the past decade, researchers have focused on designing a diverse range of complexes based on CDDP, incorporating various axial ligands such as NSAIDs, histone deacetylase inhibitors, and alkylating agents [2, 3, 27]. COX inhibitor, including celecoxib, nimesulide, etodolac, carprofen, and bendazac, are increasingly acknowledged as potential drug targets for cancer therapies. Researchers have discovered that COX inhibitors are able to suppress tumor growth, inhibit metastasis, and improving the tumor inflammatory microenvironment [10]. Platinum(IV) complexes targeting COX-2 have gained significant attention in the pharmaceutical field owing to their low toxicity, high bioavailability, and oral availability [22, 25]. Veratric acid, a kind of COX-2 inhibitor, has been reported to have wide-ranging therapeutic potentials. Given that, we constructed a novel platinum(IV) prodrugs veratricplatin based on veratric acid and evaluated its anti-tumor activities both in vitro and in vivo, especially in FaDu cell. This finding may unveil a promising prodrug for the treatment of HNSCC.
In our research, the complex veratricplatin not only exhibited a significantly lower IC50 value of (1.68 ± 0.26) μM compared to CDDP (6.86 ± 0.77) μM in FaDu cell, but showed lower cytotoxicity in human normal cells (MRC-5). Furthermore, it is well acknowledged that veratric acid enhances the inflammatory environment and exhibits a synergistic effect when combined with cisplatin, thereby augmenting its anti-tumor efficacy. Therefore, we next examined the ability of cells to uptake platinum and the results showed veratricplatin could increase the intracellular platinum content and enhance the inhibitory effect of platinum on tumor cells. Subsequently, we assessed the cytotoxicity of veratricplatin, showing an optimal inhibitory effect on FaDu cells through inhibiting migration and angiogenesis. The apoptosis was also detected using flow cytometry, the results indicating pro-apoptotic effect of veratricplatin in FaDu cells. Given the important role of EMT transformation in tumor progression, we then examined its marker protein E-cad. The results showed veratricplatin increased the expression of E-cad, prompting enhancement of EMT. Finally, the study in vivo provided more solid evidence for its good anti-tumor activity. Overall, these results demonstrate the excellent anti-tumor effects of veratricplatin, providing an experimental basis for the application of tetravalent platinum-derivative drugs in the clinical treatment of HNSCC, thereby expanding the available treatment options.
Our preliminary research also found that veratricplatin elicits robust cytotoxicity in FaDu cells, while causing comparatively milder cytotoxicity in cochlea cell. Nevertheless, the mechanisms underlying this phenomenon remain a topic for further research. Giving that ototoxicity and nephrotoxicity inevitably constrain the clinical application of cisplatin, these become crucial areas of focus for the subsequent stages of our research.
Conclusion
To summarize, veratricplatin demonstrated remarkable drug efficacy, in terms of increased cytotoxicity in vitro, reversal of CDDP resistance, promoting apoptosis, and high efficiency with low toxicity in vivo. Notably, this study presents the first application of a Pt(IV) prodrug in HNSCC therapy, with substantial activity both in vitro and in vivo. Besides, the molecular mechanism associated with veratricplatin drug in inhibiting EMT and tumor progression has been elucidated. Our findings provide promising future implementations for effective HNSCC and other solid tumor therapies in clinical settings.
Data availability
On reasonable request, the corresponding author will provide the data generated during and/or analyzed during this study.
References
Achkar IW, Abdulrahman N, Al-Sulaiti H, Joseph JM, Uddin S, Mraiche F (2018) Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med 16:96
Almotairy A, Montagner D, Morrison L, Devereux M, Howe O, Erxleben A (2020) Pt(IV) pro-drugs with an axial HDAC inhibitor demonstrate multimodal mechanisms involving DNA damage and apoptosis independent of cisplatin resistance in A2780/A2780cis cells. J Inorg Biochem 210:111125
Aputen AD, Elias MG, Gilbert J, Sakoff JA, Gordon CP, Scott KF, ALDRICH-Wright JR (2022) Potent chlorambucil-platinum(IV) prodrugs. Int J Mol Sci 23
Bhat GR, Hyole RG, Li J (2021) Head and neck cancer: Current challenges and future perspectives. Adv Cancer Res 152:67–102
Catsburg C, Gunter MJ, Chen C, Cote ML, Kabat GC, Nassir R, Tinker L, Wactawski-Wende J, Page DL, Rohan TE (2014) Insulin, estrogen, inflammatory markers, and risk of benign proliferative breast disease. Cancer Res 74:3248–3258
Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Garneau JC, Bakst RL, Miles BA (2018) Hypopharyngeal cancer: a state of the art review. Oral Oncol 86:244–250
Ghosh S (2019) Cisplatin: the first metal based anticancer drug. Bioorg Chem 88:102925
Gibson D (2016) Platinum(iv) anticancer prodrugs - hypotheses and facts. Dalton Trans 45:12983–12991
Harini G, Shree GS, Anushikaa R, Bharathi R, Aravind S, Vatsala K, Shanmugavadivu A, Selvamurugan N (2023) Antiproliferative and apoptotic effects of pH-responsive veratric acid-loaded polydopamine nanoparticles in human triple negative breast cancer cells. Chem Biodivers e202201006
He S, Li C, Zhang Q, Ding J, Liang XJ, Chen X, Xiao H, Chen X, Zhou D, Huang Y (2018) Tailoring platinum(IV) amphiphiles for self-targeting all-in-one assemblies as precise multimodal theranostic nanomedicine. ACS Nano 12:7272–7281
Jamieson ER, Lippard SJ (1999) Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev 99:2467–2498
Jia-Jun T, Su-Mei L, Liang Y, Ju-Ke M, Ya-Kui M, Hai-Bo W, Wei X (2012) Nimesulide inhibited the growth of hypopharyngeal carcinoma cells via suppressing Survivin expression. Head Neck Oncol 4:7
Johnstone TC, Suntharalingam K, Lippard SJ (2016) The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem Rev 116:3436–3486
Li X, Liu Y, Tian H (2018) Current developments in Pt(IV) prodrugs conjugated with bioactive ligands. Bioinorg Chem Appl 2018:8276139
Ma J, Wang Q, Huang Z, Yang X, Nie Q, Hao W, Wang PG, Wang X (2017) Glycosylated platinum(IV) complexes as substrates for glucose transporters (GLUTs) and organic cation transporters (OCTs) exhibited cancer targeting and human serum albumin binding properties for drug delivery. J Med Chem 60:5736–5748
Najjar A, Rajabi N, Karaman R (2017) Recent approaches to platinum(IV) prodrugs: a variety of strategies for enhanced delivery and efficacy. Curr Pharm Des 23:2366–2376
Neil JR, Johnson KM, Nemenoff RA, Schiemann WP (2008) Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-beta through a PGE2-dependent mechanisms. Carcinogenesis 29:2227–2235
Porcheri C, Meisel CT, Mitsiadis T (2019) Multifactorial contribution of notch signaling in head and neck squamous cell carcinoma. Int J Mol Sci 20
Posner M, Vermorken JB (2008) Induction therapy in the modern era of combined-modality therapy for locally advanced head and neck cancer. Semin Oncol 35:221–228
Rai V, Aggarwal SK, Verma SS, Awasthee N, Dhasmana A, Aggarwal S, Das SN, Nair MS, Yadav S, Gupta SC (2020) Epoxyazadiradione exhibit activities in head and neck squamous cell carcinoma by targeting multiple pathways. Apoptosis 25:763–782
Ravera M, Gabano E, McGlinchey MJ, Osella D (2022) Pt(IV) antitumor prodrugs: dogmas, paradigms, and realities. Dalton Trans 51:2121–2134
Redon CE, Dickey JS, Bonner WM, Sedelnikova OA (2009) gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv Space Res 43:1171–1178
Saito S, Ozawa H, Imanishi Y, Sekimizu M, Watanabe Y, Ito F, Ikari Y, Nakahara N, Kameyama K, Ogawa K (2021) Cyclooxygenase-2 expression is associated with chemoresistance through cancer stemness property in hypopharyngeal carcinoma. Oncol Lett 22:533
Schmidt C, Babu T, Kostrhunova H, Timm A, Basu U, Ott I, Gandin V, Brabec V, Gibson D (2021) Are Pt(IV) prodrugs that release combretastatin A4 true multi-action prodrugs? J Med Chem 64:11364–11378
Shin SW, Jung E, Kim S, Lee KE, Youm JK, Park D (2013) Antagonist effects of veratric acid against UVB-induced cell damages. Molecules 18:5405–5419
Spector D, Krasnovskaya O, Pavlov K, Erofeev A, Gorelkin P, Beloglazkina E, Majouga A (2021) Pt(IV) Prodrugs with NSAIDs as axial ligands. Int J Mol Sci 22
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Viallard C, Larrivee B (2017) Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20:409–426
Wang D, Zhu G, Huang X, Lippard SJ (2010) X-ray structure and mechanism of RNA polymerase II stalled at an antineoplastic monofunctional platinum-DNA adduct. Proc Natl Acad Sci U S A 107:9584–9589
Wang X, Wang X, Guo Z (2015) Functionalization of platinum complexes for biomedical applications. Acc Chem Res 48:2622–2631
Zhang D, Nie J, Wang D, Wu H, Sun L, Wang X, Wu J (2022) Engineering a multi-target therapy nanoplatform against tumor growth and metastasis via a novel NSAID-Pt(IV) prodrug. Chem Commun (Camb) 58:3803–3806
Zhang JL, Wang Z, Hu W, Chen SS, Lou XE, Zhou HJ (2013) DHA regulates angiogenesis and improves the efficiency of CDDP for the treatment of lung carcinoma. Microvasc Res 87:14–24
Zhang R, Song XQ, Liu RP, Ma ZY, Xu JY (2019) Fuplatin: an efficient and low-toxic dual-prodrug. J Med Chem 62:4543–4554
Acknowledgements
We thank KetenEdit (www.Ketengedit.com) for English language editing service during the preparation of the manuscript.
Funding
This work was supported by the Health Commission of Shandong Province (202113010614).
Author information
Authors and Affiliations
Contributions
Conceptualization, Jing Nie and Jiyong Wu; methodology, Dongbo Wang; validation, Huina Wu; writing—original draft preparation, Qian Wu; writing—review and editing, Qi Liu; supervision, Yamei Li; all the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Wu, H., Wu, Q. et al. Veratricplatin inhibits the progression of hypopharyngeal squamous cell carcinoma FaDu cells in vitro and in vivo. Cancer Chemother Pharmacol 92, 211–221 (2023). https://doi.org/10.1007/s00280-023-04560-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-023-04560-5