Abstract
Purpose
New chemotherapies have become available for the treatment of advanced pancreatic cancer and have led to changes in its standard treatments. Since pancreatic cancer is becoming more common as a result of population aging, there is a need for diversification of chemotherapy.
Methods
Between March 2014 and April 2017, FOLFIRINOX (FFX) or gemcitabine plus nab-paclitaxel (G-nab) was used as first-line therapy to treat 27 patients with locally advanced and metastatic pancreatic cancer at our hospital. In this study, we retrospectively evaluated their clinical characteristics, survival outcomes and adverse events.
Results
Twelve of the 27 patients were treated with FFX, and the other 15 patients were treated with G-nab. The disease control rate was 86.7% in the G-nab group and 75% in the FFX group. Median OS time was 8.9 months in the FFX group and 11.8 months in the G-nab group. The 1-year survival rate was 46.6% in the G-nab group and 16.6% in the FFX group. The second-line treatment rate was 40% in the G-nab group and 66.7% in the FFX group. The grade 3–4 neutropenia rate was 20% in the G-nab group and 25% in the FFX group. No patients developed febrile neutropenia, or severe nausea, diarrhea, or anorexia. The peripheral sensory neuropathy rate was 73.3% in the G-nab group and 75% in the FFX group.
Conclusions
Although G-nab and FFX are effective treatments for advanced pancreatic cancer, the G-nab group had a higher 1-year survival rate, and G-nab can be more safely administered to older patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of pancreatic cancer patients in Japan has been increasing, and pancreatic cancer is now the fifth most common cause of cancer-related deaths in the country, with approximately 30,000 new cases reported annually [1]. Unfortunately, pancreatic cancer has a poor prognosis, and in Japan its morbidity rate is almost equal to its mortality rate [2]. Although surgery is the only curative treatment for pancreatic cancer, most cases are diagnosed at an advanced stage that precludes surgery, and surgery alone is associated with a low survival rate. Since most patients treated surgically receive adjuvant chemotherapy, chemotherapy plays an important role in the management of pancreatic cancer. Historically, gemcitabine monotherapy has been the standard treatment for advanced pancreatic cancer. However, FOLFIRINOX (FFX) and gemcitabine plus nab-paclitaxel (G-nab) are two of several new regimens that have been developed to treat advanced pancreatic cancer, and they are expected to decrease tumor burden and increase survival in advanced pancreatic cancer cases [3, 4]. Because elderly patients typically have many complications and poor performance status (PS), it is important for physicians to have access to a variety of chemotherapies that can be used to effectively treat them, and in this retrospective study we evaluated the efficacy and safety of FFX and G-nab as a first-line therapy for locally advanced and metastatic pancreatic cancer.
Methods
Patients
The subjects of this retrospective study were 27 patients who during the period between March 2014 and April 2017 had been diagnosed with pancreatic cancer at Tokyo Women’s Medical University Hospital and treated with FFX or G-nab as first-line therapy: 14 of the patients had been diagnosed with locally advanced and the other 13 patients had been diagnosed with metastatic pancreatic cancer. All 27 patients had an Eastern Cooperative Oncology Group PS of 0–1, and their objective response to treatment was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST). A diagnosis of adenocarcinoma was histologically and cytologically confirmed in every case. The eligibility criteria for FFX therapy included a total bilirubin level that was within the normal range, platelet count > 100,000/mm3, and neutrophil count > 2000/mm3. Patients were considered ineligible for FFX if they had diarrhea or watery stools were homozygous for UGT1A1*6 or UGT1A1*28, or were heterozygous for UGT1A1*6 and UGT1A1*28. The eligibility criteria for G-nab therapy included leukocyte count < 12,000/mm3, neutrophil count > 1500/mm3, platelet count > 100,000/mm3, hemoglobin concentration > 9.0 g/dl, serum aspartate transaminase and alanine transaminase levels less than 2.5 times their maximum normal values, and total serum bilirubin value less than 1.5 times its maximum normal value. All patients had normal renal function and less than grade 1 peripheral sensory neuropathy.
Treatment and toxicity
Patients in the G-nab group received a 30-min intravenous infusion of nab-paclitaxel at a dose of 125 mg/m2 followed by a 30-min intravenous infusion of gemcitabine at a dose of 1000 mg/m2 on days 1, 8, and 15 every 4 weeks in 1 cycle. If treatment on day 15 was skipped, a new cycle was started the following week. If treatment on day 8 was skipped, the patient received the regular treatment on day 15. Neutropenia was treated with granulocyte colony-stimulating factor (GCS-F). Both the nab-paclitaxel dose (100 mg/m2) and the gemcitabine dose (800 mg/m2) were reduced, if toxicity other than peripheral sensory neuropathy was detected; the nab-paclitaxel dose alone was reduced, if peripheral sensory neuropathy was detected.
Patients in the FFX group received a 2-h intravenous infusion of oxaliplatin at a dose of 85 mg/m2, 2-h intravenous infusion of l-leucovorin at 200 mg/m2, and a 90-min intravenous infusion of irinotecan at a dose of 180 mg/m2, followed by an intravenous bolus of 5-FU at a dose of 400 mg/m2, and then a 46-h continuous intravenous infusion of 5-FU at dose of 2400 mg/m2. This treatment was repeated every 2 weeks and continued until disease progression or a severe adverse event was detected. Neutropenia was treated with GCS-F. If neutropenia was detected, the dose of irinotecan was reduced to 150 mg/m2, and the intravenous bolus of 5-FU was omitted. The oxaliplatin alone was reduced, if peripheral sensory neuropathy was detected.
Toxicity was evaluated using the Common Terminology Criteria for Adverse Events (version 4.0). The all-grade and grade 3–4 toxicities rates of neutropenia, anemia, thrombocytopenia, diarrhea, and peripheral sensory neuropathy in the two groups were compared.
Evaluation and statistical analysis
Enhanced computed tomography was used to evaluate the pancreatic lesion at 8–12 weeks after treatment, and the response was graded according to the RECIST criteria as partial response (PR), stable disease (SD), or progressive disease (PD). Overall survival (OS) time and progression-free survival (PFS) time were analyzed using the Kaplan–Meier method and the Wilcoxon test. OS was defined as the time from the diagnosis of pancreatic cancer until death. The response rate (RR) and disease control rate (DCR) were calculated based on the values for PR and SD. The tumor marker (CA19-9 or DUPAN2) reduction rates were evaluated by comparing the values before and after chemotherapy. All analyses were performed using JMP Pro software (version 12.1).
Results
Patient characteristics
Table 1 shows the patients’ characteristics. Twelve patients were treated with FFX (7 male and 5 female, median age 62 years), and 15 patients were treated with G-nab (8 male and 7 female, median age 63 years). Three of the 12 patients in the FFX group received modified FFX (mFFX) as their first treatment. In the G-nab group, 12 patients received full treatment doses and 3 patients received reduced doses. Two patients who received the reduced G-nab doses were > 75 years old, and the third patient was > 80 years old. One of the 15 patients in the G-nab group underwent surgery after 2 cycles of G-nab therapy.
In the FFX group, the tumor arose in the head of the pancreas in six cases, the body of the pancreas in four cases, and the tail of the pancreas in two cases. In the G-nab group, the tumor arose in the head of the pancreas in ten cases, the body of the pancreas in four cases, and the tail of the pancreas in one case. There was a family history of carcinoma in three cases in the G-nab group and in four cases in the FFX group. Two patients in the G-nab group had diabetes mellitus (Table 1).
Efficacy
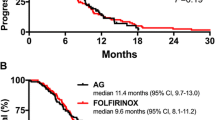
In the G-nab group, 2 patients had a PR, 11 patients had a SD, and 2 patients had PD. In the FFX group, 3 patients had a PR, 6 patients had SD, and 3 patients had PD. The RR was 13.3% in the G-nab group and 25% in the FFX group. The DCR was 86.7% in the G-nab group and 75% in the FFX group. The percentage of patients whose serum tumor marker values decreased was 73.3% in the G-nab group and 50% in the FFX group (Table 2). Median OS in the FFX group was 9.7 months, but median OS was not calculated in the G-nab group because there were many survivors (Fig. 1). The median OS of the 13 patients who were diagnosed with metastatic pancreatic cancer was 9.7 months in the FFX group and 9.8 months in the G-nab group (Fig. 2). The 1-year survival rate of all patients was 46.7% in the G-nab group and 25% in the FFX group. Median PFS of all patients was 8.8 months in the FFX group, and many of patients as a whole who developed PD during first-line chemotherapy were started on second-line therapy (Fig. 3). The proportion of the pancreatic cancer patient group as a whole that received second-line therapy was 33.3% in the G-nab group and 66.7% in the FFX group. In the G-nab group, three patients received S1, one patient received FFX, and one patient underwent surgery. In the FFX group, five patients received G-nab combination therapy, two patients received gemcitabine alone, and one patient received S1 with heavy particle therapy (Table 3).
Adverse events
The most frequent toxicity was neutropenia. Neutropenia was diagnosed in 85.7% of the patients in the G-nab group and in 88.9% of the patients in the FFX group. The grade 3–4 neutropenia rate was 60% in the G-nab group and 58.3% in the FFX group. No patients developed febrile neutropenia (FN). Table 4 shows the adverse events in detail. The peripheral sensory neuropathy rate was 73.3% in the G-nab group and 75% in the FFX group. One patient developed severe peripheral sensory neuropathy, but it resolved after chemotherapy was discontinued. There were no cases of severe nausea, diarrhea, or anorexia. Interstitial pneumonia is a severe complication associated with gemcitabine and nab-paclitaxel treatment, and it developed in 26.7% of the patients in the G-nab group. No patients in the FFX group developed interstitial pneumonia. The thrombosis rate was much higher in the FFX group than in the G-nab group (41.6 vs. 6.7%) (Table 4).
Discussion
Historically, the standard chemotherapy for advanced pancreatic cancer has been gemcitabine monotherapy; however, since more effective alternatives, including FFX or G-nab [5,6,7,8], have been developed, recent guidelines recommend first-line treatment with FFX or G-nab [9]. The guidelines’ recommendations are supported by the results of the MPACT trial and ACCORD 11 trials, which confirmed that FFX and G-nab were superior to gemcitabine monotherapy [3, 4]. However, since which of these two treatments was the superior treatment for advanced pancreatic cancer was unclear, in the present study we compared the outcomes and toxicities of these two first-line therapies in patients with locally advanced or metastatic pancreatic cancer who had been diagnosed and treated at our hospital.
Although the difference in median age between the FFX group and G-nab group in the present study was not statically significant, the age range of the G-nab group (44–82 vs. 50–72 years in the FFX group) was broader, possibly indicating that elderly patients tolerate G-nab better than FFX. In contrast, the FFX group tended to be younger and have better PS, and that may have been related to adequate age (i.e., < 70 years old) and higher rate of adverse events. The mFFX regimen (no 5-FU bolus and an irinotecan dose of 150 mg/m2) is an alternative regimen designed to minimize the risk of adverse events, and since its efficacy is recognized as being nearly equal to that of FFX [10], members of the Japan Clinical Oncology Group (JCOG) recommend mFFX as a treatment for advanced pancreatic cancer. However, many patients in the FFX group received the full FFX regimen before this approach was recommended, and that is likely to explain why most patients required a dose reduction because of a severe adverse event (e.g., neutropenia). Only one patient in the FFX group in our study received mFFX from the beginning. By contrast, most patients in the G-nab group received the full doses, and only two older patients in the G-nab group required dose reductions: one was effectively treated with the lower dose regimen, and the other was treated surgically after the chemotherapy. However, the distant metastasis rate tended to be higher in the G-nab group, because there were many cases of metastatic pancreatic cancer in the G-nab group.
The FFX group had a DCR of 75% and a RR of 25%, and these results were similar to the results of a phase II study of Japanese patients (DCR 69.4%, RR 38.9%) [11]. By contrast, the G-nab group had a DCR of 86.7% and an RR of 13.3%, both of which were lower than the rates in the phase II study (DCR 94%, RR 58.8%) [12]. The median OS of 9.8 months of the patients with metastasis in the G-nab group was lower than the median OS of 13.5 months in the phase II study. The poorer results in the present study may be related to the relatively low rate of transitioning to second-line treatment after G-nab therapy, which may have been attributable to the relatively poor PS, poor general condition of the patients, and poorer cancer prognosis (vs. the FFX group). Moreover, the median OS of the FFX group in this study was 9.7 months, and lower than the median OS of 10.7 months in the phase II study. By contrast, the median OS in the G-nab group was not calculated because most patients survived while receiving G-nab treatment. The 1-year survival rate of the G-nab group in the phase II study was 61.8%, and comparable to the 67.5% in the present study, but the 1-year survival rate of the FFX group in our study was 25%, and lower than the 41.5% in the previous phase II study. This discrepancy between 1-year survival rate of the FFX group in our study and phase II study may be related to the small number of patients in the present study. Interestingly, the rates of transition to second-line treatment in the present study were lower than in the previous phase II study (FFX 66.7 vs. 92%, G-nab 33.3 vs. 97%), and these differences may be related to the small number of patients who had many previous histories, and to the timing of the transition to second-line treatment.
The grade 3–4 neutropenia rates in the G-nab group and FFX group in the present study were the same: 58.3%, but lower than the rates in the phase II study [13,14,15], possibly because many of the patients in the FFX group in the present study had received mFFX. Moreover, there were no cases of febrile neutropenia or grade 3–4 nausea or diarrhea in the present study, and these findings may related to the administration of preventive treatment, such as selective NK1 receptor antagonist antiemetic drugs and dexamethasone during chemotherapy [9]. Taxane drugs are known to cause peripheral sensory neuropathy as an adverse effect; however, the incidences of grade 3–4 peripheral sensory neuropathy in the present study were similar in both groups [15]. The lower rate of severe peripheral sensory neuropathy in the two groups is likely to have been because treatment was discontinued or the doses were carefully reduced whenever a patient developed peripheral sensory neuropathy. There have been several reports of a correlation between drugs for chemotherapy and the occurrence of interstitial pneumonia. Interstitial pneumonia is a known complication in treatment with G-nab, and it was diagnosed in 26.7% of the patients in the G-nab group. However, none of the patients in the FFX group developed interstitial pneumonia. Generically, interstitial pneumonia induced nab-paclitaxel was reported about 1.0%, and there was a high rate of complication by interstitial pneumonia in the present study. Several cancers are associated with an increased risk of thrombosis, and pancreatic cancer, in particular, has been associated with higher rate of occurrence of thrombosis and Trousseau syndrome [10]. The thrombosis rate during treatment in the present study was 41.6% in the FFX group and 6.7% in the G-nab group, and the thrombosis may have been caused by treatment-related coagulation abnormalities. The thrombosis in the ACCORD11 study was 6.6%, and thrombosis rate in our study was higher. Many of the sites of thrombosis were near central venous catheters that had been inserted to administer FFX therapy, and the thrombosis tended to involve abnormalities of the blood coagulation system. In additional limitation, it was a retrospective single-center study of only 27 patients, and has bias than prospective studies. We should investigate a prospective study of a large number of pancreatic cancer patients in the future.
In conclusion, no significant differences were found between the efficacy of FFX and G-nab in the present study. However, G-nab therapy was associated with relatively tolerable toxicities and was used to treat older patients and patients with poorer PS. Furthermore, since FFX therapy has age-based limitations, requires genetic testing, and requires placement of a central venous catheter, G-nab may be more appropriate as a first-line therapy for advanced pancreatic cancer. If patients do not respond to first-line G-nab therapy, it may be prudent to consider S1 monotherapy, based on the likelihood that the patient has poor PS and may develop neutropenia.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics 2012. CA Cancer J Clin 65:87–108
Japanese Ministry of Health, Labour and Welfare (2012) Statistical investigation result
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Von Hoff D, Ervin T, Arena F et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Von Hoff DD, Ramanathan RK, Borad MJ, LAheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M (2011) Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 29:4548–4554
Ghosn M, Ibrahim T, Assi T, EI Rassy E, Kourie HR, Kattan J (2016) Dilemma of first-line regimens in metastatic pancreatic adenocarcinoma. World J Gastroenterol 22:10124–10130
Hashimoto M, Hikichi T, Suzuki T, Tai M, Ichii O, Matsuhashi N, Kita E, Takahashi S, Okubo Y, Hakozaki H, Ejiri Y, Ohira H (2017) Successful chemotherapy with modified FOLFIRINOX for pancreatic acinar cell carcinoma. Clin J Gastroenterol 10:564–569
Date K, Ettelale C, Maraveyas A (2017) Tissue factor-bearing microparticles and inflammation: a potential mechanism for the development of venous thromboembolism in cancer. J Thromb Haemost 15:2289–2299
Lakatos G, Petranyi A, Szucs A, Nehez L, Harsanyi L, Hegyi P, Bodoky G (2017) Efficacy and safety of FOLFIRINOX in locally advanced pancreatic cancer. A single center experience. Pathol Oncol Res 23:753–759
Ansari D, Ansari D, Andersson R, Andren-Sandberg A (2015) Pancreatic cancer and thromboembolic disease, 150 years after Trousseau. Hepatobiliary Surg Nutr 4:325–335
Okusaka T, Ikeda M, Fukutomi A, Ioka T, Ohkawa S, Isayama H, Boku N (2014) Phase II study FOLFIRINOX for chemotherapy-naïve Japanese. Cancer Sci 105:1321–1326
Ueno H, Ikeura M, Ueno M, Mizuno N, Ioka T, Omura Y, Nakajima TE, Furuse J (2016) Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 77:595–603
Ando Y, Saka H, Ando M et al (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetics analysis. Cancer Res 60:6921–6929
Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Stomiolo AM, Tarassoff P, Neison R, Dorr FA, Stephens CD, Von Hoff DD (1997) Improvement in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: a randomized trial. J Clin Oncol 15:2403–2413
Alistar A, Morris BB, Desnoyer R et al (2017) Safety and tolerability of the first-in-class agent CPI-613 in combination with modified FOLFIRINOX in patients with metastatic pancreatic cancer: a single-centre, open-label, dose-escalation, phase 1 trial. Lancet Oncol 18:770–778
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendment or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was not obtained, because our study was a retrospective analysis.
Rights and permissions
About this article
Cite this article
Tahara, J., Shimizu, K., Otsuka, N. et al. Gemcitabine plus nab-paclitaxel vs. FOLFIRINOX for patients with advanced pancreatic cancer. Cancer Chemother Pharmacol 82, 245–250 (2018). https://doi.org/10.1007/s00280-018-3611-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-018-3611-y