Abstract
Objective
The aim of this study was to evaluate the safety and efficacy of combined nimotuzumab and neoadjuvant chemoradiotherapy followed by surgery in locally advanced esophageal cancer.
Methods
Patients with clinically resectable, locally advanced esophageal cancer treated with neoadjuvant chemoradiotherapy plus nimotuzumab were eligible for study participation. Radiotherapy was administered in 1.8 Gy once daily for 5 days per week up to a total dose of 41.4 Gy. Weekly nimotuzumab (200 mg/week) was administered following paclitaxel and carboplatin on the same day for 5 weeks. The primary end-point was the pathological complete response (pCR) rate and the secondary end-point was the safety, progression-free survival (PFS) and overall survival (OS).
Results
A total of 64 patients with a median age of 58 years were enrolled in this study. pCR was observed in 51.6% patients. Grade 3 acute toxicities were observed in 6 patients (9.4%), shown as bone marrow suppression. 7 patients experienced grade 1 transient skin rash during nimotuzumab treatment. The median PFS time and OS time were 64.6 and 68.2 months.
Conclusions
Combined nimotuzumab and neoadjuvant chemoradiotherapy for clinically resectable, locally advanced esophageal cancer showed a significant anticancer effect with tolerable toxicities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal squamous cell carcinoma is one of the commonest malignant tumors of digestive system in China. Recently, preoperative neoadjuvant chemoradiotherapy is a hot treatment choice for locally advanced esophageal squamous cell carcinoma. Clinical evidences showed that neoadjuvant chemoradiotherapy combined with surgery can significantly increase the local and distant control and achieve better survival, compared with surgery alone. More and more attention has been paid to neoadjuvant chemoradiotherapy and this treatment modality has incorporated into the recommendations of the guidelines.
Cisplatin combined with 5-Fu has been the standard regimen for neoadjuvant chemotherapy in esophageal cancer. However, in recent years, many scholars have tried to optimize the neoadjuvant chemotherapy regimen, trying to apply paclitaxel combined with carboplatin in neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma patients. According to a meta-analysis published in 2015 [1], paclitaxel plus platinum regimen could significantly improve overall survival, compared with platinum plus 5-fluorouracil regimen for locoregional esophageal cancer, especially for squamous cell carcinoma. The result of CROSS study [2], in which paclitaxel and carboplatin was used, indicated the significant survival benefit of neoadjuvant chemoradiotherapy. The hazard ratio (HR) was only 0.42 for squamous cell carcinoma.
Epidermal growth factor receptor (EGFR, also named as ERBB1) is reported to be closely associated with tumor cell growth, proliferation, tumor invasion and apoptosis [3]. Precious studies indicated that EGFR was overexpressed in esophageal squamous cell carcinoma and could be a promising treatment target [4, 5]. However, most targeted therapies based on EGFR showed no efficacy in non-selected esophageal cancer patients [6,7,8]. Nimotuzumab is an IgG humanized monoclonal antibody directed against EGFR and thereby blocking the binding of EGF and finally inhibiting the EGFR signaling pathway [9]. In a vitro study [10], nimotuzumab could increase the radiosensitivity of esophageal cancer cell by up-regulating the expression IGFBP-3 and activating the EGFR pathway. Several clinical phase I–II studies indicated that nimotuzumab combined with radical radiotherapy or chemoradiotherapy enhanced the antitumor effect in patients with esophageal cancer and significantly prolonged the survival time [11, 12]. In a recent randomized phase II study [13], 109 patients with locally advanced esophageal cancer received chemoradiotherapy with or without nimotuzumab. 93% of patients in this study were squamous cell carcinoma. Addition of nimotuzumab to chemoradiotherapy significantly increased the endoscopic pathological complete response (epCR) rate and improved the survival. However, there is no study investigating the role of nimotuzumab in neoadjuvant setting in esophageal squamous cell carcinoma. The present study was designed to confirm the tolerability and efficacy of nimotuzumab in combination with paclitaxel- and carboplatin-based chemoradiotherapy in esophageal squamous cell carcinoma.
Methods
Patient eligibility
The eligibility criteria of this study were as follows: newly confirmed to have histologically or cytologically proven esophageal squamous cell carcinoma; age 20–70 years; Eastern Cooperative Oncology Group (ECOG) performance status ≤ 1; a life expectancy of 3 months or more; stages II and III (based on UICC-TNM classification [14]); adequate hepatic function [total serum bilirubin ≤ 1.5 mg d/L; aspartate transaminase (AST) and alanine transaminase (ALT) < 2.5 fold the upper limit of the normal range]; adequate renal function (creatinine ≤ 1.2 mg/dL, creatinine clearance ≥ 30 mL/min); adequate bone marrow function (white blood cell count ≥ 4000 cells/ml, neutrophile count ≥ 2000 cells/ml, hemoglobin ≥ 10 g/dL, platelet count ≥ 100,000 cells/ml); patients are willing to receive chemoradiotherapy and followed by surgical resection. Patients with any following characteristics were excluded: suffering from co-existing primary malignant; a concomitant serious illness such as uncontrolled bacterial or viral infection,heart failure, pulmonary embolism and uncontrolled diabetes mellitus. This study was approved by the Institutional Review Board of Ningbo Yinzhou people’s hospital. All patients signed informed consent before treatment. Before treatment, all patients received chest CT scan, esophageography, esophageoscope and Endoscopic ultrasonography. The extent of the disease was determined by AJCC 6th TNM staging system.
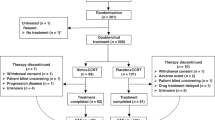
A total of 70 patients diagnosed with esophageal squamous cell carcinoma, between January 2010 and December 2016, were enrolled in the present study. After the neoadjuvant chemoradiotherapy, 64 patients underwent radical transthoracic esophagectomy and regional lymphadenectomy. Two patients refused surgery and four patients were found with tumor that could not be resected after neoadjuvant therapy. They were treated with definitive chemoradiotherapy and excluded from this further analysis.
Radiotherapy
Patients lied down of a wide aperture CT simulator couch in a supine position. A lightweight thermoplastic body mask was used to cover the lower neck, supraclavicular, chest and upper abdomen. Intravenous contrast CT with 5-mm interval was performed. Intensity-modulated radiation therapy (IMRT) plans were designed by Philips Radiation Oncology Systems (Pinnacle version 8.0).
The gross tumor volume (GTV) was delineated according to CT scans, endoscopy and esophageal barium radiography, plus enlarged lymph nodes in the mediastinum. The clinical target volume (CTV) was contoured as GTV plus an area bounded by a margin of 3 cm in superior and inferior directions, 1 cm in horizontal directions. The plan target volume (PTV) was defined as the GTV plus 5–10 mm to account for the daily setup variation and respiratory movement. IMRT was administered in 1.8 Gy once daily for 5 days per week up to a total dose of 41.4 Gy. Lung, heart and spinal cord were contoured as the surrounding organs at risk. The dose limitation was as follows: the lung mean dose was of below 16.0 Gy, V20(volume receiving above 20 Gy) < 30%, V30 (volume receiving above 30 Gy) < 20% and V5 (volume receiving above 5 Gy) < 60%, respectively. The V40 of the heart was below 40%. The maximum spinal cord dose was of 45.0 Gy. The PTV encompassed at least 95% isodose line. The dose volume histogram (DVH) was obtained for PTV, spinal cord, lung and heart.
Chemoradiotherapy plus nimotuzumab treatment
All patients were treated with paclitaxel and carboplatin weekly for 5 weeks. Patients received paclitaxel at 60 mg/m2 intravenously (1 h) with standard premedications. Standard premedications consisted of dexamethasone, diphenhydramine, and cimetidine. Carboplatin was administered at a fixed dose of area under the plasma concentration time curve (AUC) 2 mg/ml/min. Thoracic IMRT started on the same day. Chemotherapy was suspended if ≥ grade 3 hematotoxicity, esophagitis or pneumonitis were observed or patients’ willingness.
According to a phase II clinical trial [12], a 200 mg/week of nimotuzumab was the safe and effective dose on the basis of serum levels, tumor response and patients’ survival. Therefore, weekly nimotuzumab (200 mg/week), diluted in 250 mL 0.9% sodium chloride, was administered by intravenous infusion over 1 h for 5 weeks. Nimotuzumab was given after an interval of 30 min following paclitaxel and carboplatin on the same day.
Adverse events and therapeutic effect evaluation
Acute toxic effects were scored weekly, using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Chemotherapy was suspended if ≥ grade 3 hematotoxicity, esophagitis or pneumonitis was observed. Clinical treatment response was assessed according to Response Evaluation Criteria in Solid Tumor RECIST criteria (RECIST), version 1.1, combined with esophageal endoscopy. The CR (complete remission), PR (partial remission), SD (stable disease) and PD (progressive disease) were evaluated 4 weeks after chemoradiotherapy. CR is the disappearance of primary tumor with short axis of regional metastatic lymph nodes measuring < 10 mm. A decrease in the sum of target disease of ≥ 30% represents PR. SD lies between partial response and progressive disease.
Surgery and pathological workup
Radical transthoracic esophagectomy and regional lymphadenectomy were performed after a period of 4–8 weeks following the completion of chemoradiotherapy. Pathological evaluation to neoadjuvant chemoradiotherapy was carried out according to the examination of primary esophageal resected specimen and removed regional lymph nodes.
Statistical analysis
The primary endpoint of this study was pCR rate. According to the historical data, the average pCR rate was 30% and the standard deviation was 20%. The pCR rate of interest was set as 40% (α = 0.05, type II error β = 0.10). Based on this statistical hypothesis, at least 35 assessable patients were planned to be enrolled in the study. The second endpoint was survival time and toxicities. Progression-free survival (PFS) was defined as the duration from the date of first day of chemoradiotherapy to the disease progression. Overall survival (OS) was calculated as the time from the date of first day of chemoradiotherapy to death or censoring. Survival curves were estimated by the univariate Kaplan–Meier method. All statistical calculations were performed with SPSS 13.0 for Windows (Chicago, IL). p < 0.05 were considered as statistically significant.
Results
Patients’ characteristics
Finally, 64 patients were enrolled in this study. The baseline clinical characteristics are shown in Table 1. The age distribution ranged from 42 to 69 years. The median age was 58 years. 52 cases were males and 12 cases were females. The ratio of men to women is 4.33:1. Most patients (47/64, 73.4%) had a history of smoking. Before chemoradiotherapy, endoscopic ultrasonography revealed that 53 cases invaded the adventitia and 11 cases invaded the muscularis propria. According to the AJCC 6th staging system, 39 patients were N1 and 25 were N0 before treatment.
Patients received 222 cycles of weekly chemotherapy, with a median 4 cycles (range 2–6 courses). All patients completed radiotherapy without interruption. All patients were administered with five cycles of nimotuzumab. The efficacy of chemoradiotherapy according to chest CT and esophageal barium radiography was classified as PR in 10 cases (15.6%) and no change (SD) in 54 cases (84.4%).
Pathological evaluation
The median interval between chemoradiotherapy and surgery was 6 weeks (range 4–8 weeks). A total of 1344 lymph nodes were dissected, with a median number of 20 lymph nodes per patient. After careful examination by two experienced pathologists, pathological CR and no pCR were recorded in 33 (51.6%) and 31 (48.5%) patients, respectively. In patients who had no pCR resection, T down-staging was observed in 16 patients (53.3%). N down-staging was found in 38 patients (59.4%).
Adverse events
In general, the treatment-related toxicities were manageable and acceptable (Shown in Table 2). No patients died as a result of treatment-related side effects. The most common type of non-hematotoxicity was radiation-induced esophagitis. Chemotherapy was suspended in 21 patients because of intolerable esophagitis. However, all patients completed the nimotuzumab, especially in patients who had severe esophagitis. Grade 3 acute toxicities were observed in six patients (9.4%), shown as bone marrow suppression. Seven patients experienced grade 1 transient skin rash during nimotuzumab treatment. It was cured by intravenous injection of dexamethasone. Full dose of thoracic radiotherapy was completed in all patients. A grade 1/2 radiation-induced pneumonitis was observed in 19 patients. There was no difference in incidence of acute toxicities, grade 3 acute toxicities between patients treated with different cycles of chemotherapy (≤ 3 vs >3).
After surgery, patients were hospitalized for a median of 10 days (range 7–71 days). Post-operation complication occurred in 7.8% (5/64) patients, including surgical incision infection (3/64) and pneumonitis (2/64). No patients developed anastomotic leak and bleeding. No patient died during the perioperative period.
Survival
The median follow-up time was 22 months. 22 patients experienced treatment failure. Eighteen patients developed regional lymph node metastasis. Three patients had anastomotic recurrence. 15 patients experienced lung metastasis. Liver, brain, bone and pleura metastasis were observed in 2, 3, 5 and 3 patients, respectively. Three patients developed second malignancies. 23 patients died during follow-up. One patient died of esophageal fistula 1 month after operation. Three patients died because of incurable infection.
The 3-year PFS and OS were 68.4% and 65.8%. The median PFS time and OS time were 64.6 and 68.2 months (Show in Fig. 1). Pathological response was associated with better PFS (pCR vs No pCR: 64.6 m vs 29.2 m, p = 0.036) and OS (pCR vs No pCR: 68.2 m vs 30.8 m, p = 0.027). The survival curve is shown in Fig. 2. Oppositely, chemotherapy cycle, pretreatment tumor length and therapeutic effect evaluation were not related with prognosis (p > 0.05).
The median PFS time and OS time in patients who did not receive surgery (six patients, excluded from study) were 16.4 and 26.6 months. Four patients experienced treatment failure and three patients died of disease progression.
Discussion
The present study was conducted to evaluate the efficacy and safety of neoadjuvant radiotherapy with paclitaxel, carboplatin and nimotuzumab in patients with resectable esophageal squamous cell carcinoma. The result showed that this treatment modality were tolerable and effective. No patients died during neoadjuvant treatment. All patients completed the preplanned concurrent chemoradiotherapy. Five cycles of nimotuzumab were achieved in all patients. Most patients received more than 4 cycles of weekly paclitaxel and carboplatin. A pCR in both the primary tumor and lymph nodes was observed in 51.6%. The pCR rate in our study was higher than the CROSS trial [15] and in line with the recent NEOCRTEC5010 [16]. The median PFS time and OS time were 64.6 and 68.2 months. These promising results make it necessary for further investigation of this treatment protocol in randomized clinical trials.
Over the past decade, more and more studies have investigated the benefit and toxicity of nimotuzumab for esophageal cancer (Shown in Table 3). The first clinical trial of nimotuzumab in esophageal cancer was conducted by Zhao et al. [17], initiated in 2012. 11 locally advanced esophageal cancer patients received weekly nimotuzumab concurrently with chemoradiotherapy. The main treatment-related side effects were esophagitis, leucocytopenia and neutrocytopenia. These side effects were common in concurrent chemoradiotherapy and may not be associated with nimotuzumab. One-year progression-free survival and overall survival rate were 100% and 67%, respectively. The result was supported by the subsequent phase I–II clinical trials [11, 12, 18, 19]. Even in patients with metastatic esophageal cancer, the objective response rate was 42.1% [20]. In most trials, 200-mg nimotuzumab was a commonly used dose and had been proven safe and tolerable. Wang et al. [21] tried to increase the dose of nimotuzumab from 200 mg/week to 1200 mg/week and found that high dose of nimotuzumab showed limited toxicities. The toxicities did not significantly differ between high-dose group and low-dose group. Furthermore, patients in high-dose group had better prognosis, compared with patients in low-dose group (5-year OS rate 66.7% vs 31.5%, p = 0.039). Guo et al. [22] conducted a phase I trial to investigate the efficacy and toxicity of nimotuzumab combined with radiotherapy in elderly patients (> 70 years) with esophageal cancer. Only grade 1–2 radiation-induced esophagitis were reported in all patients. The overall objective response rate was 68.8%. As a monoclonal antibody against EGFR, the expression of EGFR is related to the therapeutic effect as expectation. However, Jia et al. [23] analyzed the relationship of EGFR expression and clinical response of combination treatment of nimotuzumab and chemotherapy in esophageal squamous cell carcinoma in a phase I trial. The expression of EGFR was detected by immunohistochemistry in 55 tumor samples. The objective response rate was 55.6% in high-EGFR expression group and 54.1% in low-EGFR group. Both the median PFS time and OS time was much longer in low-EGFR group than that in high-EGFR group. Between 2009 and 2011, de Castro Junior et al. [13] randomized 107 patients with esophageal cancer between chemoradiotherapy and nimotuzumab versus chemoradiotherapy alone. The endoscopic complete response (eCR) rate was 62.3% in nimotuzumab group and 37.0% in control group p = 0.002). With a median follow-up of 14.7 months, a significant survival advantage was observed in nimotuzumab group (Median overall survival time: 15.9 months vs 11.5 months, p = 0.03).
So far, most of the studies on nimotuzumab mainly focused on locally advanced or metastatic esophageal cancer. Between 2011 and 2013, Lu et al. [24] conducted a phase II study to investigate the benefit of chemotherapy plus nimotuzumab as the first-line treatment for pathologically confirmed unresectable locally advanced or metastatic esophageal cancer. A total of 59 patients were enrolled in this study. After treatment, seven patients received radical surgery. Although the pCR rate was not reported in this literature, the median duration of disease control time was 23.0 months and median OS time was not reached by the time of last follow-up. In this study, neoadjuvant chemoradiotherapy with nimotuzumab is well tolerated, with potentially promising efficacy. Although the efficacy of chemoradiotherapy according to chest CT and esophageal barium radiography was classified as PR in 10 cases (15.6%) and SD in 54 cases (84.4%), the pCR rate was 51.6%. The pCR rate was higher than that in CROSS study [2] and NEOCRTEC5010 [16]. After a median follow-up of 22 months, the 3-year PFS and OS were 68.4% and 65.8%. The median PFS time and OS time were 64.6 and 68.2 months. A survival benefit was demonstrated for patients who had high pathological response, which is in line with recent meta-analysis based on 17 studies [25].
Skin toxicity was regarded as a common side effect in patients who received anti-EGFR monoclonal antibody. According to a recent meta-analysis [26], there was no significant difference between chemoradiotherapy with nimotuzumab and without nimotuzumab. Patients with esophageal cancer underwent chemoradiotherapy plus nimotuzumab demonstrated a grade ≥ 3 skin rash in 1.5% [19] and grade ≥ 3 dermatological toxicity in 9.5% [12]. In our study, seven patients with grade one skin rash were reported and no grade ≥ 3 skin rash occurred. There were no hand–foot syndrome, dry skin, oral mucositis and alopecia occurred in this study, which is consistent with other findings [13, 18]. Furthermore, patients with skin rash were not related with better therapeutic effect or longer survival time, contrary to the results of other EGFR antibodies [27, 28].
We have to admit that there are several limitations in this study. The sample size in the present study was relatively small. Second, this study was conducted at a single institute study, and did not have control patients who received chemoradiotherapy without nimotuzumab. Therefore, a well-designed multicenter randomized trial is needed to confirm the results of this study in the future. Due to geographical factors, only patients with esophageal squamous cell carcinoma were enrolled in this study. It is unknown whether nimotuzumab is also suitable for esophageal adenocarcinoma. Pathology reports were reviewed and individually searched by two experienced pathologist; however, the pCR rate might be overestimated because not all resected specimens were detected slice by slice.
In summary, concurrent paclitaxel- and carboplatin-plus-nimotuzumab-based neoadjuvant chemoradiotherapy could achieve a promising pathological response and better overall survival. Further clinical investigation is warranted to evaluate the efficacy of this regimen for esophageal cancer.
References
Huang TC, Hsu CH, Lin CC, Tu YK (2015) Systematic review and network meta-analysis: neoadjuvant chemoradiotherapy for locoregional esophageal cancer. Jpn J Clin Oncol 45(11):1023–1028. https://doi.org/10.1093/jjco/hyv119
van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A, Group C (2012) Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366(22):2074–2084
Zhen Y, Guanghui L, Xiefu Z (2014) Knockdown of EGFR inhibits growth and invasion of gastric cancer cells. Cancer Gene Ther 21(11):491–497. https://doi.org/10.1038/cgt.2014.55
Song J, Shi W, Zhang Y, Sun M, Liang X, Zheng S (2016) Epidermal growth factor receptor and B7-H3 expression in esophageal squamous tissues correlate to patient prognosis. OncoTargets Ther 9:6257–6263. https://doi.org/10.2147/OTT.S111691
Yu W, Yang X, Chu L, Zhao K, Chen H, Xiang J, Zhang Y, Li H, Zhao W, Sun M, Wei Q, Fu X, Xie C, Zhu Z (2018) Prognostic value of EGFR family expression in lymph node-negative esophageal squamous cell carcinoma patients. Pathol Res Pract 214(7):1017–1023. https://doi.org/10.1016/j.prp.2018.04.017
Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar H, Horiba N, Dubey A, Greenberger JS, Raben A, Giguere J, Roof K, Videtic G, Pollock J, Safran H, Crane CH (2017) Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 phase 3 randomized clinical trial. JAMA Oncol 3(11):1520–1528. https://doi.org/10.1001/jamaoncol.2017.1598
Janjigian YY, Ku GY, Campbell JC, Shah MA, Capanu M, Kelsen DP, Ilson DH (2014) Phase II trial of cetuximab plus cisplatin and irinotecan in patients with cisplatin and irinotecan-refractory metastatic esophagogastric cancer. Am J Clin Oncol 37(2):126–130. https://doi.org/10.1097/COC.0b013e318271b14f
Ilson DH, Kelsen D, Shah M, Schwartz G, Levine DA, Boyd J, Capanu M, Miron B, Klimstra D (2011) A phase 2 trial of erlotinib in patients with previously treated squamous cell and adenocarcinoma of the esophagus. Cancer 117(7):1409–1414. https://doi.org/10.1002/cncr.25602
Mazorra Z, Chao L, Lavastida A, Sanchez B, Ramos M, Iznaga N, Crombet T (2018) Nimotuzumab: beyond the EGFR signaling cascade inhibition. Semin Oncol 45(1–2):18–26. https://doi.org/10.1053/j.seminoncol.2018.04.008
Zhao L, He LR, Xi M, Cai MY, Shen JX, Li QQ, Liao YJ, Qian D, Feng ZZ, Zeng YX, Xie D, Liu MZ (2012) Nimotuzumab promotes radiosensitivity of EGFR-overexpression esophageal squamous cell carcinoma cells by upregulating IGFBP-3. J Transl Med 10:249. https://doi.org/10.1186/1479-5876-10-249
Kato K, Ura T, Koizumi W, Iwasa S, Katada C, Azuma M, Ishikura S, Nakao Y, Onuma H, Muro K (2018) Nimotuzumab combined with concurrent chemoradiotherapy in Japanese patients with esophageal cancer: a phase I study. Cancer Sci 109(3):785–793. https://doi.org/10.1111/cas.13481
Liang J, Mingyan E, Wu G, Zhao L, Li X, Xiu X, Li N, Chen B, Hui Z, Lv J, Fang H, Tang Y, Bi N, Wang W, Zhai Y, Li T, Chen D, Zou S, Lu N, Perez-Rodriguez R, Zheng J, Wang L (2013) Nimotuzumab combined with radiotherapy for esophageal cancer: preliminary study of a Phase II clinical trial. OncoTargets Ther 6:1589–1596. https://doi.org/10.2147/ott.s50945
de Castro Junior G, Segalla JG, de Azevedo SJ, Andrade CJ, Grabarz D, de Araujo Lima Franca B, Del Giglio A, Lazaretti NS, Alvares MN, Pedrini JL, Kussumoto C, de Matos Neto JN, Forones NM, Fernandes Junior HJ, Borges G, Girotto G, da Silva I, Maluf-Filho F, Skare NG (2018) A randomised phase II study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: NICE trial. Eur J Cancer 88:21–30. https://doi.org/10.1016/j.ejca.2017.10.005
Mariette C, Piessen G, Briez N, Triboulet JP (2008) The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 247(2):365–371. https://doi.org/10.1097/SLA.0b013e31815aaadf
Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A, Group Cs, (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16(9):1090–1098. https://doi.org/10.1016/S1470-2045(15)00040-6
Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Yang H, Li T, Lordick F, D’Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J, Group AMETSC (2018) Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a Phase III multicenter, randomized, open-label clinical trial. J Clin Oncol 36(27):2796–2803. https://doi.org/10.1200/jco.2018.79.1483
Zhao KL, Hu XC, Wu XH, Fu XL, Fan M, Jiang GL (2012) A phase I dose escalation study of Nimotuzumab in combination with concurrent chemoradiation for patients with locally advanced squamous cell carcinoma of esophagus. Invest New Drugs 30(4):1585–1590. https://doi.org/10.1007/s10637-011-9735-0
Ramos-Suzarte M, Lorenzo-Luaces P, Lazo NG, Perez ML, Soriano JL, Gonzalez CE, Hernadez IM, Albuerne YA, Moreno BP, Alvarez ES, Callejo IP, Alert J, Martell JA, Gonzalez YS, Gonzalez YS, Astudillo de la Vega H, Ruiz-Garcia EB, Ramos TC (2012) Treatment of malignant, non-resectable, epithelial origin esophageal tumours with the humanized anti-epidermal growth factor antibody nimotuzumab combined with radiation therapy and chemotherapy. Cancer Biol Ther 13(8):600–605. https://doi.org/10.4161/cbt.19849
Ma NY, Cai XW, Fu XL, Li Y, Zhou XY, Wu XH, Hu XC, Fan M, Xiang JQ, Zhang YW, Chen HQ, Lai ST, Jiang GL, Zhao KL (2014) Safety and efficacy of nimotuzumab in combination with radiotherapy for patients with squamous cell carcinoma of the esophagus. Int J Clin Oncol 19(2):297–302. https://doi.org/10.1007/s10147-013-0564-3
Ling Y, Chen J, Tao M, Chu X, Zhang X (2012) A pilot study of nimotuzumab combined with cisplatin and 5-FU in patients with advanced esophageal squamous cell carcinoma. J Thorac Dis 4(1):58–62. https://doi.org/10.3978/j.issn.2072-1439.2011.08.02
Wang C, Fu X, Cai X, Wu X, Hu X, Fan M, Xiang J, Zhang Y, Chen H, Jiang G, Zhao K (2016) High-dose nimotuzumab improves the survival rate of esophageal cancer patients who underwent radiotherapy. OncoTargets Ther 9:117–122. https://doi.org/10.2147/OTT.S89592
Guo JH, Chen MQ, Chen C, Lu HJ, Xu BH (2015) Efficacy and toxicity of nimotuzumab combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma. Mol Clin Oncol 3(5):1135–1138. https://doi.org/10.3892/mco.2015.606
Jia J, Cui Y, Lu M, Wang X, Li J, Li J, Li Y, Zhang X, Gao J, Zhou J, Lu Z, Gong J, Yu J, Sun Z, Liu C, Shen L, Zhang X (2016) The relation of EGFR expression by immunohistochemical staining and clinical response of combination treatment of nimotuzumab and chemotherapy in esophageal squamous cell carcinoma. Clin Transl Oncol 18(6):592–598. https://doi.org/10.1007/s12094-015-1406-8
Lu M, Wang X, Shen L, Jia J, Gong J, Li J, Li J, Li Y, Zhang X, Lu Z, Zhou J, Zhang X (2016) Nimotuzumab plus paclitaxel and cisplatin as the first line treatment for advanced esophageal squamous cell cancer: a single centre prospective phase II trial. Cancer Sci 107(4):486–490. https://doi.org/10.1111/cas.12894
Tomasello G, Petrelli F, Ghidini M, Pezzica E, Passalacqua R, Steccanella F, Turati L, Sgroi G, Barni S (2017) Tumor regression grade and survival after neoadjuvant treatment in gastro-esophageal cancer: a meta-analysis of 17 published studies. Eur J Surg Oncol 43(9):1607–1616. https://doi.org/10.1016/j.ejso.2017.03.001
Li J, Yan H (2018) Skin toxicity with anti-EGFR monoclonal antibody in cancer patients: a meta-analysis of 65 randomized controlled trials. Cancer Chemother Pharmacol. https://doi.org/10.1007/s00280-018-3644-2
Kainis I, Syrigos N, Kopitopoulou A, Gkiozos I, Filiou E, Nikolaou V, Papadavid E (2018) Erlotinib-associated rash in advanced non-small cell lung cancer: relation to clinicopathological characteristics, treatment response, and survival. Oncol Res 26(1):59–69. https://doi.org/10.3727/096504017X14913452320194
Steffens M, Paul T, Hichert V, Scholl C, von Mallek D, Stelzer C, Sorgel F, Reiser B, Schumann C, Rudiger S, Boeck S, Heinemann V, Kachele V, Seufferlein T, Stingl J (2016) Dosing to rash?—the role of erlotinib metabolic ratio from patient serum in the search of predictive biomarkers for EGFR inhibitor-mediated skin rash. Eur J Cancer 55:131–139. https://doi.org/10.1016/j.ejca.2015.11.022
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qi, S., Mao, Y. & Jiang, M. A phase I study evaluating combined nimotuzumab and neoadjuvant chemoradiotherapy followed by surgery in locally advanced esophageal cancer. Cancer Chemother Pharmacol 84, 1115–1123 (2019). https://doi.org/10.1007/s00280-019-03944-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03944-w